Bioactivity and Delivery Strategies of Phytochemical Compounds in Bone Tissue Regeneration
Abstract
1. Introduction
2. Bone Structure and Function
3. Bone Regeneration Process
4. Bioactive Phytochemicals and Bone Signaling Pathways
4.1. Anti-Inflammatory Activity
4.2. Antioxidants Activity
4.3. Bone Cells Differentiation Activity
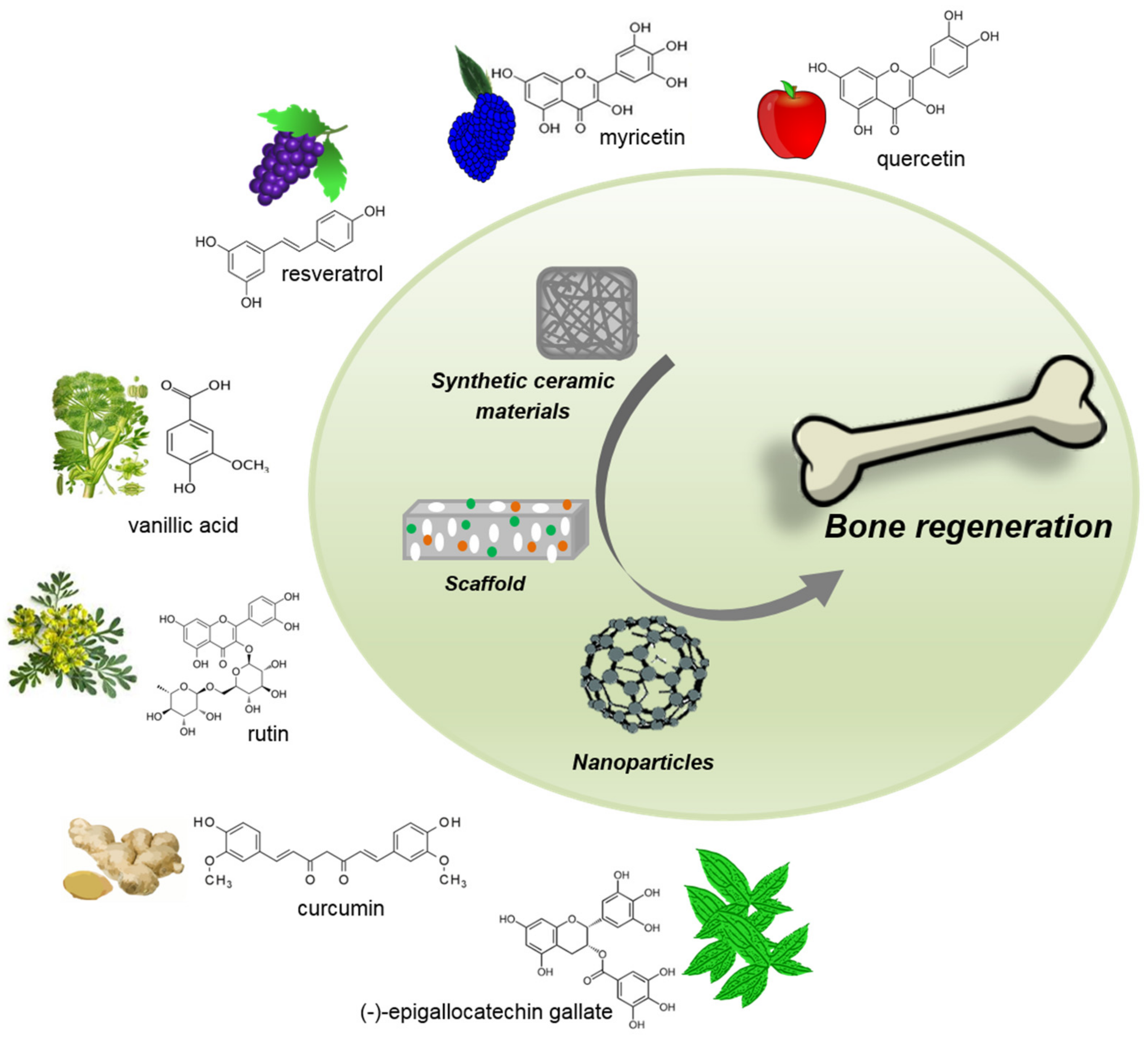
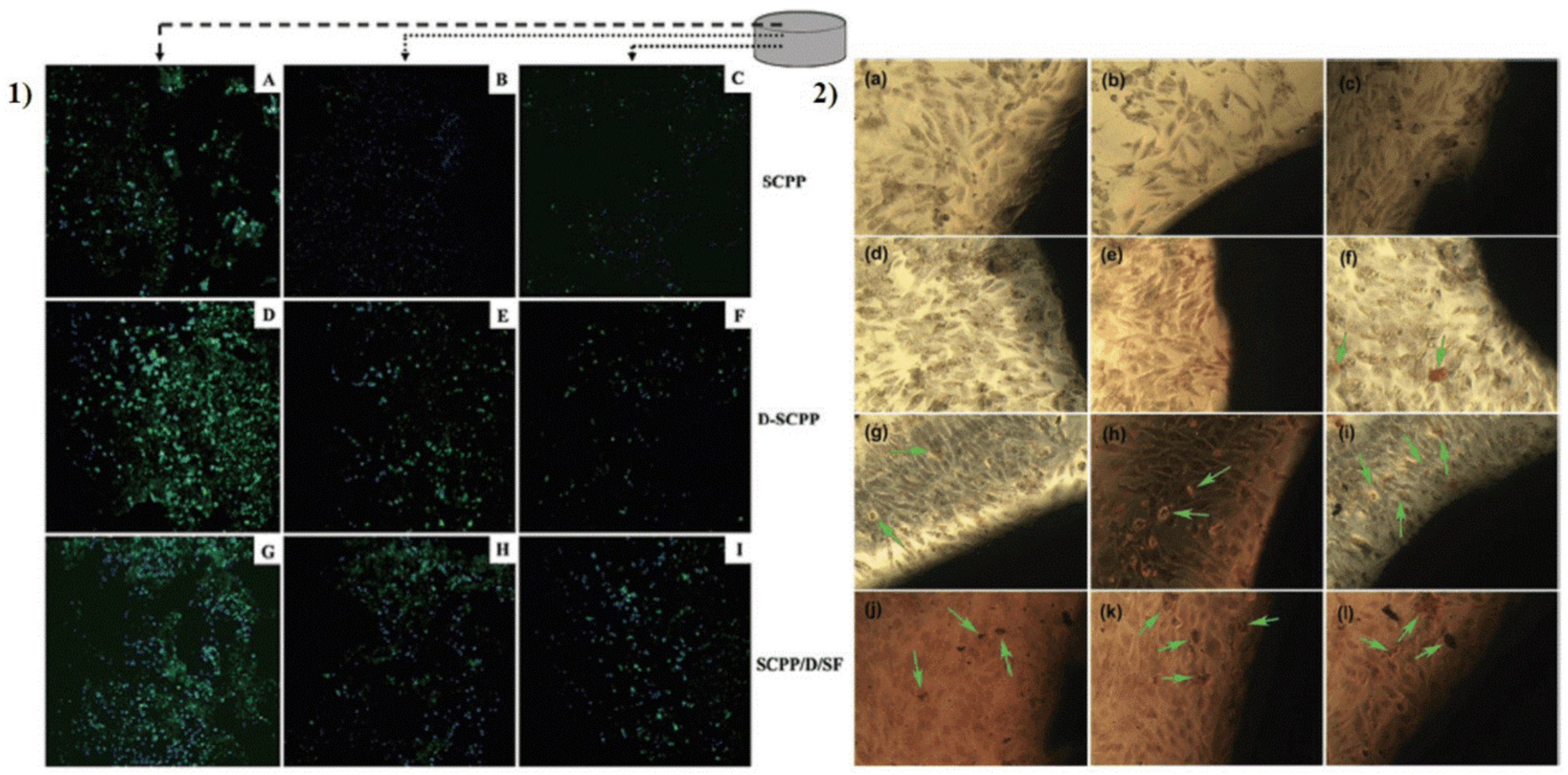
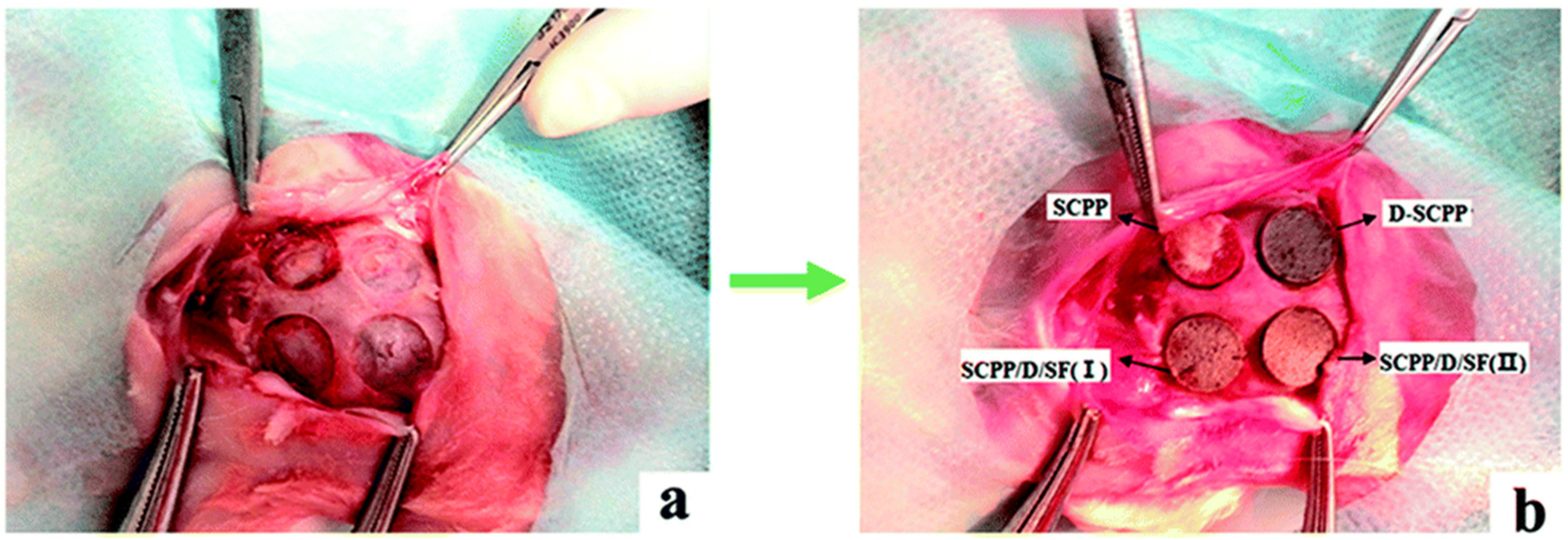
5. Phytochemical-Delivery Vehicles in Bone Tissue Regeneration
5.1. Ceramics
5.2. Scaffolds
5.3. Nanoparticles
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Liu, C. Nanomaterial-based bone regeneration. Nanoscale 2017, 9, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, M.; Leigheb, M.; Brooks, R.A.; Boccafoschi, F.; Cannas, M.F. Regulation of osteoblast and osteoclast functions by FGF-6. J. Cell. Physiol. 2010, 225, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Ur Rehman, F.; Zhao, C.; Liu, B.; He, N. Recent advances in nano scaffolds for bone repair. Bone Res. 2016, 4, 16050. [Google Scholar] [CrossRef]
- Webber, M.J.; Khan, O.F.; Sydlik, S.A.; Tang, B.C.; Langer, R. A perspective on the clinical translation of scaffolds for tissue engineering. Ann. Biomed. Eng. 2015, 43, 641–656. [Google Scholar] [CrossRef]
- Bosetti, M.; Lloyd, A.W.; Santin, M.; Denyer, S.P.; Cannas, M. Effects of phosphatidylserine coatings on titanium on inflammatory cells and cell-induced mineralisation in vitro. Biomaterials 2005, 26, 7572–7578. [Google Scholar] [CrossRef]
- Bosetti, M.; Boccafoschi, F.; Calarco, A.; Leigheb, M.; Gatti, S.; Piffanelli, V.; Peluso, G.; Cannas, M. Behaviour of human mesenchymal stem cells on a polyelectrolyte-modified HEMA hydrogel for silk-based ligament tissue engineering. J. Biomater. Sci. Polym. Ed. 2008, 19, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Byberg, L.; Bellavia, A.; Larsson, S.C.; Orsini, N.; Wolk, A.; Michaëlsson, K. Mediterranean Diet and Hip Fracture in Swedish Men and Women. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 31, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 2564. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130 (Suppl. 8S), 2073s–2085s. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef]
- Rosenberg, N.; Rosenberg, O.; Soudry, M. Osteoblasts in bone physiology-mini review. Rambam Maimonides Med. J. 2012, 3, e0013. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A Transcriptional Activator of Osteoblast Differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Fakhry, M.; Hamade, E.; Badran, B.; Buchet, R.; Magne, D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J. Stem Cells 2013, 5, 136–148. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- James, R.; Deng, M.; Laurencin, C.T.; Kumbar, S.G. Nanocomposites and bone regeneration. Front. Mater. Sci. 2011, 5, 342–357. [Google Scholar] [CrossRef]
- Sikavitsas, V.I.; Temenoff, J.S.; Mikos, A.G. Biomaterials and bone mechanotransduction. Biomaterials 2001, 22, 2581–2593. [Google Scholar] [CrossRef]
- Brannigan, K.; Griffin, M. An Update into the Application of Nanotechnology in Bone Healing. Open Orthop. J. 2016, 10, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; de Saint-Georges, L.; Bowman, B.M.; Jee, W.S. Bone lining cells: Structure and function. Scanning Microsc. 1989, 3, 953–960, discussion 60-1. [Google Scholar] [PubMed]
- Aarden, E.M.; Nijweide, P.J.; Burger, E.H. Function of osteocytes in bone. J. Cell. Biochem. 1994, 55, 287–299. [Google Scholar] [CrossRef]
- Andersen, T.L.; Sondergaard, T.E.; Skorzynska, K.E.; Dagnaes-Hansen, F.; Plesner, T.L.; Hauge, E.M.; Plesner, T.; Delaisse, J.M. A physical mechanism for coupling bone resorption and formation in adult human bone. Am. J. Pathol. 2009, 174, 239–247. [Google Scholar] [CrossRef]
- Everts, V.; Delaissé, J.M.; Korper, W.; Jansen, D.C.; Tigchelaar-Gutter, W.; Saftig, P.; Beertsen, W. The Bone Lining Cell: Its Role in Cleaning Howship’s Lacunae and Initiating Bone Formation. J. Bone Miner. Res. 2002, 17, 77–90. [Google Scholar] [CrossRef]
- Boyce, B.F.; Hughes, D.E.; Wright, K.R.; Xing, L.; Dai, A. Recent advances in bone biology provide insight into the pathogenesis of bone diseases. Lab. Investig. J. Tech. Methods Pathol. 1999, 79, 83–94. [Google Scholar]
- Crockett, J.C.; Mellis, D.J.; Scott, D.I.; Helfrich, M.H. New knowledge on critical osteoclast formation and activation pathways from study of rare genetic diseases of osteoclasts: Focus on the RANK/RANKL axis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2011, 22, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; Yovos, J.G. Osteoclastogenesis—Current knowledge and future perspectives. J. Musculoskelet. Neuronal Interact. 2008, 8, 204–216. [Google Scholar]
- Phan, T.C.; Xu, J.; Zheng, M.H. Interaction between osteoblast and osteoclast: Impact in bone disease. Histol. Histopathol. 2004, 19, 1325–1344. [Google Scholar] [CrossRef]
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J. An overview of the regulation of bone remodeling at the cellular level. Clin. Biochem. 2012, 45, 863–873. [Google Scholar] [CrossRef]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.R.; Garla, V.V. Fracture Healing Overview; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Kostenuik, P.; Mirza, F.M. Fracture healing physiology and the quest for therapies for delayed healing and nonunion. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2017, 35, 213–223. [Google Scholar] [CrossRef]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Sweetwyne, M.T.; Hankenson, K.D. PKCδ is required for Jagged-1 induction of human mesenchymal stem cell osteogenic differentiation. Stem Cells 2013, 31, 1181–1192. [Google Scholar] [CrossRef]
- Dishowitz, M.I.; Zhu, F.; Sundararaghavan, H.G.; Ifkovits, J.L.; Burdick, J.A.; Hankenson, K.D. Jagged1 immobilization to an osteoconductive polymer activates the Notch signaling pathway and induces osteogenesis. J. Biomed. Mater. Res. Part A 2014, 102, 1558–1567. [Google Scholar] [CrossRef]
- Chakravorty, N.; Hamlet, S.; Jaiprakash, A.; Crawford, R.; Oloyede, A.; Alfarsi, M.; Xiao, Y.; Ivanovski, S. Pro-osteogenic topographical cues promote early activation of osteoprogenitor differentiation via enhanced TGFβ, Wnt, and Notch signaling. Clin. Oral Implants Res. 2014, 25, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Herford, A.S.; Stoffella, E.; Tandon, R. Reconstruction of mandibular defects using bone morphogenic protein: Can growth factors replace the need for autologous bone grafts? A systematic review of the literature. Plast. Surg. Int. 2011, 2011, 165824. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.Q.; Xiao, J.; Xie, J.; Lu, P.P.; Ding, X. bFGF enhances activation of osteoblast differentiation and osteogenesis on titanium surfaces via PI3K/Akt signaling pathway. Int. J. Clin. Exp. Pathol. 2016, 9, 4680–4692. [Google Scholar]
- Devi, R.; Dixit, J. Clinical Evaluation of Insulin like Growth Factor-I and Vascular Endothelial Growth Factor with Alloplastic Bone Graft Material in the Management of Human Two Wall Intra-Osseous Defects. J. Clin. Diagn. Res. JCDR 2016, 10, ZC41–ZC46. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Marie, P.J. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002, 16, 1446–1465. [Google Scholar] [CrossRef]
- Furuya, H.; Tabata, Y.; Kaneko, K. Bone regeneration for murine femur fracture by gelatin hydrogels incorporating basic fibroblast growth factor with different release profiles. Tissue Eng. Part A 2014, 20, 1531–1541. [Google Scholar] [CrossRef]
- Liu, T.M.; Lee, E.H. Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Eng. Part B Rev. 2013, 19, 254–263. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Lin, L.; Chen, P.; Ma, K.T.; Zhou, C.Y.; Ao, Y.F. Runx2 overexpression enhances osteoblastic differentiation and mineralization in adipose--derived stem cells in vitro and in vivo. Calcif. Tissue Int. 2006, 79, 169–178. [Google Scholar] [CrossRef]
- Conte, R.; Luca, I.D.; Luise, A.D.; Petillo, O.; Calarco, A.; Peluso, G. New Therapeutic Potentials of Nanosized Phytomedicine. J. Nanosci. Nanotechnol. 2016, 16, 8176–8187. [Google Scholar] [CrossRef]
- Bonadies, I.; Di Cristo, F.; Valentino, A.; Peluso, G.; Calarco, A.; Di Salle, A. pH-Responsive Resveratrol-Loaded Electrospun Membranes for the Prevention of Implant-Associated Infections. Nanomaterials 2020, 10, 1175. [Google Scholar] [CrossRef]
- Amrati, F.E.; Bourhia, M.; Slighoua, M.; Ibnemoussa, S.; Bari, A.; Ullah, R.; Amaghnouje, A.; Di Cristo, F.; El Mzibri, M.; Calarco, A.; et al. Phytochemical Study on Antioxidant and Antiproliferative Activities of Moroccan Caralluma europaea Extract and Its Bioactive Compound Classes. Evid. Based Complement. Altern. Med. 2020, 2020, 8409718. [Google Scholar] [CrossRef] [PubMed]
- Conte, R.; Marturano, V.; Peluso, G.; Calarco, A.; Cerruti, P. Recent Advances in Nanoparticle-Mediated Delivery of Anti-Inflammatory Phytocompounds. Int. J. Mol. Sci. 2017, 18, 709. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Agustin-Salazar, S.; Berg, A.L.; Setaro, B.; Micillo, R.; Pizzo, E.; Weber, F.; Gamez-Meza, N.; Schieber, A.; Cerruti, P. Pecan (Carya illinoinensis (Wagenh.) K. Koch) Nut Shell as an Accessible Polyphenol Source for Active Packaging and Food Colorant Stabilization. ACS Sustain. Chem. Eng. 2020, 8, 6700–6712. [Google Scholar] [CrossRef] [PubMed]
- Marturano, V.; Bizzarro, V.; De Luise, A.; Calarco, A.; Ambrogi, V.; Giamberini, M.; Tylkowski, B.; Cerruti, P. Essential oils as solvents and core materials for the preparation of photo-responsive polymer nanocapsules. Nano Res. 2018, 11, 2783–2795. [Google Scholar] [CrossRef]
- Agustin-Salazar, S.; Gamez-Meza, N.; Medina-Juárez, L.A.; Malinconico, M.; Cerruti, P. Stabilization of Polylactic Acid and Polyethylene with Nutshell Extract: Efficiency Assessment and Economic Evaluation. ACS Sustain. Chem. Eng. 2017, 5. [Google Scholar] [CrossRef]
- Agustin-Salazar, S.; Gamez-Meza, N.; Medina-Juàrez, L.À.; Soto-Valdez, H.; Cerruti, P. From Nutraceutics to Materials: Effect of Resveratrol on the Stability of Polylactide. ACS Sustain. Chem. Eng. 2014, 2, 1534–1542. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Ogino, C.; Kondo, A. Green synthesis of thiolated graphene nanosheets by alliin (garlic) and its effect on the deposition of gold nanoparticles. RSC Adv. 2013, 4, 5986. [Google Scholar] [CrossRef]
- Elif, Ö.; Belma, Ö.; İlkay, Ş. Production of biologically safe and mechanically improved reduced graphene oxide/hydroxyapatite composites. Mater. Res. Express 2017, 4, 015601. [Google Scholar] [CrossRef]
- Alegria, E.C.B.A.; Ribeiro, A.P.C.; Mendes, M.; Ferraria, A.M.; do Rego, A.M.B.; Pombeiro, A.J.L. Effect of Phenolic Compounds on the Synthesis of Gold Nanoparticles and its Catalytic Activity in the Reduction of Nitro Compounds. Nanomaterials 2018, 8, 320. [Google Scholar] [CrossRef]
- Swilam, N.; Nematallah, K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef]
- Conte, R.; Calarco, A.; Napoletano, A.; Valentino, A.; Margarucci, S.; Di Cristo, F.; Di Salle, A.; Peluso, G. Polyphenols Nanoencapsulation for Therapeutic Applications. Biomol. Res. Ther. 2016, 5:2. [Google Scholar] [CrossRef]
- Torre, E. Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, D.; Li, Q.; Sun, X.; Song, Y.; Wang, C. Curcumin inhibits inflammatory response and bone loss during experimental periodontitis in rats. Acta Odontol. Scand. 2013, 71, 349–356. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Lion, J.M.; Mentaverri, R.; Ricupero, D.A.; Kamel, S.; Romero, J.R.; Chattopadhyay, N. Attenuation of osteoclastogenesis and osteoclast function by apigenin. Biochem. Pharmacol. 2006, 72, 184–197. [Google Scholar] [CrossRef] [PubMed]
- La, V.D.; Howell, A.B.; Grenier, D. Cranberry proanthocyanidins inhibit MMP production and activity. J. Dent. Res. 2009, 88, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.L.; Ricupero, D.A.; Huang, S.; Fatma, N.; Singh, D.P.; Romero, J.R.; Chattopadhyay, N. Differential activity of kaempferol and quercetin in attenuating tumor necrosis factor receptor family signaling in bone cells. Biochem. Pharmacol. 2006, 71, 818–826. [Google Scholar] [CrossRef] [PubMed]
- La, V.D.; Tanabe, S.; Grenier, D. Naringenin inhibits human osteoclastogenesis and osteoclastic bone resorption. J. Periodontal Res. 2009, 44, 193–198. [Google Scholar] [CrossRef]
- Comalada, M.; Ballester, I.; Bailón, E.; Sierra, S.; Xaus, J.; Gálvez, J.; Sánchez de Medina, F.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure–activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef]
- Bharti, A.C.; Takada, Y.; Aggarwal, B.B. Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J. Immunol. 2004, 172, 5940–5947. [Google Scholar] [CrossRef]
- Chowdhury, T.T.; Salter, D.M.; Bader, D.L.; Lee, D.A. Signal transduction pathways involving p38 MAPK, JNK, NFkappaB and AP-1 influences the response of chondrocytes cultured in agarose constructs to IL-1beta and dynamic compression. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2008, 57, 306–313. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, M.; Saravanan, C.; Singh, S.K. Curcumin: A potential candidate for matrix metalloproteinase inhibitors. Expert Opin. Ther. Targets 2012, 16, 959–972. [Google Scholar] [CrossRef]
- Bu, S.Y.; Hunt, T.S.; Smith, B.J. Dried plum polyphenols attenuate the detrimental effects of TNF-alpha on osteoblast function coincident with up-regulation of Runx2, Osterix and IGF-I. J. Nutr. Biochem. 2009, 20, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, I.S.; Bajaj, K.L. Tannins in black-plum (Syzygium cumini L.) seeds. Biochem. J. 1972, 128, 56. [Google Scholar] [CrossRef]
- Tokuda, H.; Takai, S.; Matsushima-Nishiwaki, R.; Akamatsu, S.; Hanai, Y.; Hosoi, T.; Harada, A.; Ohta, T.; Kozawa, O. (−)-epigallocatechin gallate enhances prostaglandin F2α-induced VEGF synthesis via upregulating SAPK/JNK activation in osteoblasts. J. Cell. Biochem. 2007, 100, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Yeh, J.K.; Cao, J.J.; Tatum, O.L.; Dagda, R.Y.; Wang, J.S. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation-induced bone loss model. J. Nutr. Biochem. 2010, 21, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.C.; Gao, W.; Wang, J.S.; Yeh, J.K. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet-induced obese rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Asmawi, M.Z.; Vuorela, P.; Vapaatalo, H.; Moilanen, E. Effects of flavonoids on prostaglandin E2 production and on COX-2 and mPGES-1 expressions in activated macrophages. Planta Med. 2011, 77, 1504–1511. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Sekher Pannala, A.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-Ring Chemistry and Antioxidant Activity: Fast Reaction Kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, L.; Wang, X.; Zhang, T.-L.; Wang, K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007, 81, 832–840. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Ke, K.; Sul, O.J.; Kim, H.J.; Kim, S.H.; Lee, M.H.; Kim, H.J.; Kim, S.Y.; Chung, H.T.; Choi, H.S. Curcumin protects against ovariectomy-induced bone loss and decreases osteoclastogenesis. J. Cell. Biochem. 2011, 112, 3159–3166. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Wang, Z.; Xu, Z.; Zhang, Q.; Yin, M. Effects of dietary resveratrol on excess-iron-induced bone loss via antioxidative character. J. Nutr. Biochem. 2015, 26, 1174–1182. [Google Scholar] [CrossRef]
- Huang, Q.; Gao, B.; Wang, L.; Hu, Y.Q.; Lu, W.G.; Yang, L.; Luo, Z.J.; Liu, J. Protective effects of myricitrin against osteoporosis via reducing reactive oxygen species and bone-resorbing cytokines. Toxicol. Appl. Pharmacol. 2014, 280, 550–560. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal Chelation of Polyphenols. Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2001; pp. 190–203. [Google Scholar]
- Islam, S.; Islam, N.; Kermode, T.; Johnstone, B.; Mukhtar, H.; Moskowitz, R.W.; Goldberg, V.M.; Malemud, C.J.; Haqqi, T.M. Involvement of Caspase-3 in Epigallocatechin-3-gallate-Mediated Apoptosis of Human Chondrosarcoma Cells. Biochem. Biophys. Res. Commun. 2000, 270, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Wachi, M.; Woo, J.-T.; Kato, M.; Kasai, S.; Takahashi, F.; Lee, I.S.; Nagai, K. Fenton Reaction Is Primarily Involved in a Mechanism of (−)-Epigallocatechin-3-gallate to Induce Osteoclastic Cell Death. Biochem. Biophys. Res. Commun. 2002, 292, 94–101. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Kang, L.; Wang, C.-Z.; Huang, H.H.; Cheng, T.-L.; Huang, H.-T.; Lee, M.J.; Lin, Y.S.; Ho, M.L.; Wang, G.J.; et al. (-)-Epigallocatechin-3-Gallate (EGCG) Enhances Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Molecules 2018, 23, 3221. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Chang, J.-K.; Tsai, C.-H.; Chien, T.-T.C.; Kuo, P.-L. Myricetin induces human osteoblast differentiation through bone morphogenetic protein-2/p38 mitogen-activated protein kinase pathway. Biochem. Pharmacol. 2007, 73, 504–514. [Google Scholar] [CrossRef]
- Chen, J.R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J.J. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical Wnt signaling. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.J.; Choi, R.C.; Cheung, A.W.; Chen, V.P.; Xu, S.L.; Dong, T.T.; Chen, J.J.; Tsim, K.W.K. Baicalin, a flavone, induces the differentiation of cultured osteoblasts: An action via the Wnt/beta-catenin signaling pathway. J. Biol. Chem. 2011, 286, 27882–27893. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef]
- Xiao, H.H.; Gao, Q.G.; Zhang, Y.; Wong, K.C.; Dai, Y.; Yao, X.S.; Wong, M.S. Vanillic acid exerts oestrogen-like activities in osteoblast-like UMR 106 cells through MAP kinase (MEK/ERK)-mediated ER signaling pathway. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt B, 382–391. [Google Scholar] [CrossRef]
- Abdel-Naim, A.B.; Alghamdi, A.A.; Algandaby, M.M.; Al-Abbasi, F.A.; Al-Abd, A.M.; Eid, B.G.; Abdallah, H.M.; El-Halawany, A.M. Rutin Isolated from Chrozophora tinctoria Enhances Bone Cell Proliferation and Ossification Markers. Oxid. Med. Cell. Longev. 2018, 2018, 5106469. [Google Scholar] [CrossRef]
- Rassi, C.M.; Lieberherr, M.; Chaumaz, G.; Pointillart, A.; Cournot, G. Modulation of osteoclastogenesis in porcine bone marrow cultures by quercetin and rutin. Cell Tissue Res. 2005, 319, 383–393. [Google Scholar] [CrossRef]
- Lee, D.J.; Tseng, H.C.; Wong, S.W.; Wang, Z.; Deng, M.; Ko, C.-C. Dopaminergic effects on in vitro osteogenesis. Bone Res. 2015, 3, 15020. [Google Scholar] [CrossRef]
- Schneider-Stock, R.; Ghantous, A.; Bajbouj, K.; Saikali, M.; Darwiche, N. Epigenetic mechanisms of plant-derived anticancer drugs. Front. Biosci. 2012, 17, 129–173. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Shayan, P.; Busch, F.; Aldinger, C.; Buhrmann, C.; Lueders, C.; Mobasheri, A. Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS ONE 2012, 7, e35712. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.C.; Hou, S.M.; Chen, R.J.; Peng, H.W.; Hsieh, C.F.; Kuo, M.L.; Yen, M.L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Shin, S.I.; Shin, K.S.; Lee, Y.R.; Park, B.H.; Kim, E.C. The role of sirtuin 1 in osteoblastic differentiation in human periodontal ligament cells. J. Periodontal Res. 2011, 46, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Chen, G.; Feng, S.; Wang, P.; Zhu, X.; Zhang, R. Quercetin promotes the osteogenic differentiation of rat mesenchymal stem cells via mitogen-activated protein kinase signaling. Exp. Ther. Med. 2015, 9, 2072–2080. [Google Scholar] [CrossRef][Green Version]
- Moon, H.J.; Ko, W.K.; Han, S.W.; Kim, D.S.; Hwang, Y.S.; Park, H.K.; Kwon, I.K. Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochem. Biophys. Res. Commun. 2012, 418, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lim, J.; Teoh, S.-H. Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers 2020, 12, 2881. [Google Scholar] [CrossRef]
- Cojocaru, F.-D.; Botezat, D.; Gardikiotis, I.; Uritu, C.-M.; Dodi, G.; Trandafir, L.; Rezus, C.; Rezus, E.; Tamba, B.-I.; Mihai, C.-T. Nanomaterials Designed for Antiviral Drug Delivery Transport across Biological Barriers. Pharmaceutics 2020, 12, 171. [Google Scholar] [CrossRef]
- Newman, M.R.; Benoit, D.S. Local and targeted drug delivery for bone regeneration. Curr. Opin. Biotechnol. 2016, 40, 125–132. [Google Scholar] [CrossRef]
- Bosetti, M.; Bianchi, A.E.; Zaffe, D.; Cannas, M. Comparative in vitro study of four commercial biomaterials used for bone grafting. J. Appl. Biomater. Funct. Mater. 2013, 11, e80–e88. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Fracchia, L.; Marchetti, A.; Rinaldi, M.; Bosetti, M. Injectable Scaffolds Enriched with Silver to Inhibit Bacterial Invasion in Tissue Regeneration. Materials 2019, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Susmita, B.; Naboneeta Sa Dishary, B. Natural medicine delivery from biomedical devices to treat bone disorders: A review. Acta Biomater. 2021, 126, 63–91. [Google Scholar] [CrossRef]
- Tadic, D.; Epple, M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, Z.; Li, Y.; Zhang, X.; De Bruijn, J.D.; De Groot, K. Osteoinduction by calcium phosphate biomaterials. J. Mater. Sci. Mater. Med. 1998, 9, 723–726. [Google Scholar] [CrossRef]
- Ramseier, C.A.; Rasperini, G.; Batia, S.; Giannobile, W.V. Advanced reconstructive technologies for periodontal tissue repair. Periodontology 2000 2012, 59, 185–202. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Aichelmann-Reidy, M.E.; Branch-Mays, G.L. Regeneration of periodontal tissue: Bone replacement grafts. Dent. Clin. N. Am. 2010, 54, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.T.; Nares, S.; Reynolds, M.A. Periodontal regeneration—Intrabony defects: A systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86 (Suppl. 2), S77–S104. [Google Scholar] [CrossRef]
- Houde, V.; Grenier, D.; Chandad, F. Protective effects of grape seed proanthocyanidins against oxidative stress induced by lipopolysaccharides of periodontopathogens. J. Periodontol. 2006, 77, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, M.; Takada, K.; Makimura, M.; Otake, S. Improvement of periodontal status by green tea catechin using a local delivery system: A clinical pilot study. J. Periodontal Res. 2002, 37, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Kushiyama, M.; Shimazaki, Y.; Murakami, M.; Yamashita, Y. Relationship between intake of green tea and periodontal disease. J. Periodontol. 2009, 80, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rodriguez, P.; Sánchez, M.; Landin, M. Drug-Loaded Biomimetic Ceramics for Tissue Engineering. Pharmaceutics 2018, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.C.C.; Bollati, D.; Iviglia, G. Inventor Compositions for Filling Bone and Periodontal Defects WO 2015/014872, 5 February 2015.
- Cazzola, M.; Corazzari, I.; Prenesti, E.; Bertone, E.; Vernè, E.; Ferraris, S. Bioactive glass coupling with natural polyphenols: Surface modification, bioactivity and anti-oxidant ability. Appl. Surf. Sci. 2016, 367, 237–248. [Google Scholar] [CrossRef]
- Zhou, K.; Ren, X.; Zhao, M.; Mei, X.; Zhang, P.; Chen, Z.; Zhu, X. Promoting proliferation and differentiation of BMSCs by green tea polyphenols functionalized porous calcium phosphate. Regener. Biomater. 2018, 5, 35–41. [Google Scholar] [CrossRef]
- Preethi Soundarya, S.; Sanjay, V.; Haritha Menon, A.; Dhivya, S.; Selvamurugan, N. Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Sundar, R.; John, A.; Abraham, A. Phytochemical Incorporated Drug Delivery Scaffolds for Tissue Regeneration. Regen. Eng. Transl. Med. 2018, 4, 167–176. [Google Scholar] [CrossRef]
- Rekulapally, R.; Udayachandrika, K.; Hamlipur, S.; Nair, A.S.; Pal, B.; Singh, S. Tissue engineering of collagen scaffolds crosslinked with plant based polysaccharides. Prog. Biomater. 2021, 10, 29–41. [Google Scholar] [CrossRef]
- Rambhia, K.J.; Ma, P.X. Controlled drug release for tissue engineering. J. Control. Release 2015, 219, 119–128. [Google Scholar] [CrossRef]
- Santin, M.; Morris, C.; Standen, G.; Nicolais, L.; Ambrosio, L. A new class of bioactive and biodegradable soybean-based bone fillers. Biomacromolecules 2007, 8, 2706–2711. [Google Scholar] [CrossRef]
- Merolli, A.; Nicolais, L.; Ambrosio, L.; Santin, M. A degradable soybean-based biomaterial used effectively as a bone filler in vivo in a rabbit. Biomed. Mater. 2010, 5, 015008. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, L.; Zhang, P.; Song, J.; Liu, W. An anti-inflammatory cell-free collagen/resveratrol scaffold for repairing osteochondral defects in rabbits. Acta Biomater. 2014, 10, 4983–4995. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dånmark, S.; Edlund, U.; Finne-Wistrand, A.; He, X.; Norgård, M.; Blomén, E.; Hultenby, K.; Andersson, G.; Lindgren, U. Resveratrol-conjugated poly-ε-caprolactone facilitates in vitro mineralization and in vivo bone regeneration. Acta Biomater. 2011, 7, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.S.; Ahmed, S.S.; Dhanasekaran, M.; Santosh, S.W. Polycaprolactone scaffold engineered for sustained release of resveratrol: Therapeutic enhancement in bone tissue engineering. Int. J. Nanomed. 2014, 9, 183–195. [Google Scholar] [CrossRef]
- Riccitiello, F.; De Luise, A.; Conte, R.; D’Aniello, S.; Vittoria, V.; Di Salle, A.; Calarco, A.; Peluso, G. Effect of resveratrol release kinetic from electrospun nanofibers on osteoblast and osteoclast differentiation. Eur. Polym. J. 2018, 99, 289–297. [Google Scholar] [CrossRef]
- Wang, C.C.; Wang, C.H.; Chen, H.C.; Cherng, J.H.; Chang, S.J. Combination of resveratrol-containing collagen with adipose stem cells for craniofacial tissue-engineering applications. Int. Wound J. 2018, 15, 660–672. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Xu, J.; Wang, K.; Lu, X.; Zhang, H.; Qu, S.; Zhen, G.; Ren, F. Bioadhesive Microporous Architectures by Self-Assembling Polydopamine Microcapsules for Biomedical Applications. Chem. Mater. 2015, 27, 848–856. [Google Scholar] [CrossRef]
- Ko, E.; Yang, K.; Shin, J.; Cho, S.-W. Polydopamine-Assisted Osteoinductive Peptide Immobilization of Polymer Scaffolds for Enhanced Bone Regeneration by Human Adipose-Derived Stem Cells. Biomacromolecules 2013, 14, 3202–3213. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, J.S.; Lee, M.S.; An, S.; Yang, K.; Lee, K.; Yang, H.S.; Lee, H.; Cho, S.W. Plant Flavonoid-Mediated Multifunctional Surface Modification Chemistry: Catechin Coating for Enhanced Osteogenesis of Human Stem Cells. Chem. Mater. 2017, 29, 4375–4384. [Google Scholar] [CrossRef]
- Pasche, S.; Vörös, J.; Griesser, H.J.; Spencer, N.D.; Textor, M. Effects of ionic strength and surface charge on protein adsorption at PEGylated surfaces. J. Phys. Chem. B 2005, 109, 17545–17552. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Deng, D.; Zhou, X.; Cheng, L.; ten Cate, J.M.; Li, J.; Li, X.; Crielaard, W. Novel tea polyphenol-modified calcium phosphate nanoparticle and its remineralization potential. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, L. Plant polyphenols mediated synthesis of gold nanoparticles for pain management in nursing care for dental tissue implantation applications. J. Drug Deliv. Sci. Technol. 2020, 58, 101753. [Google Scholar] [CrossRef]
- Felice, F.; Zambito, Y.; Belardinelli, E.; D’Onofrio, C.; Fabiano, A.; Balbarini, A.; Di Stefano, R. Delivery of natural polyphenols by polymeric nanoparticles improves the resistance of endothelial progenitor cells to oxidative stress. Eur. J. Pharm. Sci. 2013, 50, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Luisa, D.P.-A.M.; Griselda, R.-M.; Valentín, M.-L.; Carmina, O.-S.; Cristina, V.-M.; J., M.; Maykel, G.T.; David, Q.G.; Sánchez-Sánchez, R.; Leyva-Gómez, G. Curcumin-loaded poly-ε-caprolactone nanoparticles show antioxidant and cytoprotective effects in the presence of reactive oxygen species. J. Bioact. Compat. Polym. 2020, 35, 270–285. [Google Scholar] [CrossRef]
- Malathy, S.; Iyer, P.R. Naringin Loaded Chitosan Nanoparticle for Bone Regeneration: A Preliminary in vitro Study. J. Nanomed. Nanotechnol. 2018, 9, 1–7. [Google Scholar]

| Phytochemical | Signaling Pathway | Reference |
|---|---|---|
| Curcumin | NF-kB pathways, Redox-sensitive signaling pathways | [75,89,90] |
| EGCG | MAPKs signaling pathways | [82] |
| Quercetin | COX2/HIF1αsignaling ERK1-2/MAPKs signaling | [83,84,108] |
| Resveratrol | Redox-sensitive signaling pathways Sirt/RUNX2 signaling MAPKs pathways | [89,90,109] |
| Incaritin | NFATc signaling | [87] |
| Naringin | Glutathione Pathway (GSH) | [88] |
| Myricetin | SMAD/BMP signaling Wnt/β-catenin signaling | [97] |
| Vanillic acid | ERs pathways | [101] |
| Rutin | ERs pathways | [102,103] |
| Bioactive Compound | Device | Effect | Ref |
|---|---|---|---|
| - Gallic acid - Tea polyphenols | Synthetic ceramic materials | - pro-osteogenic - anti-inflammatory - antioxidant | [128,134] |
| - Resveratrol - Phlorotannins - Catechins | Scaffolds -hydrogel - PLGA - PCL and PLA | - antioxidant - pro-differentiating - pro-osteogenic - wound healing | [135,136,137,142] |
| - EGCG - Catechin - Pro-anthocyanidins - Curcumin - Naringin | Nanoparticles - TP-CaP - Au-NPs - GSE-NP - Cur–PCL | - osteopromotive - osteoblast differentiation - osteoblast proliferation - cytoprotective - remineralization - antioxidant - anti - inflammatory | [147,148,149,150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentino, A.; Di Cristo, F.; Bosetti, M.; Amaghnouje, A.; Bousta, D.; Conte, R.; Calarco, A. Bioactivity and Delivery Strategies of Phytochemical Compounds in Bone Tissue Regeneration. Appl. Sci. 2021, 11, 5122. https://doi.org/10.3390/app11115122
Valentino A, Di Cristo F, Bosetti M, Amaghnouje A, Bousta D, Conte R, Calarco A. Bioactivity and Delivery Strategies of Phytochemical Compounds in Bone Tissue Regeneration. Applied Sciences. 2021; 11(11):5122. https://doi.org/10.3390/app11115122
Chicago/Turabian StyleValentino, Anna, Francesca Di Cristo, Michela Bosetti, Amal Amaghnouje, Dalila Bousta, Raffaele Conte, and Anna Calarco. 2021. "Bioactivity and Delivery Strategies of Phytochemical Compounds in Bone Tissue Regeneration" Applied Sciences 11, no. 11: 5122. https://doi.org/10.3390/app11115122
APA StyleValentino, A., Di Cristo, F., Bosetti, M., Amaghnouje, A., Bousta, D., Conte, R., & Calarco, A. (2021). Bioactivity and Delivery Strategies of Phytochemical Compounds in Bone Tissue Regeneration. Applied Sciences, 11(11), 5122. https://doi.org/10.3390/app11115122






