Identification and Quantification of Valuable Compounds in Red Grape Seeds
Abstract
:1. Introduction
1.1. The Benefits of Grape Seeds
1.2. The Antioxidant and Antiradical Capacity of Grape Seeds
2. Materials and Methods
2.1. Materials
2.2. Procedure Methods
2.2.1. Determination of the Total Polyphenol Content of the Grape Seeds
2.2.2. Determination of the Antioxidant Capacity of the Grape Seeds
2.2.3. Determination of the Antiradical Capacity of the Grape Seeds
2.2.4. Determination of the Phenolic Compounds of the Grape Seeds
2.2.5. Statistical Analysis
3. Results
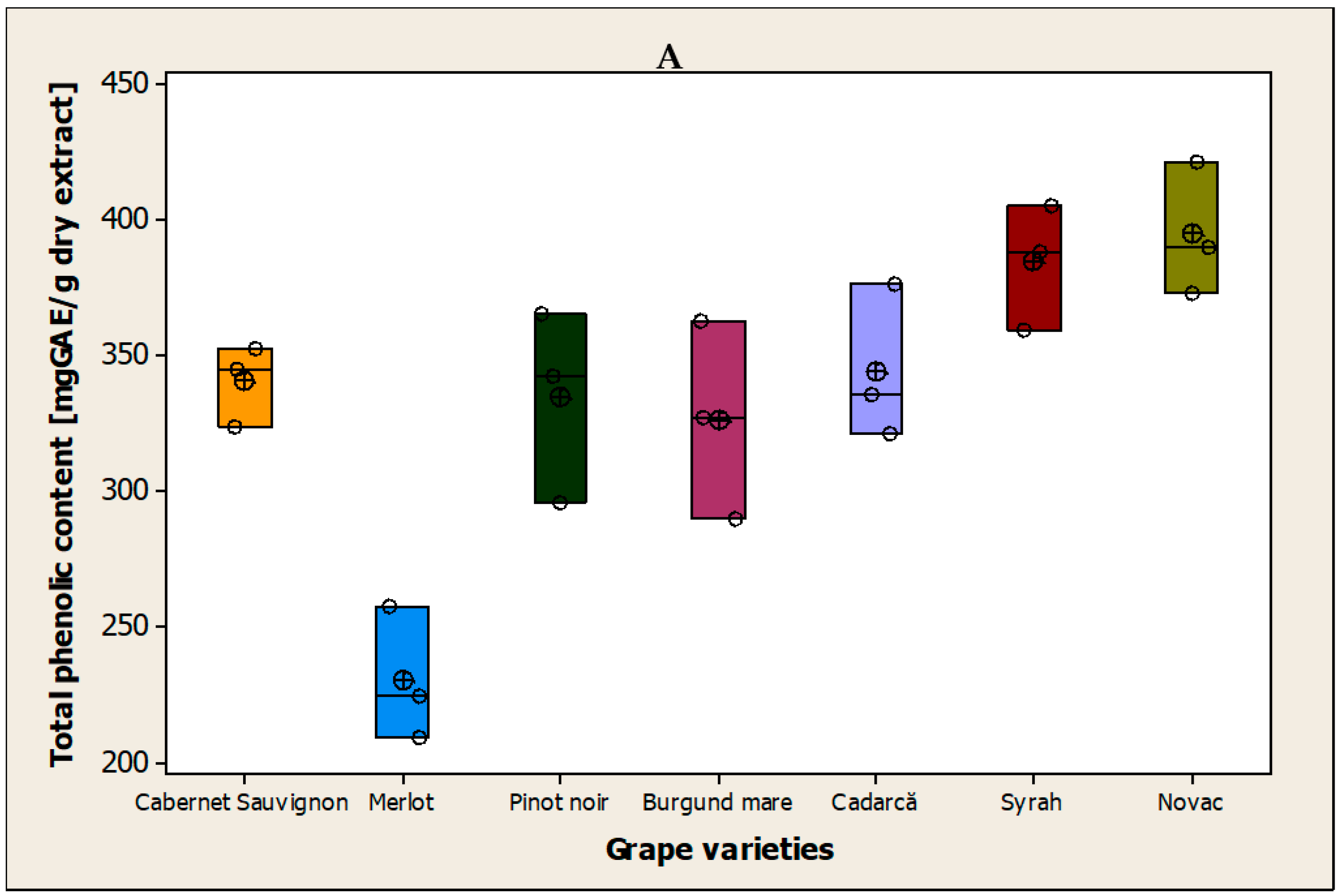
3.1. Determination of the Total Polyphenol Content of the Grape Seeds
3.2. Determination of the Antioxidant Capacity of the Grape Seeds
3.3. Determination of the Antiradical Capacity of the Grape Seeds
3.4. Determination of the Phenolic Compounds of the Grape Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.; Silva, V.; Igrejas, G.; Gaivão, I.; Aires, A.; Klibi, N.; Dapkevicius, M.E.; Valentão, P.; Falco, V.; Poeta, P. Valorization of winemaking by-products as a novel source of antibacterial properties: New strategies to fight antibiotic resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Ladas, D.; Mavromatis, A. Wine Waste management: Treatment methods and potential uses of treated waste. In Waste Management for the Food Industries; Arvanitoyannis, I.S., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 413–452. [Google Scholar]
- Xiuzhen, H.; Tao, S.; Hongxiang, L. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar]
- Sano, A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food Chem. Toxicol. 2017, 108, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.; Tamil, S.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Y.-M.; Leblanc, M.H.; Bhatt, A.J.; Rhodes, P.G. Grape seed extract given three hours after injury suppresses lipid peroxidation and reduces hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 2007, 61, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Feringa, H.H.; Laskey, D.; Dickson, J.E.; Coleman, C. The effect of grape seed extract on cardiovascular risk markers: A meta-analysis of randomized controlled trials. J. Am. Diet. Assoc. 2011, 111, 1173–1181. [Google Scholar] [CrossRef]
- Jin, H.; Liu, M.; Zhang, X.; Pan, J.; Han, J.; Wang, Y.; Lei, H.; Ding, Y.; Yuan, Y. Grape seed procyanidin extract attenuates hypoxic pulmonary hypertension by inhibiting oxidative stress and pulmonary arterial smooth muscle cells proliferation. J. Nutr. Biochem. 2016, 36, 81–88. [Google Scholar] [CrossRef]
- Fine, A.M. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Altern. Med. Rev. 2000, 5, 144–151. [Google Scholar]
- Song, Q.; Shi, Z.; Bi, W.; Liu, R.; Zhang, C.; Wang, K.; Dang, X. Beneficial effect of grape seed proanthocyanidin extract in rabbits with steroid-induced osteonecrosis via protecting against oxidative stress and apoptosis. J. Orthop. Sci. 2015, 20, 196–204. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y.; Kawachi, Y.; Otsuka, F. Oral intake of proanthocyanidin-rich extract from grape seeds improves chloasma. F. Phytother Res. 2004, 18, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Sut, A.; Kosiorek, A.; Saluk-Bijak, J.; Golanski, J. Dual anticoagulant/antiplatelet activity of polyphenolic grape seeds extract. Nutrients 2019, 11, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifton, P.M. Effect of grape seed extract and quercetin on cardiovascular and endothelial parameters in high-risk subjects. J. Biomed. Biotechnol. 2004, 5, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WWang, Y.-J.; Thomas, P.; Zhong, J.-H.; Bi, F.-F.; Kosaraju, S.; Pollard, A.; Fenech, M.; Zhou, X.-F. Consumption of grape seed extract prevents amyloid-β deposition and attenuates inflammation in brain of an alzheimer’s disease mouse. Neurotox. Res. 2009, 15, 3–14. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Lobo, J.K.; Janle, E.M.; Cooper, B.; Simon, J.E.; Wu, Q.L.; Welch, C.; Ho, L.; Weaver, C.; Pasinetti, G.M. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: Implications for treatment in Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 18, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleymani, S.; Iranpanah, A.; Najafi, F.; Belwal, T.; Ramola, S.; Abbasabadi, Z.; Momtaz, S.; Farzaei, M.H. Implications of grape extract and its nanoformulated bioactive agent resveratrol against skin disorders. Arch. Dermatol. Res. 2019, 311, 577–588. [Google Scholar] [CrossRef]
- Argani, H.; Ghorbanihaghjo, A.; Vatankhahan, H.; Rashtchizadeh, N.; Raeisi, S.; Ilghami, H. The effect of red grape seed extract on serum paraoxonase activity in patients with mild to moderate hyperlipidemia. Sao Paulo Med. J. 2016, 134, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Asbaghi, O.; Nazarian, B.; Reiner, Ž.; Amirani, E.; Kolahdooz, F.; Chamani, M.; Asemi, Z. The effects of grape seed extract on glycemic control, serum lipoproteins, inflammation, and body weight: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2020, 34, 239–253. [Google Scholar] [CrossRef]
- Rajput, S.A.; Sun, L.; Zhang, N.; Khalil, M.M.; Ling, Z.; Chong, L.; Wang, S.; Rajput, I.R.; Bloch, D.M.; Khan, F.A.; et al. Grape seed proanthocyanidin extract alleviates aflatoxinb1-induced immunotoxicity and oxidative stress via modulation of NF-κB and Nrf2 signaling pathways in broilers. Toxins 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terra, X.; Valls, J.; Vitrac, X.; Mérrillon, J.-M.; Arola, L.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvado, J.; et al. Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting NFkB signaling pathway. J. Agric. Food Chem. 2007, 55, 4357–4365. [Google Scholar] [CrossRef]
- El-Awdan, S.A.; Abdel Jaleel, G.A.; Saleh, D.O. Grape seed extract attenuates hyperglycaemia-induced in rats by streptozotocin. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Kar, P.; Laight, D.; Rooprai, H.K.; Shaw, K.M.; Cummings, M. Effects of grape seed extract in type 2 diabetic subjects at high cardiovascular risk: A double blind randomized placebo-controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet Med. 2009, 26, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Turki, K.; Charradi, K.; Boukhalfa, H.; Belhaj, M.; Limam, F.; Aouani, E. Grape seed powder improves renal failure of chronic kidney disease patients. EXCLI J. 2016, 15, 424–433. [Google Scholar]
- Visser, J.; Van Staden, P.J.; Soma, P.; Buys, A.V.; Pretorius, E. The stabilizing effect of an oligomeric proanthocyanidin on red blood cell membrane structure of poorly controlled Type II diabetes. Nutr. Diabetes. 2017, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Longo, C.; Gerardi, C.; Trosko, J.E. Pro-apoptotic effect of grape seed extract on MCF-7 involves transient increase of gap junction intercellular communication and Cx43 up-regulation: A mechanism of chemoprevention. Int. J. Mol. Sci. 2019, 20, 3244. [Google Scholar] [CrossRef] [Green Version]
- Hamza, A.A.; Heeba, G.H.; Elwy, H.M.; Murali, C.; El-Awady, R.; Amin, A. Molecular characterization of the grape seeds extract’s effect against chemically induced liver cancer: In vivo and in vitro analyses. Sci. Rep. 2018, 8, 1270. [Google Scholar] [CrossRef] [Green Version]
- Al-Habib, A.; Al-Saleh, E.; Safer, A.-M.; Afzal, M. Bactericidal effect of grape seed extract on methicillin-resistant Staphylococcus aureus (MRSA). J. Toxicol. Sci. 2010, 35, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, D.; Swaroop, A.; Preuss, H.G.; Bagchi, M. Free radical scavenging, antioxidant, and cancer chemoprevention by grape seed proanthocyanin: An overview. Mutat. Res. 2014, 768, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.T.; Xue, B.; Smoake, J.; Lu, Q.-Y.; Park, H.; Henning, S.M.; Burns, W.; Bernabei, A.; Elashoff, D.; Serio, K.J.; et al. MicroRNA-19a/b mediates grape seed procyanidin extract-induced anti-neoplastic effects against lung cancer. J. Nutr. Biochem. 2016, 34, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, H.; Uehara, K.; Nagashima, T.; Nakata, A.; Sato, K.; Mihara, Y.; Komatsu, K.I.; Takanari, J.; Shimizu, S.; Wakame, K. Global Liver gene expression analysis on a murine metabolic syndrome model treated by low-molecular-weight lychee fruit polyphenol [Oligonol(R)]. Anticancer Res. 2016, 36, 3705–3713. [Google Scholar] [PubMed]
- Kadri, S.; El Ayed, M.; Kadri, A.; Limam, F.; Aouani, E.; Mokni, M. Protective effect of grape seed extract and orlistat co-treatment against stroke: Effect on oxidative stress and energy failure. Biomed. Pharmacother. 2021, 136, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Y.; Liu, H.; Hu, N.; Zhang, Y.; Wang, S. Grape seed extract ameliorates PhIP-induced colonic injury by modulating gut microbiota, lipid metabolism, and NF-κB signaling pathway in rats. J. Funct. Foods 2021, 78, 1–12. [Google Scholar] [CrossRef]
- Carle, R.; Claus, A.; Kammerer, D.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar]
- Xu, Z. Important antioxidant phytochemicals in agricultural food products. In Analysis of Antioxidant-Rich Phytochemicals, 1st ed.; Xu, Z., Howard, L.R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 1–24. [Google Scholar]
- Biagi, M.; Miraldi, E.; Figura, N.; Giachetti, D. Antiradical Activity and in vitro Inhibition of Helicobacter pylori by Italian Red Wines. Nat. Prod. Commun. 2009, 4, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Karasu, S.; Başlar, M.; Karaman, S.; Kiliçli, M.; Abdullah, A.U.; Yaman, H.; Sağdiç, O. Characterization of some bioactive compounds and physicochemical properties of grape varieties grown in turkey: Thermal degradation kinetics of anthocyanin. Turk. J. Agric. For. 2016, 40, 177–185. [Google Scholar] [CrossRef]
- Carbone, K.; Fiordiponti, L. Colour evaluation, bioactive compound content, phenolic acid profiles and in vitro biological activity of Passerina del Frusinate white wines: Influence of pre-fermentative skin contact times. Molecules 2016, 21, 960. [Google Scholar] [CrossRef] [Green Version]
- Cabernet Sauvignon. Available online: https://www.rewine.ro/blog/cabernet-sauvignon/ (accessed on 3 November 2020).
- Tobar, M.; Fiore, N.; Pérez-Donoso, A.G.; León, R.; Rosales, I.M.; Gambardella, M. Divergent molecular and growth responses of young “Cabernet Sauvignon” (Vitis vinifera) plants to simple and mixed infections with Grapevine rupestris stem pitting-associated virus. Hort. Res. 2020, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De ce Merlot Este Vinul Prieten cu Toate Gusturile? Available online: https://vinlavin.ro/vin-merlot-pentru-gusturi-pretentioase/ (accessed on 3 November 2020).
- HHu, B.; Gao, J.; Xu, S.; Zhu, J.; Fan, X.; Zhou, X. Quality evaluation of different varieties of dry red wine based on nuclear magnetic resonance metabolomics. J. Appl. Biol. Chem. 2020, 63, 1–8. [Google Scholar]
- Fii “Snob” Vara Asta. Degusta Pinot Noir! Available online: https://www.rewine.ro/blog/fi-snob-vara-asta-degusta-pinot-noir/ (accessed on 3 November 2020).
- Martin, D.; Grab, F.; Grose, C.; Stuart, L.; Scofield, C.; McLachlan, A.; Rutan, T. Vintage by vine interactions most strongly influence Pinot noir grape composition in New Zealand. OENO One 2020, 54, 881–902. [Google Scholar] [CrossRef]
- Blaufraenkisch…Soiuri de Struguri. Available online: http://vinpenet.blogspot.com/2013/02/despre-blaufraenkisch-2010-lacerta.html (accessed on 3 November 2020).
- Balla Géza a Reinventat Cadarca și Nu s-a Oprit Aici. Available online: https://vinul.ro/balla-geza-a-reinventat-cadarca-si-nu-s-a-oprit-aici.html (accessed on 4 November 2020).
- Syrah sau Shiraz. Available online: https://www.rewine.ro/blog/syrah-sau-shiraz/ (accessed on 4 November 2020).
- Novac, Soi de Perspectivă pentru Vinuri Roșii. Available online: https://www.lumeasatului.ro/articole-revista/agrotehnica/6169-novac-soi-de-perspectiva-pentru-vinuri-rosii (accessed on 4 November 2020).
- Lamuela-Raventós, R.M. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications, 1st ed.; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 107–114. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin, E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Yalcin, H.; Kavuncuoglu, H.; Ekici, L.; Sagdic, O. Determination of fatty acid composition, volatile components, physico-chemical and bioactive properties of grape (Vitis Vinifera) seed and seed oil. J. Food Process. Preserv. 2016, 41, 1–7. [Google Scholar] [CrossRef]
- Salas, P.G.; Soto, A.M.; Carretero, A.S.; Gutierrez, A.F. Phenolic-compound extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.; Kumar, C.S. Syringic acid (SA)–A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Rosas, E.C.; Correa, L.B.; Henriques, M.D.G. Anti-inflammatory properties of Schinus terebinthifolius and its use in arthritic conditions. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 489–505. [Google Scholar]
- Janel, N.; Noll, C. Polyphenols in chronic diseases and their mechanisms of action. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 1401–1419. [Google Scholar]
- Dias, T.R.; Alves, M.G.; Silva, B.M.; Oliveira, P.F. Nutritional factors and male reproduction. In Encyclopedia of Reproduction, Second Edition; Skinner, M., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 458–464. [Google Scholar]
- Kim, J.; Lee, K.W. Coffee and its active compounds are neuroprotective. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 423–427. [Google Scholar]
- Calabrese, V.; Mancuso, C.; De Marco, C.; Stella, A.M.G.; Butterfield, D.A. Nitric oxide and cellular stress response in brain aging and neurodegenerative disorders. In Oxidative Stress and Neurodegenerative Disorders; Qureshi, G.A., Parvez, S.H., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 115–134. [Google Scholar]
- Abramovič, H. Antioxidant properties of hydroxycinnamic acid derivatives: A focus on biochemistry, physicochemical parameters, reactive species, and biomolecular interactions. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 843–852. [Google Scholar]
- Risuleo, G. Resveratrol: Multiple activities on the biological functionality of the cell. In Nutraceuticals Efficacy, Safety and Toxicity; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 453–464. [Google Scholar]
- Ay, M.; Charli, A.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Quercetin. Nutraceuticals Efficacy, Safety and Toxicity; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 447–452. [Google Scholar]
- Zuo, J.; Tang, W.; Xu, Y. Anti-hepatitis B virus activity of chlorogenic acid and its related compounds. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 607–613. [Google Scholar]
- Patel, K.; Patel, D.K. The beneficial role of rutin, a naturally occurring flavonoid in health promotion and disease prevention: A systematic review and update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 457–579. [Google Scholar]
- Bătușaru, C.M. Sustainability of the small business environment in Romania in the context of increasing economic competitiveness. Manag. Sustain. Dev. 2019, 11, 37–41. [Google Scholar]





| Compound | Composition (mg/L ppm (Parts per Million)) | ||||||
|---|---|---|---|---|---|---|---|
| Cabernet Sauvignon | Merlot | Pinot noir | Burgund Mare | Cadarcă | Syrah | Novac | |
| Catechin | 8.17 ± 0.01 | 7.28 ± 0.01 | 6.98 ± 0.01 | 7.15 ± 0.01 | 8.12 ± 0.01 | 8.46 ± 0.01 | 8.78 ± 0.01 |
| Epicatismin | 5.11 ± 0.01 | 5.12 ± 0.01 | 5.16 ± 0.01 | 4.82 ± 0.01 | 4.43 ± 0.01 | 5.98 ± 0.01 | 6.17 ± 0.01 |
| Epicatechin gallate | 2.15 ± 0.01 | 2.18 ± 0.01 | 2.26 ± 0.01 | 1.95 ± 0.01 | 2.24 ± 0.01 | 2.02 ± 0.01 | 2.46 ± 0.01 |
| Gallic acid | 26.34 ± 0.05 | 0.71 ± 0.01 | 31.46 ± 0.05 | 31.02 ± 0.05 | 32.46 ± 0.05 | 33.80 ± 0.05 | 35.46 ± 0.05 |
| P-hydroxybenzoic acid | 1.85 ± 0.05 | 1.23 ± 0.05 | 1.77 ± 0.05 | 2.04 ± 0.05 | 2.09 ± 0.05 | 2.22 ± 0.05 | 2.30 ± 0.05 |
| Vanilic acid | 18.02 ± 0.01 | 15.57 ± 0.01 | 16.15 ± 0.01 | 13.24 ± 0.01 | 18.65 ± 0.01 | 18.45 ± 0.01 | 20.08 ± 0.01 |
| Syringic acid | 122.87 ± 0.25 | 130.13 ± 0.25 | 129.40 ± 0.25 | 129.81 ± 0.25 | 133.20 ± 0.25 | 134.26 ± 0.25 | 134.12 ± 0.25 |
| M-hydroxybenzoic acid | 4.58 ± 0.01 | 4.60 ± 0.01 | 4.20 ± 0.01 | 3.69 ± 0.01 | 3.44 ± 0.01 | 5.32 ± 0.01 | 5.26 ± 0.01 |
| Caffeic acid | 1.01 ± 0.01 | 1.08 ± 0.01 | 0.96 ± 0.01 | 1.19 ± 0.01 | 1.27 ± 0.01 | 1.33 ± 0.01 | 1.35 ± 0.01 |
| Ferulic acid | 0.59 ± 0.01 | 0.47 ± 0.01 | 0.90 ± 0.01 | 0.61 ± 0.01 | 0.70 ± 0.01 | 0.62 ± 0.01 | 0.54 ± 0.01 |
| Chlorogenic acid | 7.67 ± 0.01 | 6.95 ± 0.01 | 8.24 ± 0.01 | 5.32 ± 0.01 | 6.54 ± 0.01 | 6.67 ± 0.01 | 7.90 ± 0.01 |
| P-coumaric acid | 5.22 ± 0.01 | 3.50 ± 0.01 | 4.49 ± 0.01 | 4.68 ± 0.01 | 4.45 ± 0.01 | 5.27 ± 0.01 | 6.01 ± 0.01 |
| Resveratrol | 2.27 ± 0.01 | 1.22 ± 0.01 | 0.84 ± 0.01 | 1.74 ± 0.01 | 2.01 ± 0.01 | 2.28 ± 0.01 | 2.41 ± 0.01 |
| Rutin | 0.15 ± 0.01 | 0.41 ± 0.01 | 0.21 ± 0.01 | 0.36 ± 0.01 | 0.37 ± 0.01 | 0.36 ± 0.01 | 0.31 ± 0.01 |
| Quercetin | 0.82 ± 0.01 | 32.43 ± 0.05 | 2.14 ± 0.01 | 0.47 ± 0.01 | 5.84 ± 0.01 | 2.33 ± 0.01 | 1.06 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tița, O.; Lengyel, E.; Stegăruș, D.I.; Săvescu, P.; Ciubara, A.B.; Constantinescu, M.A.; Tița, M.A.; Rață, D.; Ciubara, A. Identification and Quantification of Valuable Compounds in Red Grape Seeds. Appl. Sci. 2021, 11, 5124. https://doi.org/10.3390/app11115124
Tița O, Lengyel E, Stegăruș DI, Săvescu P, Ciubara AB, Constantinescu MA, Tița MA, Rață D, Ciubara A. Identification and Quantification of Valuable Compounds in Red Grape Seeds. Applied Sciences. 2021; 11(11):5124. https://doi.org/10.3390/app11115124
Chicago/Turabian StyleTița, Ovidiu, Ecaterina Lengyel, Diana Ionela Stegăruș, Petre Săvescu, Alexandru Bogdan Ciubara, Maria Adelina Constantinescu, Mihaela Adriana Tița, Diana Rață, and Anamaria Ciubara. 2021. "Identification and Quantification of Valuable Compounds in Red Grape Seeds" Applied Sciences 11, no. 11: 5124. https://doi.org/10.3390/app11115124








