Using CO2 as a Cooling Fluid for Power Plants: A Novel Approach for CO2 Storage and Utilization
Abstract
:1. Introduction
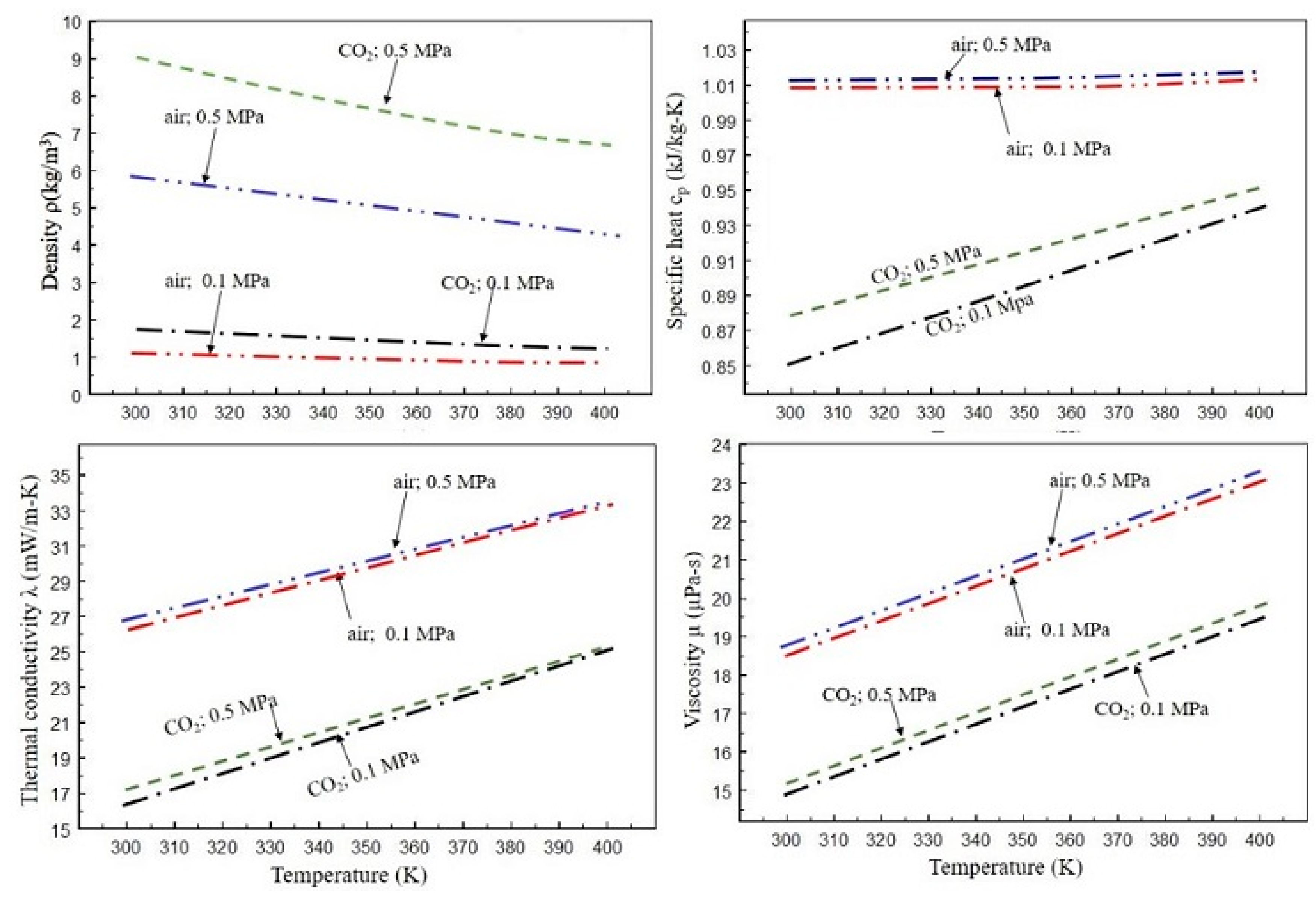
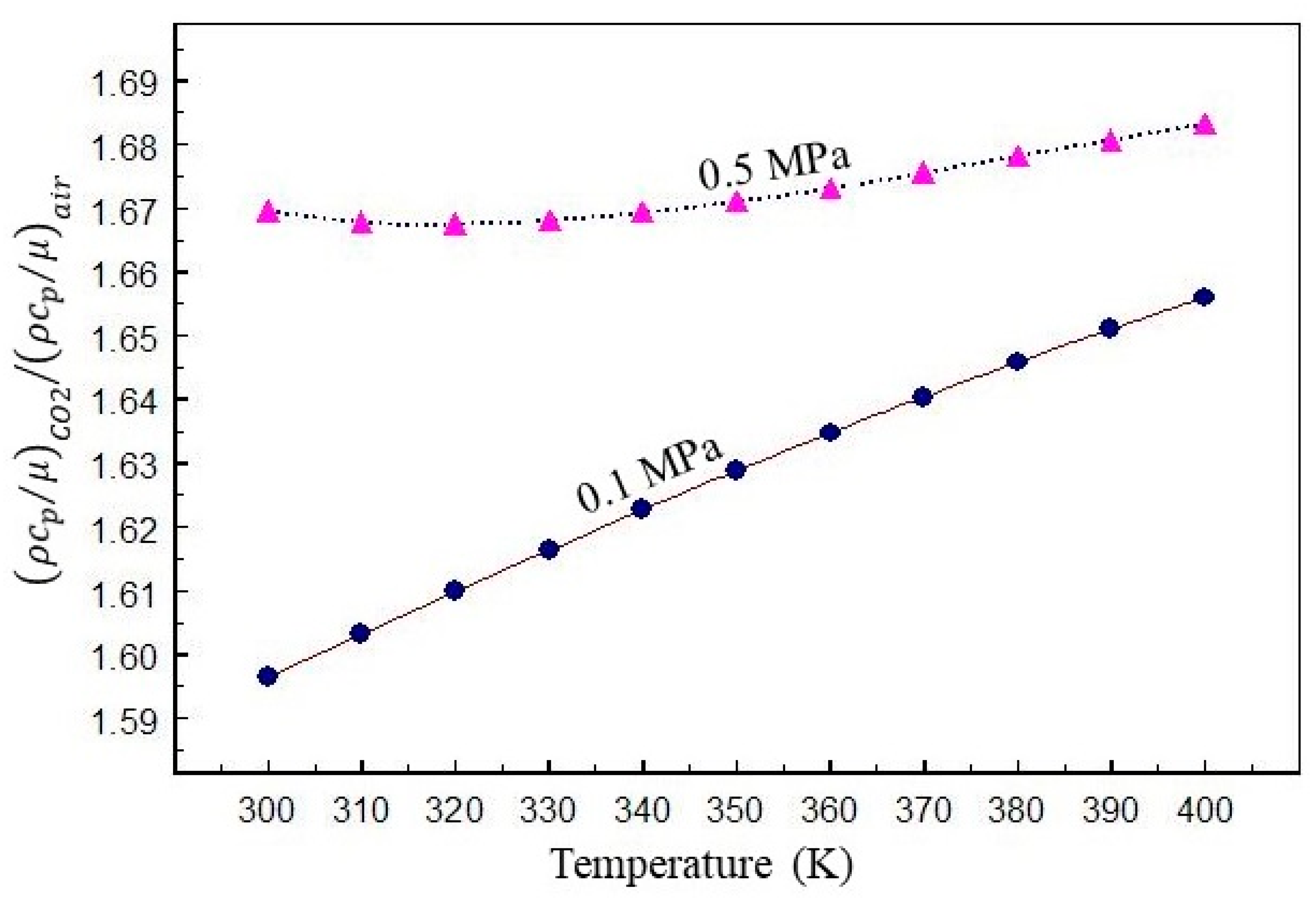
2. Thermodynamic and Transport Properties
3. Steam Condensing Performance
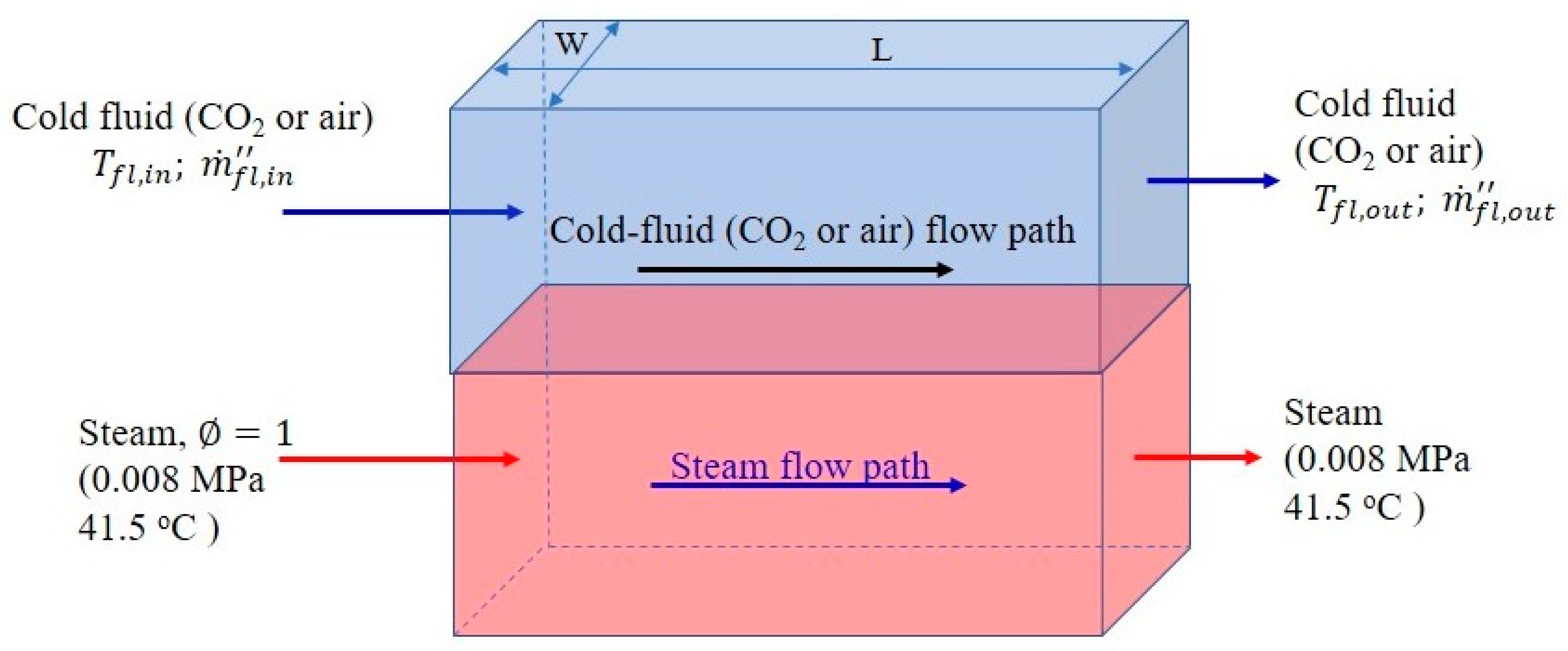
3.1. Mass Conservation Equation for the Condensing Steam
3.2. Vapor Conservation Equation
3.3. Energy Conservation Equation for the Cooling Stream
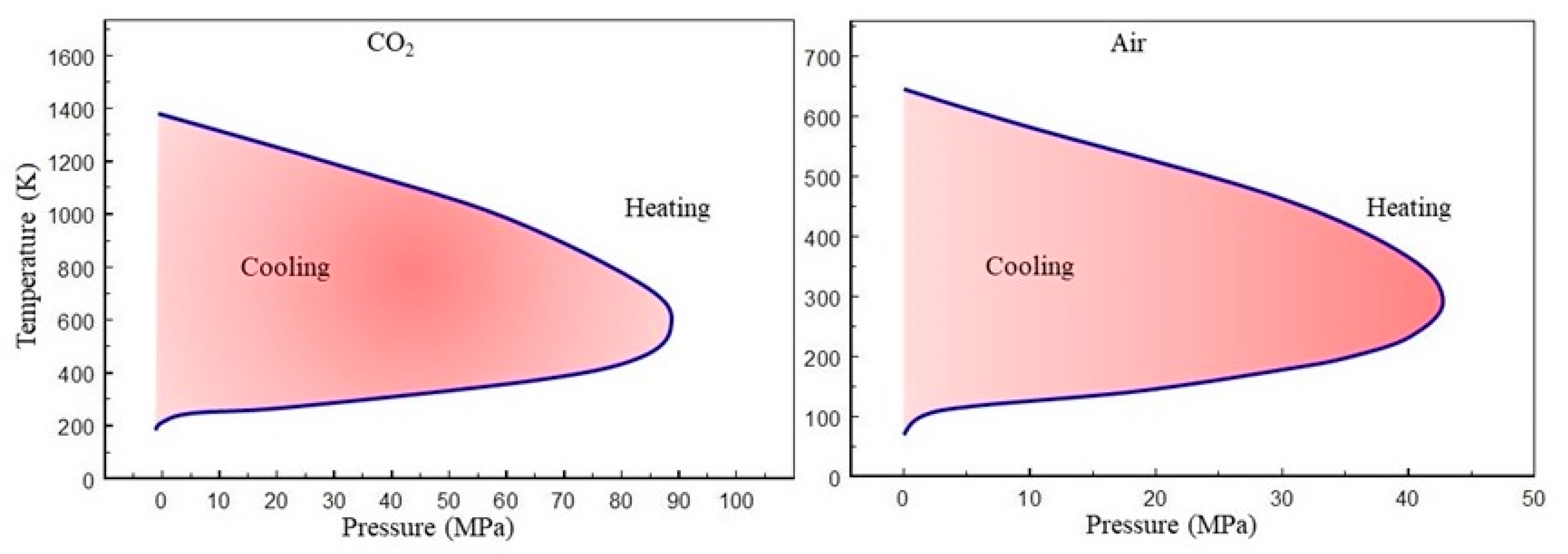
4. CO2 Storage and Utilization
5. Conclusions
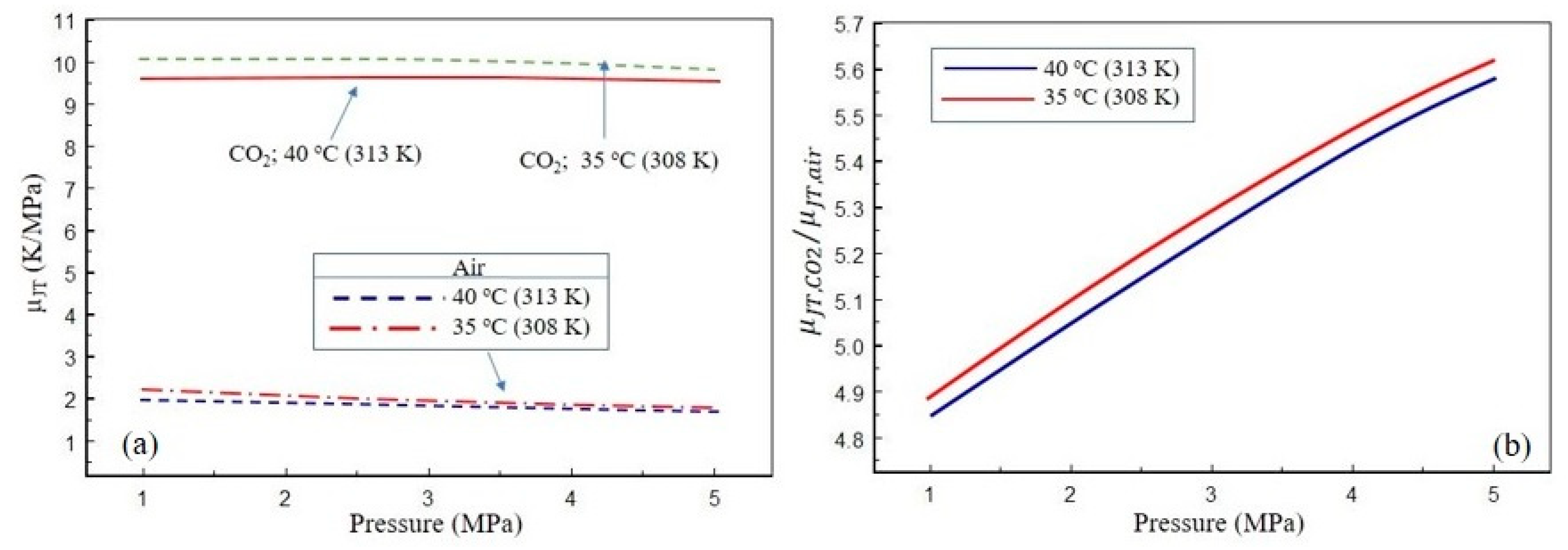
- With its high heat capacity and excellent Joule–Thomson cooling effect, CO2 is a better and more effective fluid for removing heat from a thermal power plant than air.
- The condenser is an important component in power plants. Its primary function is to produce saturated liquid water before pumping it back into the boiler while maintaining the back pressure on the exhaust side of the turbine. Sample calculations carried out for a simple steam-condensing device shown in Figure 5 indicated that CO2 is a better heat-removing fluid than air for a condenser to meet these functions.
- The condensing surface area was also estimated, and the results show that when CO2 is used, the condensing surface is 50% to 60% less than the case if air is used. This leads to significant reductions in the condenser size and capital costs.
- We roughly estimated the amount of CO2 that can be stored and utilized for a steam power plant that operates with steam of 540 °C (813 K) and 10 MPa at the turbine inlet and saturated-vapor steam at 0.008 MPa at the turbine outlet. The results indicate that if CO2 is used as a cooling fluid, the CO2 emitted from a 1000 MW power plant during a period of 250 days can be stored and utilized.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
Nomenclature
| Ast (m2) | Cross-sectional area of the steam path |
| (J/kg-K) | Fluid-specific heat at constant pressure |
| (J/kg-K) | CO2-specific heat at constant pressure |
| D (m) | Heat transfer characteristic length of the cold-fluid stream |
| (J/kg) | Latent heat of condensation |
| (J/m2–s-K) | Convective heat transfer coefficient |
| (J/kg) | Specific enthalpy of the steam at the turbine inlet |
| (J/kg) | Specific enthalpy of the steam at the turbine exit |
| L (m) | Length |
| (kg/m2–s) | Fluid mass flow rate per unit area |
| (kg/m3-s) | Condensation rate |
| (kg/s) | Steam mass flow rate |
| (kg/s) | CO2 mass flow rate |
| Nu | Nusselt number, Nu |
| P (m) | Perimeter of the cold-fluid flow path |
| (Pa) | Storage container pressure |
| (Pa) | Fluid pressure in the condenser |
| (J/s) | Isothermal heat released rate during steam condensation |
| (J/s) | Rate of heat absorbed by CO2 stream |
| (K) | CO2 temperature at the condenser inlet |
| (K) | CO2 temperature at the condenser outlet |
| (K) | Steam temperature |
| (K) | Cold-fluid temperature at the condenser inlet |
| (K/Pa) | Joule–Thomson coefficient |
| (K) | Fluid temperature in the condenser |
| (K) | Fluid temperature in the storage container |
| (kg/m3) | Fluid density |
| x (m) | x-direction |
| (m/s) | Steam velocity in x-direction |
| (J/s) | Turbine work |
| λfl (J/m-s-K) | The fluid thermal conductivity |
| Dimensionless distance | |
| (kg/m3) | Density of liquid water |
| (kg/m3) | Density of the vapor |
| Volume fraction of the vapor | |
| Vapor fraction of the steam at the condenser inlet |
References
- Cho, J.; Park, G.; Kwon, S.; Lee, K.S.; Lee, H.S.; Min, B. Compositional Modeling to Analyze the Effect of CH4 on Coupled Carbon Storage and Enhanced Oil Recovery Process. Appl. Sci. 2020, 10, 4272. [Google Scholar] [CrossRef]
- Hou, S.-S.; Chiang, C.-Y.; Lin, T.-H. Oxy-Fuel Combustion Characteristics of Pulverized Coal under O2/Recirculated Flue Gas Atmospheres. Appl. Sci. 2020, 10, 1362. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, R.; Ohkura, M.; Nakamata, T.; Wakui, T. Numerical Analysis for Performance Evaluation of a Multi-Functional CO2 Heat Pump Water Heating System. Appl. Sci. 2018, 8, 1829. [Google Scholar] [CrossRef] [Green Version]
- O’Donovan, A.; Grimes, R. A theoretical and experimental investigation into the thermodynamic performance of a 50 MW power plant with a novel modular air-cooled,” Condenser. Appl. Therm. Eng. 2014, 71, 119–129. [Google Scholar] [CrossRef]
- Lin, J.; Mahvi, A.J.; Kunke, T.S.; Garimella, S. Improving air-side heat transfer performance in air-cooled power plant condensers. Appl. Therm. Eng. 2020, 170, 114913. [Google Scholar] [CrossRef]
- Mahvi, A.J.; Kunke, T.; Crystal, R.V.; Garimella, S. Enhanced Power Plan Air-Cooled Condenser Using Auto-Fluttering Reads. Appl. Therm. Sci. 2021, 193, 116956. [Google Scholar] [CrossRef]
- Bustamante, J.G.; Rattner, A.S.; Garimella, S. Achieving near-water-cooled power plant performance with air-cooled condensers. Appl. Therm. Eng. 2016, 105, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Pritchett, J.W. On the relative effectiveness of H2O and CO2 as reservoir working fluids for egs heat mining. Geotherm. Resour. Counc. Trans. 2009, 33, 235–239. [Google Scholar]
- Pruess, K. Enhanced geothermal systems (egs) using CO2 as working fluid—A novel approach for generating renewable energy with simultaneous sequestration of carbon. Geothermics 2006, 35, 351–367. [Google Scholar] [CrossRef] [Green Version]
- Pruess, K. On production behavior of enhanced geothermal systems with CO2 as working fluid. Energy Convers. Manag. 2008, 49, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Pruess, K.; Spycher, N. Enhanced Geothermal Systems (EGS) with CO2 as Heat Transmission Fluid—A Scheme for Combining Recovery of Renewable Energy with Geologic Storage of CO2 World Geothermal Congress; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2010. [Google Scholar]
- Randolph, J.B.; Saar, M.O. Coupling carbon dioxide sequestration with geothermal energy capture in naturally permeable, porous geologic formations: Implications for CO2 sequestration. In Proceedings of the 10th International Conference on Greenhouse Gas Control Technologies, Amsterdam, The Netherlands, 19–23 September 2011; Gale, J., Hendriks, C., Turkenberg, W., Eds.; Elsevier Procedia: Amsterdam, The Netherlands, 2011; Volume 4, pp. 2206–2213. [Google Scholar]
- Randolph, J.B.; Saar, M.O. Combining geothermal energy capture with geologic carbon dioxide sequestration. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Feng, G.; Shi, Y.; Lei, H. Use of CO2 as Heat Transmission Fluid to Extract Geothermal Energy: Advantages and Disadvantages in Comparison with Water; World Geothermal Congress: Melbourne, Australia, 2015. [Google Scholar]
- Zhang, L.; Feng, L.; Xu, R.; Jiang, P.; Liu, H. Heat transfer and fluid transport of supercritical CO2 in enhanced geothermal system with local thermal non-equilibrium model. Energy Procedia 2014, 63, 7644–7650. [Google Scholar] [CrossRef] [Green Version]
- Phuoc, T.X.; Massoudi, M.; Wang, P.; McKoy, M.L. Heat losses associated with the upward flow of air, water, CO2 in geothermal production wells. Int. J. Heat Mass Transf. 2019, 132, 249–258. [Google Scholar] [CrossRef]
- Phuoc, T.X.; Massoudi, M.; Wang, P.; McKoy, M.L. Exergy of air, CO2, and H2O for use as Geothermal Fluids. Int. J. Heat Mass Transf. 2018, 126, 448–456. [Google Scholar] [CrossRef]
- Phuoc, T.X.; Massoudi, M. Pumping Gaseous CO2 into a High-pressure, Constant-Volume Storage Cylinder: A Thermodynamics and Parametric Analysis. J. Energy Storage 2021. submitted for publication. [Google Scholar]
- Phuoc, T.X.; Massoudi, M. On Joule-Thomson cooling capability of compressed CO2 for energy storage and CO2 utilization. J. Energy Storage 2021. submitted for publication. [Google Scholar]
- Span, R.; Wagner, W. Equations of state for technical applications. III. Results for polar fluids. Int. J. Thermophys. 2003, 24, 111–162. [Google Scholar] [CrossRef]
- Vesovic, V.; Wakeham, W.A.; Olchowy, G.A.; Sengers, J.V.; Watson, J.T.R.; Millat, J. The transport-properties of carbon-dioxide. J. Phys. Chem. Ref. Data 1990, 19, 763–808. [Google Scholar] [CrossRef]
- Fenghour, A.; Wakeham, W.A.; Vesovic, V. The viscosity of carbon dioxide. J. Phys. Chem. Ref. Data 1998, 27, 31–44. [Google Scholar] [CrossRef]
- NIST. Nist Chemistry Webbook. Available online: http://webbook.Nist.Gov/ (accessed on 30 March 2021).
- Kadoya, K.; Matsunaga, N.; Nagashima, A. Viscosity and thermal-conductivity of dry air in the gaseous-phase. J. Phys. Chem. Ref. Data 1985, 14, 947–970. [Google Scholar] [CrossRef] [Green Version]
- Lemmon, E.W.; Jacobsen, R.T.; Penoncello, S.G.; Friend, D.G. Thermodynamic properties of air and mixtures of nitrogen, argon, and oxygen from 60 to 2000 k at pressures to 2000 MPA. J. Phys. Chem. Ref. Data 2000, 29, 331–385. [Google Scholar] [CrossRef] [Green Version]
- Lemmon, E.W.; Jacobsen, R.T. Viscosity and thermal conductivity equations for nitrogen, oxygen, argon, and air. Int. J. 2004, 25, 21–69. [Google Scholar] [CrossRef]
- Kestin, J. A Course in Thermodynamics; Revised Printing; McGraw-Hill: New York, NY, USA, 1979; Volume 1. [Google Scholar]
- Wallis, G.B. One-Dimensional Two-Phase Flow; McGraw-Hill Publishing: New York, NY, USA, 1969. [Google Scholar]
- Pattanayaka, L.; Padhib, B.N.; Kodamasinghc, B. Thermal performance assessment of steam surface condenser. Case Stud. Therm. Eng. 2019, 14, 100484. [Google Scholar] [CrossRef]


| Container | (kg/m2-s), Entering a Cooling Device | ||||
|---|---|---|---|---|---|
| P | T | Tin | |||
| (MPa) | (K) | (K) | (kg/m2-s) | ||
| CO2 | Air | CO2 | Air | ||
| 1.0 | 308 | 299 | 306 | 1265 | 1012 |
| 313 | 304 | 311 | 1253 | 1003 | |
| 2.0 | 308 | 288 | 304 | 1871 | 1475 |
| 313 | 294 | 309 | 1852 | 1462 | |
| 3.0 | 308 | 278 | 302 | 2354 | 1827 |
| 313 | 284 | 307 | 2327 | 1812 | |
| 4.0 | 308 | 268 | 300 | 2781 | 2125 |
| 313 | 275 | 306 | 2746 | 2106 | |
| 5.0 | 308 | 259 | 299 | 3176 | 2388 |
| 313 | 266 | 304 | 3132 | 2367 | |
| Pstor | (MPa) | (J/s-K) | ||
|---|---|---|---|---|
| Air | CO2 | Air | CO2 | |
| 1 | 101,884 | 107,702 | 2.635 × 10−7 | 1.55 × 10−7 |
| 2 | 148,485 | 157,302 | 1.797 × 10−7 | 1.00 × 10−7 |
| 3 | 183,906 | 195,623 | 1.443 × 10−7 | 7.68 × 10−8 |
| 4 | 213,887 | 228,415 | 1.234 × 10−7 | 6.24 × 10−8 |
| 5 | 240,350 | 258,122 | 1.095 × 10−7 | 5.26 × 10−8 |
| Pstor | ||||
|---|---|---|---|---|
| (MPa) | Air | CO2 | Air | CO2 |
| 1 | 1 | 0.82 | WL | 0.82 WL |
| 2 | 0.77 | 0.49 | 0.77 WL | 0.49 WL |
| 3 | 0.63 | 0.37 | 0.63 WL | 0.37 WL |
| 4 | 0.54 | 0.30 | 0.54 WL | 0.30 WL |
| 5 | 0.51 | 0.27 | 0.51 WL | 0.27 WL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuoc, T.X.; Massoudi, M. Using CO2 as a Cooling Fluid for Power Plants: A Novel Approach for CO2 Storage and Utilization. Appl. Sci. 2021, 11, 4974. https://doi.org/10.3390/app11114974
Phuoc TX, Massoudi M. Using CO2 as a Cooling Fluid for Power Plants: A Novel Approach for CO2 Storage and Utilization. Applied Sciences. 2021; 11(11):4974. https://doi.org/10.3390/app11114974
Chicago/Turabian StylePhuoc, Tran X., and Mehrdad Massoudi. 2021. "Using CO2 as a Cooling Fluid for Power Plants: A Novel Approach for CO2 Storage and Utilization" Applied Sciences 11, no. 11: 4974. https://doi.org/10.3390/app11114974
APA StylePhuoc, T. X., & Massoudi, M. (2021). Using CO2 as a Cooling Fluid for Power Plants: A Novel Approach for CO2 Storage and Utilization. Applied Sciences, 11(11), 4974. https://doi.org/10.3390/app11114974







