Polyphenol Rich Sugarcane Extract Reduces Body Weight in C57/BL6J Mice Fed a High Fat, High Carbohydrate Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PRSE
2.2. Animals and Housing
2.3. Measurement of Energy Expenditure and Activity
2.4. Measurement of Digestibility by Bomb Calorimetry of Feed and Faeces
2.5. Measurement of Faecal Lipid, Carbon and Nitrogen by Elemental Analyser Method
2.6. Measurement of Glycaemic Response by the Glucose Tolerance Test Method
2.7. Measurement of Body Composition by Dual Energy X-ray Absorptiometry (DEXA) Method
2.8. Post Mortem and Tissue Collection
2.9. Measurement of Adipocyte Hormones in Plasma by ELISA
2.10. Analysis of mRNA Expression by RT-PCR
2.11. Statistical Analysis
3. Results
3.1. Effect of PRSE on Body Weight, Food Intake and Water Intake
3.2. Glucose Tolerance
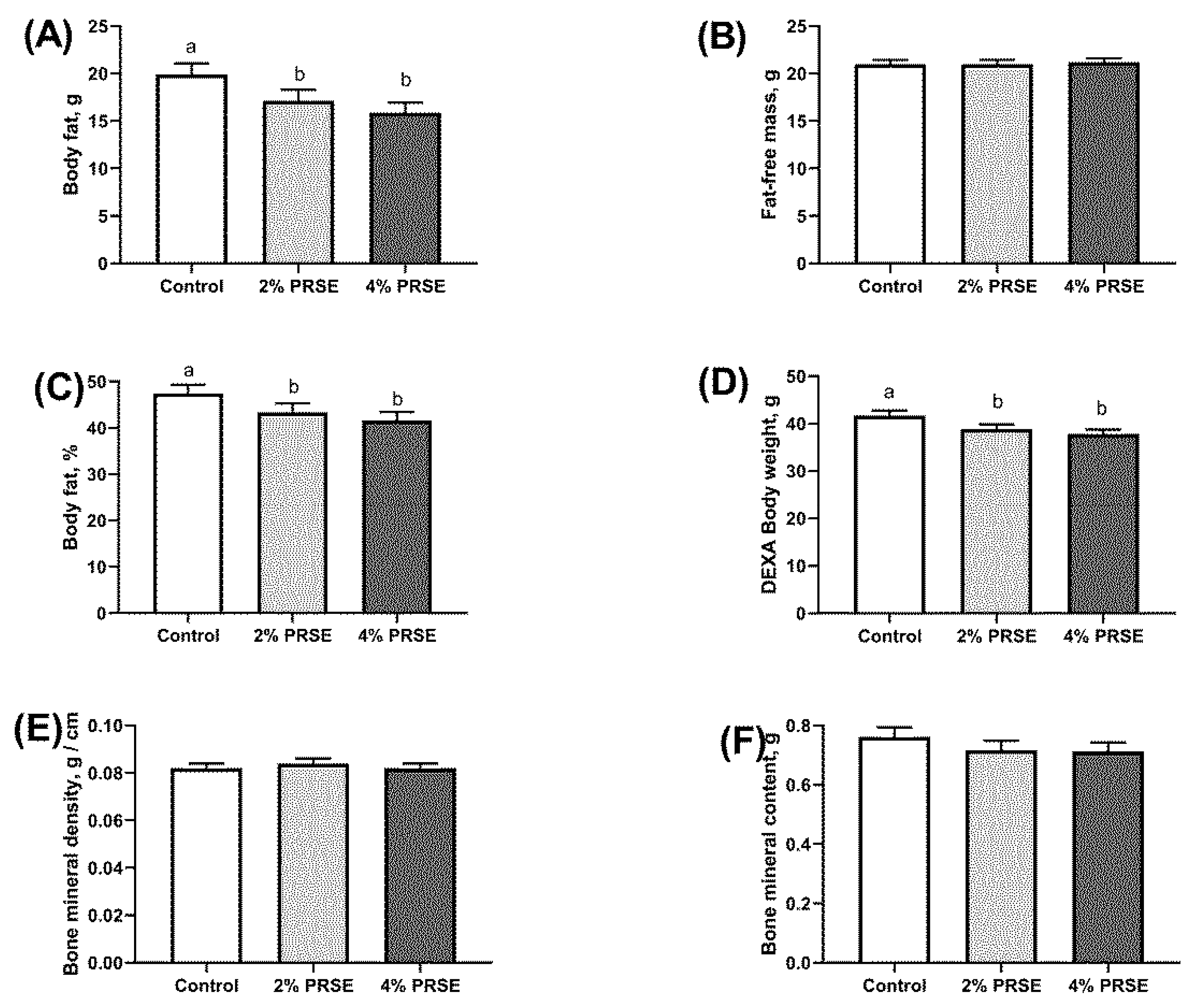
3.3. Dual Energy X-ray Absorptiometry (DEXA) Analysis of Body Composition
3.4. Energy Expenditure
3.5. Plasma Hormone Levels
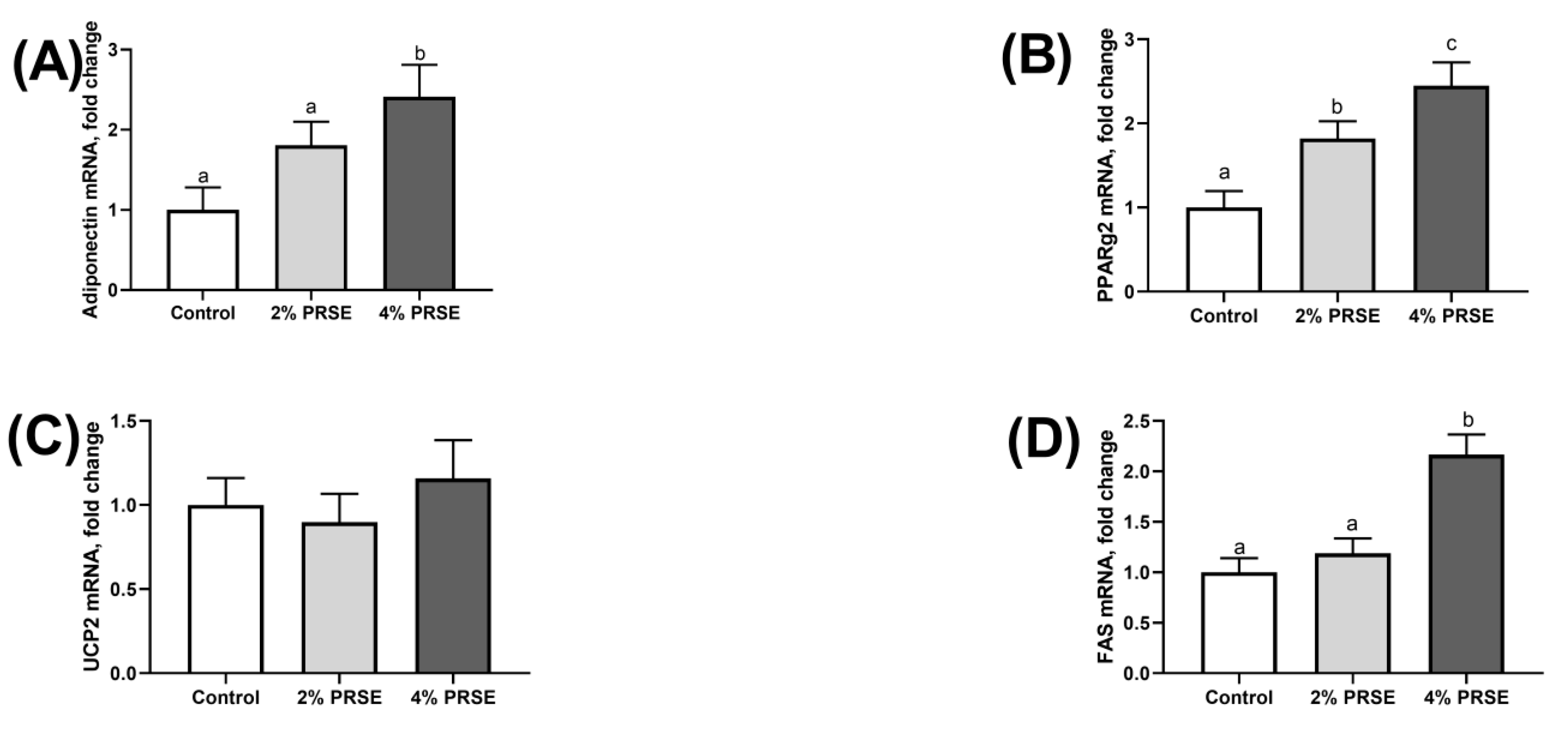
3.6. mRNA Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| AUC | Area Under the Curve |
| DEXA | Dual energy x-ray absoptiometry |
| GTT | Glucose Tolerance Test |
| PGC1α | Peroxisome proliferator-activated receptor-γ coactivator 1-α |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PRSE | Polyphenol Rich Sugarcane Extract |
| UCP2 | Mitochondrial Uncoupling Protein 2 |
| w/w | weight for weight |
References
- Meydani, M.; Hasan, S.T. Dietary Polyphenols and Obesity. Nutrients 2010, 2, 737–751. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in as-sociation with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [Green Version]
- Murase, T.; Misawa, K.; Minegishi, Y.; Aoki, M.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee polyphenols suppress di-et-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2010, 300, E122–E133. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-K.; Cheung, C.; Reuhl, K.R.; Liu, A.B.; Lee, M.-J.; Lu, Y.-P.; Yang, C.S. Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-gallate on Newly Developed High-Fat/Western-Style Diet-Induced Obesity and Metabolic Syndrome in Mice. J. Agric. Food Chem. 2011, 59, 11862–11871. [Google Scholar] [CrossRef] [Green Version]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The Major Green Tea Polyphenol, (-)-Epigallocatechin-3-Gallate, Inhibits Obesity, Metabolic Syndrome, and Fatty Liver Disease in High-Fat–Fed Mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, S.; Lambert, J.D. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. Eur. J. Nutr. 2014, 53, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Duarte-Almeida, J.M.; Novoa, A.V.; Linares, A.F.; Lajolo, F.M.; Genovese, M.I. Antioxidant Activity of Phenolics Compounds From Sugar Cane (Saccharum officinarum L.) Juice. Plant Foods Hum. Nutr. 2006, 61, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Almeida, J.M.; Salatino, A.; Genovese, M.I.; Lajolo, F.M. Phenolic composition and antioxidant activity of culms and sug-arcane (Saccharum officinarum L.) products. Food Chem. 2011, 125, 660–664. [Google Scholar] [CrossRef]
- Kong, F.; Yu, S.; Zeng, F.; Wu, X. Preparation of Antioxidant and Evaluation of the Antioxidant Activities of Antioxidants Extracted from Sugarcane Products. J. Food Nutr. Res. 2015, 3, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Luo, Z.; Zhang, Y.; Zhong, Z.; Lu, B. Phytochemical contents and antioxidant capacities of different parts of two sugar-cane (Saccharum officinarum L.) cultivars. Food Chem. 2014, 151, 452–458. [Google Scholar] [CrossRef]
- Lee, D.; Go, G.-W.; Imm, J.-Y. Tricin, a methylated cereal flavone, suppresses fat accumulation by downregulating AKT and mTOR in 3T3-L1 preadipocytes. J. Funct. Foods 2016, 26, 548–556. [Google Scholar] [CrossRef]
- Callcott, E.T.; Santhakumar, A.; Luo, J.; Blanchard, C.L. Therapeutic potential of rice-derived polyphenols on obesity-related oxidative stress and inflammation. J. Appl. Biomed. 2018, 16, 255–262. [Google Scholar] [CrossRef]
- Ando, C.; Takahashi, N.; Hirai, S.; Nishimura, K.; Lin, S.; Uemura, T.; Goto, T.; Yu, R.; Nakagami, J.; Murakami, S.; et al. Luteolin, a food-derived flavonoid, suppresses adipocyte-dependent activation of macrophages by inhibiting JNK activation. FEBS Lett. 2009, 583, 3649–3654. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y.; Igarashi, K.; Li, Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-A y mice. Biosci. Biotechnol. Biochem. 2016, 80, 1580–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Okuda, H.; Arichi, S. Effects of non-sugar fraction in black sugar on lipid and carbohydrate metabolism; Part I. Planta Med. 1984, 50, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Okuda, H.; Shoji, N.; Takemoto, T.; Arichi, S. Effects of Non-Sugar Fraction in Black Sugar on Lipid and Carbohydrate Metabolism; Part II New Compounds Inhibiting Elevation of Plasma Insulin. Planta Med. 1984, 50, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.; Kitchen, B. Antioxidant and Anti-Diabetic Functions of a Polyphenol-Rich Sugarcane Ex-tract. J. Am. Coll. Nutr. 2019, 20, 1. [Google Scholar]
- Montgomery, M.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Bray, G.A.; Popkin, B.M. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes?: Health be damned! Pour on the sugar. Diabetes Care 2014, 37, 950–956. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphe-nols and obesity. J. Nutr. Biochem. 2014, 25, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loureiro, G.; Martel, F. The effect of dietary polyphenols on intestinal absorption of glucose and fructose: Relation with obe-sity and type 2 diabetes. Food Rev. Int. 2019, 35, 390–406. [Google Scholar] [CrossRef]
- Ono, Y.; Hattori, E.; Fukaya, Y.; Imai, S.; Ohizumi, Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J. Ethnopharmacol. 2006, 106, 238–244. [Google Scholar] [CrossRef]
- Luo, H.; Kashiwagi, A.; Shibahara, T.; Yamada, K. Decreased bodyweight without rebound and regulated lipoprotein me-tabolism by gymnemate in genetic multifactor syndrome animal. Mol. Cell. Biochem. 2007, 299, 93–98. [Google Scholar] [CrossRef]
- Adekunle, I.A.; Imafidon, C.E.; Oladele, A.A.; Ayoka, A.O. Ginger polyphenols attenuate cyclosporine-induced disturbances in kidney function: Potential application in adjuvant transplant therapy. Pathophysiology 2018, 25, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.E.; El Gedaily, R.A.; Mocan, A.; Farag, M.A.; El-Seedi, H.R. Profiling Metabolites and Biological Activities of Sugarcane (Saccharum officinarum Linn.) Juice and Its Product Molasses via a Multiplex Metabolomics Approach. Molecules 2019, 24, 934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, F.; Yu, S.; Zeng, F.; Wu, X. Phenolics Content and Inhibitory Effect of Sugarcane Molasses on α-Glucosidase and α-Amylase In Vitro. Sugar Tech 2016, 18, 333–339. [Google Scholar] [CrossRef]
- Myers, M.G., Jr.; Leibel, R.L.; Seeley, R.J.; Schwartz, M.W. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol. Metab. 2010, 21, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Fukuchi, Y.; Hiramitsu, M.; Okada, M.; Hayashi, S.; Nabeno, Y.; Osawa, T.; Naito, M. Lemon Polyphenols Suppress Diet-induced Obesity by Up-Regulation of mRNA Levels of the Enzymes Involved in β-Oxidation in Mouse White Adipose Tissue. J. Clin. Biochem. Nutr. 2008, 43, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Aguilera, C.M.; Gil, A. A Systematic Review of the Efficacy of Bioactive Compounds in Cardiovascular Disease: Phenolic Compounds. Nutrients 2015, 7, 5177–5216. [Google Scholar] [CrossRef] [Green Version]
- Asterholm, I.W.; Scherer, P.E. Enhanced Metabolic Flexibility Associated with Elevated Adiponectin Levels. Am. J. Pathol. 2010, 176, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, K.; Bhattacharyya, M. Adiponectin: Probe of the molecular paradigm associating diabetes and obesity. World J. Diabetes 2015, 6, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Wei, L.; Castro-Muñozledo, F.; Koo, W.L. (−)-Epigallocatechin-3-gallate blocks 3T3-L1 adipose conversion by inhibi-tion of cell proliferation and suppression of adipose phenotype expression. Life Sci. 2011, 89, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Toda, T.; Okaniwa, T.; Ito, K.; Ochiai, W.; Sugiyama, K. Anti-Obesity and Anti-Diabetic Effects of Acacia Polyphenol in Obese Diabetic KKAy Mice Fed High-Fat Diet. Evidence-Based Complement. Altern. Med. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Herranz-López, M.; Barrajón-Catalán, E.; Segura-Carretero, A.; Menéndez, J.A.; Joven, J.; Micol, V. Lemon verbena (Lippia citriodora) polyphenols alleviate obesity-related disturbances in hypertrophic adipocytes through AMPK-dependent mechanisms. Phytomedicine 2015, 22, 605–614. [Google Scholar] [CrossRef]
- Noratto, G.; Martino, H.S.; Simbo, S.; Byrne, D.; Mertens-Talcott, S.U. Consumption of polyphenol-rich peach and plum juice pre-vents risk factors for obesity-related metabolic disorders and cardiovascular disease in Zucker rats. J. Nutr. Biochem. 2015, 26, 633–641. [Google Scholar] [CrossRef]
- Maeda, N.; Takahashi, M.; Funahashi, T.; Kihara, S.; Nishizawa, H.; Kishida, K.; Nagaretani, H.; Matsuda, M.; Komuro, R.; Ouchi, N.; et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 2001, 50, 2094–2099. [Google Scholar] [CrossRef] [Green Version]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional Regulation of Human and Rat Hepatic Lipid Metabolism by the Grapefruit Flavonoid Naringenin: Role of PPARα, PPARγ and LXRα. PLoS ONE 2010, 5, e12399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, M.M.; Larsen, J.Ø.; Hamilton-Dutoit, S.; Clasen, B.F.; Jessen, N.; Paulsen, S.K.; Kjær, T.N.; Richelsen, B.; Pedersen, S.B. Resveratrol up-regulates hepatic uncoupling protein 2 and prevents development of nonalcoholic fatty liver disease in rats fed a high-fat diet. Nutr. Res. 2012, 32, 701–708. [Google Scholar] [CrossRef]
- Lee, D.; Imm, J.-Y. Antiobesity Effect of Tricin, a Methylated Cereal Flavone, in High-Fat-Diet-Induced Obese Mice. J. Agric. Food Chem. 2018, 66, 9989–9994. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Fujimori, K. Antiadipogenic Effect of Dietary Apigenin through Activation of AMPK in 3T3-L1 Cells. J. Agric. Food Chem. 2011, 59, 13346–13352. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, L.; Dong, J.; Zhang, X.; Chen, Y.G.; Bao, B.; Liu, J. Low-dose diet supplement of a natural flavonoid, luteolin, amelio-rates diet-induced obesity and insulin resistance in mice. Mol. Nutr. Food Res. 2014, 58, 1258–1268. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flavel, M.; Ellis, T.P.; Stahl, L.; Begg, D.; Smythe, J.; Ilag, L.L.; Weisinger, R.S.; Kitchen, B.; Jois, M. Polyphenol Rich Sugarcane Extract Reduces Body Weight in C57/BL6J Mice Fed a High Fat, High Carbohydrate Diet. Appl. Sci. 2021, 11, 5163. https://doi.org/10.3390/app11115163
Flavel M, Ellis TP, Stahl L, Begg D, Smythe J, Ilag LL, Weisinger RS, Kitchen B, Jois M. Polyphenol Rich Sugarcane Extract Reduces Body Weight in C57/BL6J Mice Fed a High Fat, High Carbohydrate Diet. Applied Sciences. 2021; 11(11):5163. https://doi.org/10.3390/app11115163
Chicago/Turabian StyleFlavel, Matthew, Timothy P. Ellis, Lauren Stahl, Denovan Begg, Jason Smythe, Leodevico L. Ilag, Richard S. Weisinger, Barry Kitchen, and Markandeya Jois. 2021. "Polyphenol Rich Sugarcane Extract Reduces Body Weight in C57/BL6J Mice Fed a High Fat, High Carbohydrate Diet" Applied Sciences 11, no. 11: 5163. https://doi.org/10.3390/app11115163
APA StyleFlavel, M., Ellis, T. P., Stahl, L., Begg, D., Smythe, J., Ilag, L. L., Weisinger, R. S., Kitchen, B., & Jois, M. (2021). Polyphenol Rich Sugarcane Extract Reduces Body Weight in C57/BL6J Mice Fed a High Fat, High Carbohydrate Diet. Applied Sciences, 11(11), 5163. https://doi.org/10.3390/app11115163







