Applications of Nanosized-Lipid-Based Drug Delivery Systems in Wound Care
Abstract
1. Introduction
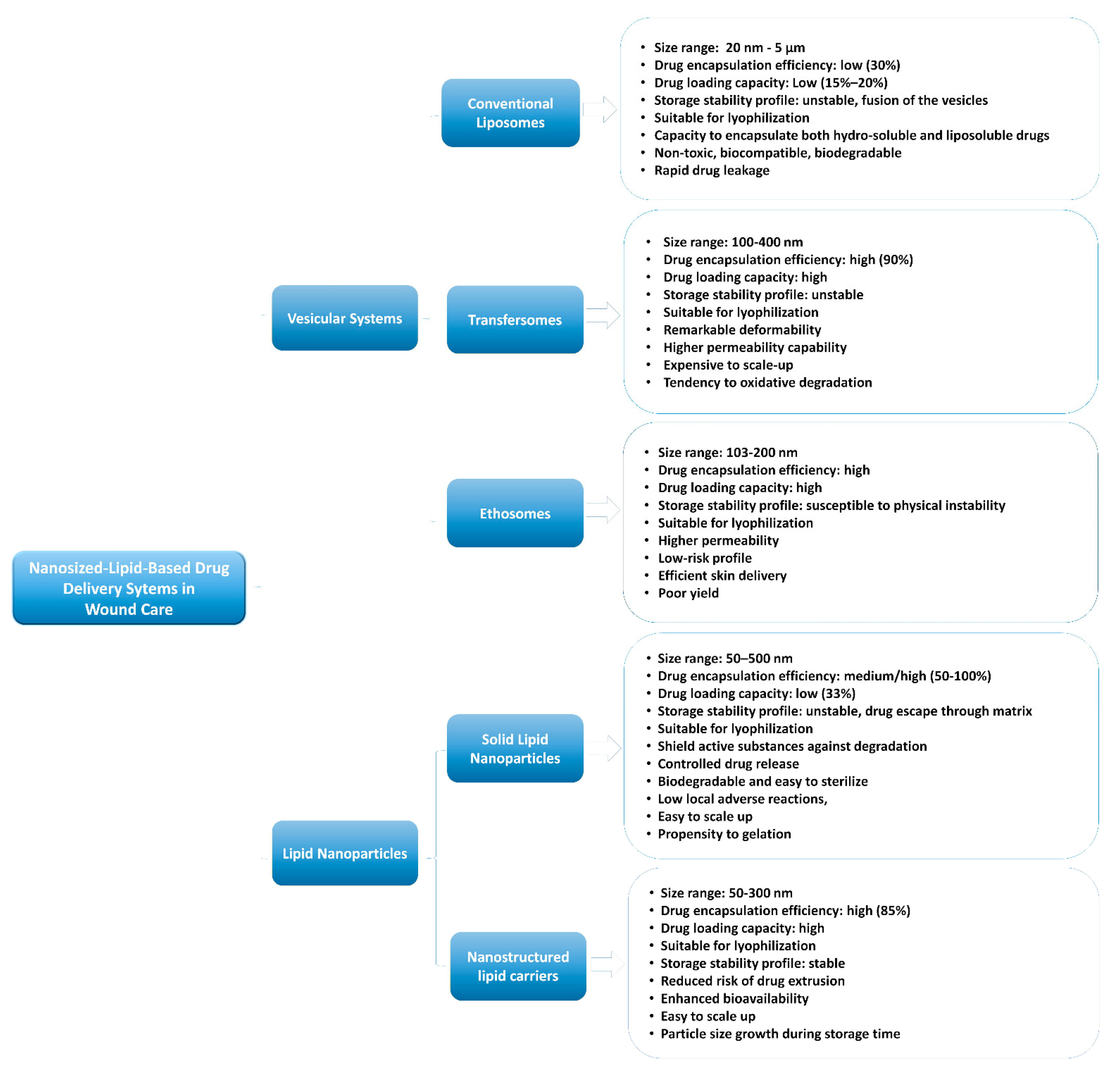
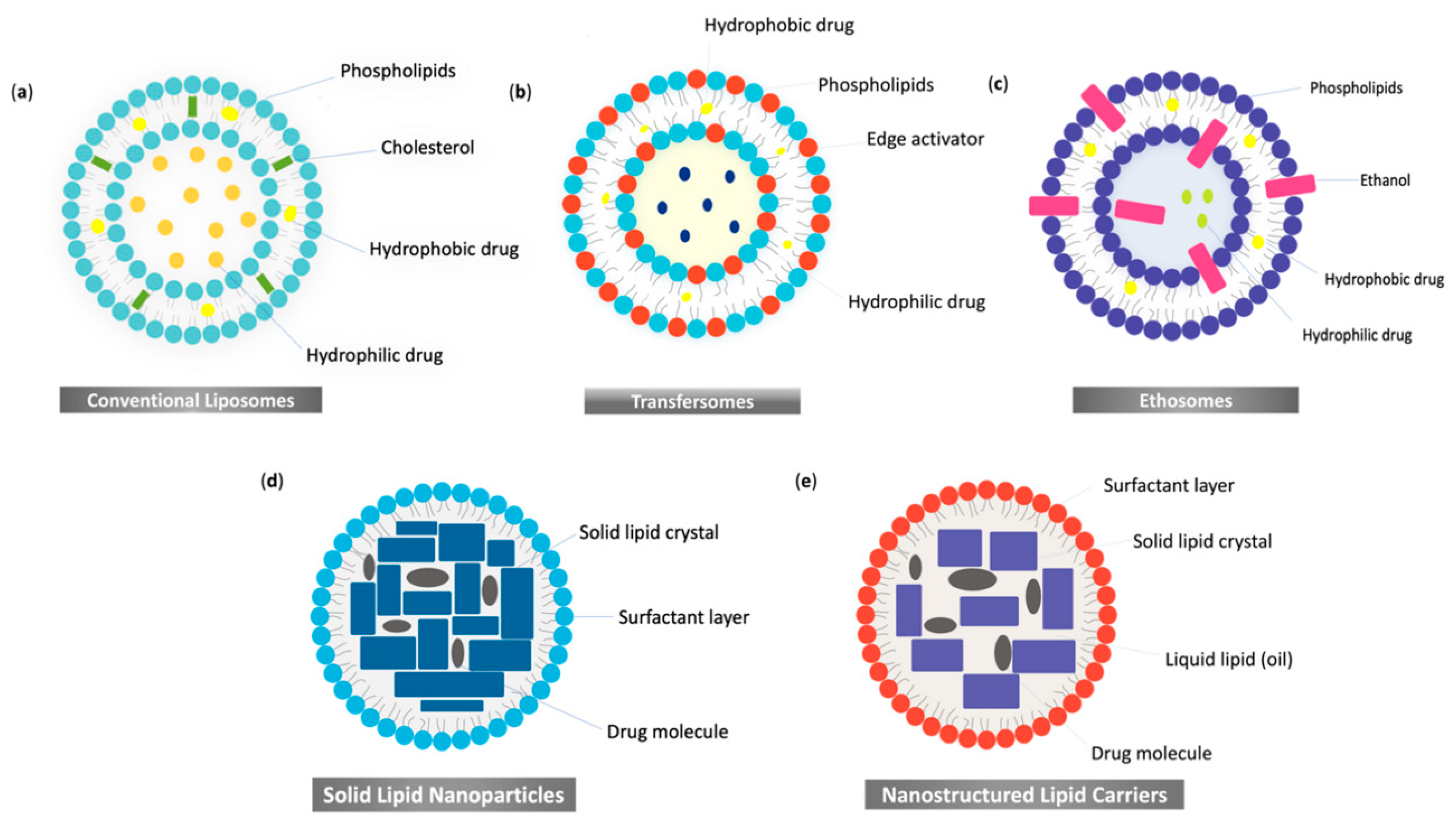
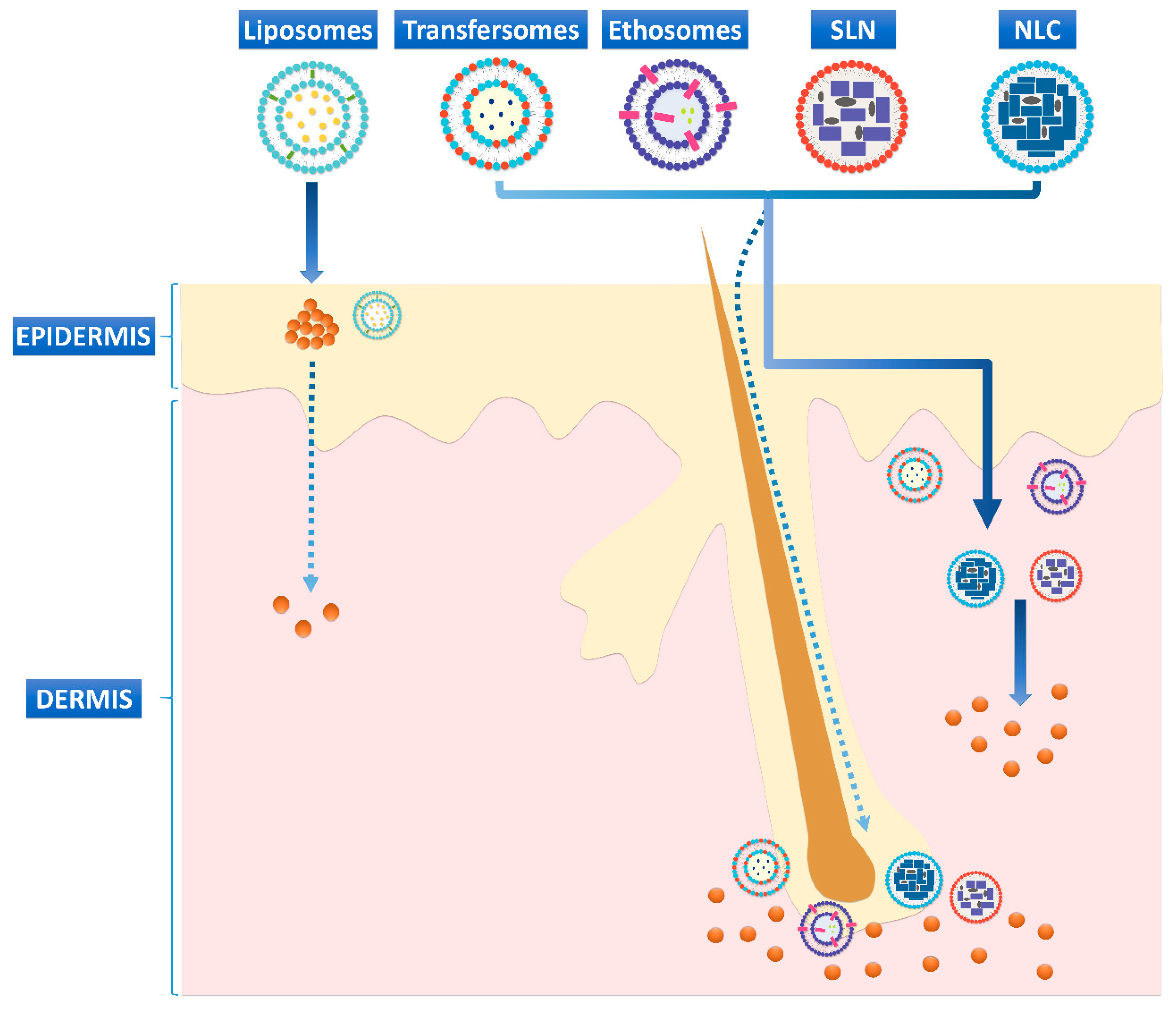
2. Nanosized-Lipid-Based Drug Delivery Systems
3. Nanosized-Lipid-Based Drug Delivery Systems in Wound Healing
3.1. Vesicular Systems
3.1.1. Conventional Liposomes in Wound Healing
3.1.2. Ultra-Deformable Liposomes or Transfersomes in Wound Healing
3.1.3. Ethosomes in Wound Healing
3.2. Lipid Nanoparticles in Wound Healing
3.2.1. Solid Lipid Nanoparticles in Wound Healing
3.2.2. Nanostructured Lipid Carriers in Wound Healing
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-Fibroblast Interactions in Wound Healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of Acute Wound Healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Petca, A.; Negoita, S.; Petca, R.-C.; Calo, O.; Diana Sinescu, R. Ether-Based Polyurethane Foam for Vacuum-Assisted Closure (V.A.C.) of Complicated Postoperative Abdominal Wound Dehiscence. Mater. Plast. 2020, 57, 32–38. [Google Scholar] [CrossRef]
- Matei, A.-M.; Draghici-Ionescu, A.-M.; Cioplea, M.; Zurac, S.; Boda, D.; Serban, I.; Caruntu, C.; Ilie, M.; Gyula, L. Skin Endometriosis: A Case Report and Review of the Literature. Exp. Ther. Med. 2021, 21, 1–5. [Google Scholar] [CrossRef]
- Fatima, K.; Khanani, S. Scar Endometriosis: An Entity Not to Be Forgotten. J. Pak. Med. Assoc. 2017, 67, 140–142. [Google Scholar]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Lindholm, C.; Searle, R. Wound Management for the 21st Century: Combining Effectiveness and Efficiency. Int. Wound J. 2016, 13, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy: PERSPECTIVE ARTICLE. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Menke, N.B.; Ward, K.R.; Witten, T.M.; Bonchev, D.G.; Diegelmann, R.F. Impaired Wound Healing. Clin. Dermatol. 2007, 25, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Vowden, K.; Vowden, P.; Posnett, J. The Resource Costs of Wound Care in Bradford and Airedale Primary Care Trust in the UK. J. Wound Care 2009, 18. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Tomic-Canic, M. Cellular and Molecular Basis of Wound Healing in Diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef]
- Grey, J.E.; Enoch, S.; Harding, K.G. Wound Assessment. BMJ 2006, 332, 285–288. [Google Scholar] [CrossRef]
- Bock, O.; Schmid-Ott, G.; Malewski, P.; Mrowietz, U. Quality of Life of Patients with Keloid and Hypertrophic Scarring. Arch. Dermatol. Res. 2006, 297, 433–438. [Google Scholar] [CrossRef]
- Bijlard, E.; Kouwenberg, C.A.E.; Timman, R.; Hovius, S.E.R.; Busschbach, J.J.V.; Mureau, M.A.M. Burden of Keloid Disease: A Cross-Sectional Health-Related Quality of Life Assessment. Acta Derm. Venereol. 2017, 97, 225–229. [Google Scholar] [CrossRef]
- Lyons, A.B.; Peacock, A.; Braunberger, T.L.; Viola, K.V.; Ozog, D.M. Disease Severity and Quality of Life Outcome Measurements in Patients with Keloids: A Systematic Review. Dermatol. Surg. 2019, 45, 1477–1483. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Wrobel, J.; Robbins, J.M. Guest Editorial: Are Diabetes-Related Wounds and Amputations Worse than Cancer? Int. Wound J. 2007, 4, 286–287. [Google Scholar] [CrossRef]
- Partridge, L.; Gems, D. Mechanisms of Ageing: Public or Private? Nat. Rev. Genet. 2002, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured Lipid Carriers: A Potential Use for Skin Drug Delivery Systems. Pharmacol. Rep. 2019, 71, 156–166. [Google Scholar] [CrossRef]
- Korrapati, P.S.; Karthikeyan, K.; Satish, A.; Krishnaswamy, V.R.; Venugopal, J.R.; Ramakrishna, S. Recent Advancements in Nanotechnological Strategies in Selection, Design and Delivery of Biomolecules for Skin Regeneration. Mater. Sci. Eng. C 2016, 67, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Pachuau, L. Recent Developments in Novel Drug Delivery Systems for Wound Healing. Expert Opin. Drug Deliv. 2015, 12, 1895–1909. [Google Scholar] [CrossRef]
- Johnson, N.; Wang, Y. Drug Delivery Systems for Wound Healing. Curr. Pharm. Biotechnol. 2015, 16, 621–629. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in Tissue Engineering and Regenerative Medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef]
- Geusens, B.; Strobbe, T.; Bracke, S.; Dynoodt, P.; Sanders, N.; Van Gele, M.; Lambert, J. Lipid-Mediated Gene Delivery to the Skin. Eur. J. Pharm. Sci. 2011, 43, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Lipid-Based Nano-Delivery Systems for Skin Delivery of Drugs and Bioactives. Front. Pharmacol. 2015, 6, 2011–2015. [Google Scholar] [CrossRef]
- Xie, G.; Lu, W.; Lu, D. Penetration of Titanium Dioxide Nanoparticles through Slightly Damaged Skin in Vitro and in Vivo. J. Appl. Biomater. Funct. Mater. 2015, 13, e356–e361. [Google Scholar] [CrossRef]
- Mauro, M.; Crosera, M.; Monai, M.; Montini, T.; Fornasiero, P.; Bovenzi, M.; Adami, G.; Turco, G.; Filon, F.L. Cerium Oxide Nanoparticles Absorption through Intact and Damaged Human Skin. Molecules 2019, 24, 3759. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jain, V.; Mahajan, S.C. Lipid Based Vesicular Drug Delivery Systems. Adv. Pharm. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Verma, S.; Singh, S.; Syan, N.; Mathur, P.; Valecha, V. Nanoparticle Vesicular Systems: A Versatile Tool for Drug Delivery. J. Chem. Pharm. Res. 2010, 2, 496–509. [Google Scholar]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A Brief Review on Solid Lipid Nanoparticles: Part and Parcel of Contemporary Drug Delivery Systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid Nanocarriers as Skin Drug Delivery Systems: Properties, Mechanisms of Skin Interactions and Medical Applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- López-García, R.; Ganem-Rondero, A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Occlusive Effect and Penetration Enhancement Ability. J. Cosmet. Dermatol. Sci. Appl. 2015, 05, 62–72. [Google Scholar] [CrossRef]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and Microparticles for Skin Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- de Souza, M.L.; dos Santos, W.M.; de Sousa, A.L.M.D.; de Albuquerque Wanderley Sales, V.; Nóbrega, F.P.; de Oliveira, M.V.G.; Rolim-Neto, P.J. Lipid Nanoparticles as a Skin Wound Healing Drug Delivery System: Discoveries and Advances. Curr. Pharm. Des. 2020, 26, 4536–4550. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.K. Burn Wound: How It Differs from Other Wounds. Indian J. Plast. Surg. 2012, 45, 364–373. [Google Scholar] [CrossRef]
- Ali, M.; Jahromi, M.; Zangabad, P.S.; Moosavi, S.M.; Zangabad, K.S.; Ghamarypour, A.; Aref, A.R.; Hamblin, M.R.; Education, U.S.; Education, U.S. Nanomedicine and Advanced Technologies for Burns: Preventing Infection and Facilitating Wound Healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Xu, H.L.; Chen, P.P.; ZhuGe, D.L.; Zhu, Q.Y.; Jin, B.H.; Shen, B.X.; Xiao, J.; Zhao, Y.Z. Liposomes with Silk Fibroin Hydrogel Core to Stabilize BFGF and Promote the Wound Healing of Mice with Deep Second-Degree Scald. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, M.; Wang, H.; Du, S. Increased Cutaneous Wound Healing Effect of Biodegradable Liposomes Containing Madecassoside: Preparation Optimization, in Vitro Dermal Permeation, and in Vivo Bioevaluation. Int. J. Nanomed. 2016, 11, 2995–3007. [Google Scholar] [CrossRef] [PubMed]
- Alemdaroǧlu, C.; Degim, Z.; Celebi, N.; Şengezer, M.; Alömeroglu, M.; Nacar, A.; Galili, U.; Deǧim, Z.; Çelebi, N.; Alemdaroǧlu, C.; et al. Investigation of Epidermal Growth Factor Containing Liposome Formulation Effects on Burn Wound Healing. J. Biomed. Mater. Res. Part A 2008, 85, 271–283. [Google Scholar] [CrossRef]
- Wasef, L.G.; Shaheen, H.M.; El-Sayed, Y.S.; Shalaby, T.I.A.; Samak, D.H.; Abd El-Hack, M.E.; Al-Owaimer, A.; Saadeldin, I.M.; El-mleeh, A.; Ba-Awadh, H.; et al. Effects of Silver Nanoparticles on Burn Wound Healing in a Mouse Model. Biol. Trace Elem. Res. 2020, 193, 456–465. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Cornaglia, A.I.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential Oil-Loaded Lipid Nanoparticles for Wound Healing. Int. J. Nanomed. 2018, 13, 175–186. [Google Scholar] [CrossRef]
- Leaper, D.J. Traumatic and Surgical Wounds. BMJ 2006, 332, 532–535. [Google Scholar] [CrossRef]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and Nanofibers for Topical Drug Delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Arantes, V.T.; Faraco, A.A.G.; Ferreira, F.B.; Oliveira, C.A.; Martins-Santos, E.; Cassini-Vieira, P.; Barcelos, L.S.; Ferreira, L.A.M.; Goulart, G.A.C. Retinoic Acid-Loaded Solid Lipid Nanoparticles Surrounded by Chitosan Film Support Diabetic Wound Healing in in Vivo Study. Colloids Surf. B Biointerfaces 2020, 188, 110749. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Guo, S.; Yang, L.; Wang, Y.; Wei, X.; Song, S.; Yang, Y.; Gan, Y.; Wang, Z. Silicone Elastomer Gel Impregnated with 20(S)-Protopanaxadiol-Loaded Nanostructured Lipid Carriers for Ordered Diabetic Ulcer Recovery. Acta Pharmacol. Sin. 2020, 41, 119–128. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, M.; Na, Y.G.; Kim, S.J.; Lee, H.K.; Cho, C.W. An EGF- And Curcumin-Co-Encapsulated Nanostructured Lipid Carrier Accelerates Chronic-Wound Healing in Diabetic Rats. Molecules 2020, 25, 4610. [Google Scholar] [CrossRef] [PubMed]
- Motawea, A.; Abd El-Gawad, A.E.G.H.; Borg, T.; Motawea, M.; Tarshoby, M. The Impact of Topical Phenytoin Loaded Nanostructured Lipid Carriers in Diabetic Foot Ulceration. Foot 2019, 40, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kasiewicz, L.N.; Whitehead, K.A. Lipid Nanoparticles Silence Tumor Necrosis Factor α to Improve Wound Healing in Diabetic Mice. Bioeng. Transl. Med. 2019, 4, 75–82. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef] [PubMed]
- Chato-Astrain, J.; Chato-Astrain, I.; Sánchez-Porras, D.; García-García, Ó.D.; Bermejo-Casares, F.; Vairo, C.; Villar-Vidal, M.; Gainza, G.; Villullas, S.; Oruezabal, R.I.; et al. Generation of a Novel Human Dermal Substitute Functionalized with Antibiotic-Loaded Nanostructured Lipid Carriers (NLCs) with Antimicrobial Properties for Tissue Engineering. J. Nanobiotechnol. 2020, 18, 1–13. [Google Scholar] [CrossRef]
- Gabriel, A.; Gupta, S.; Orgill, D.P. Challenges and Management of Surgical Site Occurrences. Plast. Reconstr. Surg. 2019, 143, 7S–10S. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.S. Recent Advances in Nanomaterial-Based Wound-Healing Therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef]
- Choi, J.U.; Lee, S.W.; Pangeni, R.; Byun, Y.; Yoon, I.S.; Park, J.W. Preparation and in Vivo Evaluation of Cationic Elastic Liposomes Comprising Highly Skin-Permeable Growth Factors Combined with Hyaluronic Acid for Enhanced Diabetic Wound-Healing Therapy. Acta Biomater. 2017, 57, 197–215. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Suliman, S.; Viljoen, A.M. The Antimicrobial Activity of Four Commercial Essential Oils in Combination with Conventional Antimicrobials. Lett. Appl. Microbiol. 2009, 48, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, H.; Manna, B. Wound Physiology. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Tran, T.-N.T. Cutaneous Drug Delivery: An Update. J. Investig. Dermatol. Symp. Proc. 2013, 16, S67–S69. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, G. Advances in Studies of Phospholipids as Carriers in Skin Topical Application. J. Nanjing Med. Univ. 2007, 21, 349–353. [Google Scholar] [CrossRef]
- Manca, M.L.; Matricardi, P.; Cencetti, C.; Peris, J.E.; Melis, V.; Carbone, C.; Escribano, E.; Zaru, M.; Fadda, A.M.; Manconi, M. Combination of Argan Oil and Phospholipids for the Development of an Effective Liposome-like Formulation Able to Improve Skin Hydration and Allantoin Dermal Delivery. Int. J. Pharm. 2016, 5052, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Deformable Liposomes and Ethosomes: Mechanism of Enhanced Skin Delivery. Int. J. Pharm. 2006, 322, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Mbah, C.C.; Builders, P.F.; Attama, A.A. Nanovesicular Carriers as Alternative Drug Delivery Systems: Ethosomes in Focus. Expert Opin. Drug Deliv. 2014, 11, 45–59. [Google Scholar] [CrossRef]
- Mortensen, L.J.; Oberdörster, G.; Pentland, A.P.; DeLouise, L.A. In Vivo Skin Penetration of Quantum Dot Nanoparticles in the Murine Model: The Effect of UVR. Nano Lett. 2008, 8, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Choe, C.-S.; Ahlberg, S.; Meinke, M.C.; Alexiev, U.; Lademann, J.; Darvin, M.E. Penetration of Silver Nanoparticles into Porcine Skin Ex Vivo Using Fluorescence Lifetime Imaging Microscopy, Raman Microscopy, and Surface-Enhanced Raman Scattering Microscopy. J. Biomed. Opt. 2014, 20, 051006. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Knorr, F.; Richter, H.; Blume-Peytavi, U.; Vogt, A.; Antoniou, C.; Sterry, W.; Patzelt, A. Hair Follicles—An Efficient Storage and Penetration Pathway for Topically Applied Substances: Summary of Recent Results Obtained at the Center of Experimental and Applied Cutaneous Physiology, Charité-Universitätsmedizin Berlin, Germany. Skin Pharmacol. Physiol. 2008, 21, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, D.; Rancan, F.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Nanoparticles in Dermatology. Arch. Dermatol. Res. 2011, 303, 533–550. [Google Scholar] [CrossRef]
- Teichmann, A.; Jacobi, U.; Ossadnik, M.; Richter, H.; Koch, S.; Sterry, W.; Lademann, J. Differential Stripping: Determination of the Amount of Topically Applied Substances Penetrated into the Hair Follicles. J. Investig. Dermatol. 2005, 125, 264–269. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef]
- Larese Filon, F.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles Skin Absorption: New Aspects for a Safety Profile Evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and Transdermal Drug Delivery Systems: Current and Future Prospects. Drug Deliv. J. Deliv. Target. Ther. Agents 2006, 13, 175–187. [Google Scholar] [CrossRef]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-Enabled Transdermal Drug Delivery Systems for Enhanced Dose Control and Tissue Targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef]
- Risaliti, L.; Kehagia, A.; Daoultzi, E.; Lazari, D.; Bergonzi, M.C.; Vergkizi-Nikolakaki, S.; Hadjipavlou-Litina, D.; Bilia, A.R. Liposomes Loaded with Salvia Triloba and Rosmarinus Officinalis Essential Oils: In Vitro Assessment of Antioxidant, Antiinflammatory and Antibacterial Activities. J. Drug Deliv. Sci. Technol. 2019, 51, 493–498. [Google Scholar] [CrossRef]
- Razavi, S.; Partoazar, A.; Takzaree, N.; Fasihi-Ramandi, M.; Bahador, A.; Darvishi, M.H. Silver Sulfadiazine Nanoethogel for Burn Healing: Characterization and Investigation of Its in Vivo Effects. Nanomedicine 2018, 13, 1319–1331. [Google Scholar] [CrossRef]
- Kakkar, V.; Kaur, I.P.; Kaur, A.P.; Saini, K.; Singh, K.K. Topical Delivery of Tetrahydrocurcumin Lipid Nanoparticles Effectively Inhibits Skin Inflammation: In Vitro and in Vivo Study. Drug Dev. Ind. Pharm. 2018, 44, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; Abd El-Rahman, F.A.A.; Hamdy, G.M. Chamomile Oil Loaded Solid Lipid Nanoparticles: A Naturally Formulated Remedy to Enhance the Wound Healing. J. Drug Deliv. Sci. Technol. 2019, 50, 329–338. [Google Scholar] [CrossRef]
- Fumakia, M.; Ho, E.A. Nanoparticles Encapsulated with LL37 and Serpin A1 Promotes Wound Healing and Synergistically Enhances Antibacterial Activity. Mol. Pharm. 2016, 13, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Girbau, C.; Alonso, R.; Aguirre, J.J.; Pedraz, J.L.; Igartua, M.; Hernandez, R.M. LL37 Loaded Nanostructured Lipid Carriers (NLC): A New Strategy for the Topical Treatment of Chronic Wounds. Eur. J. Pharm. Biopharm. 2016, 108, 310–316. [Google Scholar] [CrossRef]
- Ghodrati, M.; Farahpour, M.R.; Hamishehkar, H. Encapsulation of Peppermint Essential Oil in Nanostructured Lipid Carriers: In-Vitro Antibacterial Activity and Accelerative Effect on Infected Wound Healing. Colloids Surf. A Physicochem. Eng. Asp. 2019, 564, 161–169. [Google Scholar] [CrossRef]
- Bangham, A.D.; Haydon, D.A. Ultrastructure of Membranes: Bimolecular Organization. Br. Med. Bull. 1968, 24, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Honeywell-Nguyen, P.L.; Bouwstra, J.A. Vesicles as a Tool for Transdermal and Dermal Delivery. Drug Discov. Today Technol. 2005, 2, 67–74. [Google Scholar] [CrossRef]
- Ramanunny, A.K.; Wadhwa, S.; Gulati, M.; Singh, S.K.; Kapoor, B.; Dureja, H.; Chellappan, D.K.; Anand, K.; Dua, K.; Khursheed, R.; et al. Nanocarriers for Treatment of Dermatological Diseases: Principle, Perspective and Practices. Eur. J. Pharmacol. 2021, 890, 173691. [Google Scholar] [CrossRef]
- Yadav, A.V.; Murthy, M.S.; Shete, A.S.; Sakhare, S. Stability Aspects of Liposomes. Indian J. Pharm. Educ. Res. 2011, 45, 402–413. [Google Scholar]
- Payton, N.M.; Wempe, M.F.; Xu, Y.; Anchordoquy, T.J. Long-Term Storage of Lyophilized Liposomal Formulations. J. Pharm. Sci. 2014, 103, 3869–3878. [Google Scholar] [CrossRef] [PubMed]
- Kan, P.; Tsao, C.-W.; Wang, A.-J.; Su, W.-C.; Liang, H.-F. A Liposomal Formulation Able to Incorporate a High Content of Paclitaxel and Exert Promising Anticancer Effect. J. Drug Deliv. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Pandey, H.; Rani, R.; Agarwal, V. Liposome and Their Applications in Cancer Therapy. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Hasanovic, A.; Hollick, C.; Fischinger, K.; Valenta, C. Improvement in Physicochemical Parameters of DPPC Liposomes and Increase in Skin Permeation of Aciclovir and Minoxidil by the Addition of Cationic Polymers. Eur. J. Pharm. Biopharm. 2010, 75, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, P.S.; Zhou, A.; Borab, Z.M.; Frezzo, J.A.; Srivastava, N.; More, H.T.; Rifkin, W.J.; David, J.A.; Berens, S.J.; Chen, R.; et al. Novel Lipoproteoplex Delivers Keap1 SiRNA Based Gene Therapy to Accelerate Diabetic Wound Healing. Biomaterials 2017, 132, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, A.; Liu, X.; Meisgen, F.; Grünler, J.; Botusan, I.R.; Narayanan, S.; Erikci, E.; Li, X.; Blomqvist, L.; et al. MicroRNA-132 Enhances Transition from Inflammation to Proliferation during Wound Healing. J. Clin. Investig. 2015, 125, 3008–3026. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, D.; Wang, A.; Chu, T.; Lohcharoenkal, W.; Zheng, X.; Grünler, J.; Narayanan, S.; Eliasson, S.; Herter, E.K.; et al. MicroRNA-132 with Therapeutic Potential in Chronic Wounds. J. Investig. Dermatol. 2017, 137, 2630–2638. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Wan, R.; Mo, Y.; Li, M.; Tseng, M.T.; Chien, S. Intracellular Adenosine Triphosphate Delivery Enhanced Skin Wound Healing in Rabbits. Ann. Plast. Surg. 2009, 62, 180–186. [Google Scholar] [CrossRef]
- Roesken, F.; Uhl, E.; Curri, S.B.; Menger, M.D.; Messmer, K. Acceleration of Wound Healing by Topical Drug Delivery via Liposomes. Langenbeck’s Arch. Surg. 2000, 385, 42–49. [Google Scholar] [CrossRef]
- Das, S.; Majid, M.; Baker, A.B. Syndecan-4 Enhances PDGF-BB Activity in Diabetic Wound Healing. Acta Biomater. 2016, 42, 56–65. [Google Scholar] [CrossRef]
- Deǧim, Z.; Çelebi, N.; Alemdaroǧlu, C.; Deveci, M.; Öztürk, S.; Özoǧul, C. Evaluation of Chitosan Gel Containing Liposome-Loaded Epidermal Growth Factor on Burn Wound Healing. Int. Wound J. 2011, 8, 343–354. [Google Scholar] [CrossRef]
- Usach, I.; Margarucci, E.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Petretto, G.L.; Manconi, M.; Peris, J.E. Comparison between Citral and Pompia Essential Oil Loaded in Phospholipid Vesicles for the Treatment of Skin and Mucosal Infections. Nanomaterials 2020, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, M.; Du, S. Optimization of Madecassoside Liposomes Using Response Surface Methodology and Evaluation of Its Stability. Int. J. Pharm. 2014, 473, 280–285. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the Challenges in Administering Biopharmaceuticals: Formulation and Delivery Strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, K.J.; Yu, C.H.; Huang, Q.L.; Du, Y.Z. Nano-Drug Delivery Systems in Wound Treatment and Skin Regeneration. J. Nanobiotechnol. 2019, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jadupati, M.; Kumar, N.A. Transferosome: An opportunistic carrier for transdermal drug delivery system. Int. Res. J. Pharm. 2012, 3, 35–38. [Google Scholar]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Lipid Vesicles for Skin Delivery of Drugs: Reviewing Three Decades of Research. Int. J. Pharm. 2007, 332, 1–16. [Google Scholar] [CrossRef]
- Jain, S.; Jain, P.; Umamaheshwari, R.B.; Jain, N.K. Transfersomes—A Novel Vesicular Carrier for Enhanced Transdermal Delivery: Development, Characterization, and Performance Evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Shamma, R.N.; Elsayed, I. Transfersomal Lyophilized Gel of Buspirone HCl: Formulation, Evaluation and Statistical Optimization. J. Liposome Res. 2013, 23, 244–254. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.J.; Shafaat, K.; Faruk, A. Elastic Liposomes as Novel Carriers: Recent Advances in Drug Delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E. Transfersomes for Transdermal Drug Delivery. Expert Opin. Drug Deliv. 2006, 3, 727–737. [Google Scholar] [CrossRef]
- Kulkarni, R.; Yadav, J.D.; Vaidya, K.A.; Gandhi, P.P. Transferosomes: An Emerging Tool for Transdermal Drug Delivery. Int. J. Pharm. Sci. Res. 2011, 2, 735–741. [Google Scholar]
- Surini, S.; Leonyza, A.; Suh, C.W. Formulation and in Vitro Penetration Study of Recombinant Human Epidermal Growth Factor-Loaded Transfersomal Emulgel. Adv. Pharm. Bull. 2020, 10, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Huang, Y.B.; Wu, P.C.; Tsai, Y.H. The Evaluation of Stability of Recombinant Human Epidermal Growth Factor in Burn-Injured Pigs. Process Biochem. 2005, 40, 1661–1665. [Google Scholar] [CrossRef]
- El-Gizawy, S.A.; Nouh, A.; Saber, S.; Kira, A.Y. Deferoxamine-Loaded Transfersomes Accelerates Healing of Pressure Ulcers in Streptozotocin-Induced Diabetic Rats. J. Drug Deliv. Sci. Technol. 2020, 58, 101732. [Google Scholar] [CrossRef]
- Badhe, K.P.; Kundu, P.; Badhe, K.P.; Dube, R.K. Introduction to Ethosome as a Novel Drug Carrier System. J. Pharm. Res. 2012, 55, 5224–5227. [Google Scholar]
- Pingale, P. Ethosomes—Newer Trend in Transdermal Drug Delivery: A Review. Int. J. Pharma Res. Health Sci. 2018, 6, 2586–2590. [Google Scholar] [CrossRef]
- Iqbal, B.; Ali, J.; Baboota, S. Recent Advances and Development in Epidermal and Dermal Drug Deposition Enhancement Technology. Int. J. Dermatol. 2018, 57, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Bhalaria, M.K.; Naik, S.; Misra, A.N. Ethosomes: A Novel Delivery System for Antifungal Drugs in the Treatment of Topical Fungal Diseases. Indian J. Exp. Biol. 2009, 47, 368–375. [Google Scholar]
- Dubey, V.; Mishra, D.; Dutta, T.; Nahar, M.; Saraf, D.K.; Jain, N.K. Dermal and Transdermal Delivery of an Anti-Psoriatic Agent via Ethanolic Liposomes. J. Control. Release 2007, 123, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Pathak, K. Therapeutic and Cosmeceutical Potential of Ethosomes: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar] [CrossRef]
- Pandey, V.; Golhani, D.; Shukla, R. Ethosomes: Versatile Vesicular Carriers for Efficient Transdermal Delivery of Therapeutic Agents. Drug Deliv. 2015, 22, 988–1002. [Google Scholar] [CrossRef] [PubMed]
- Debnath, A.; Sharma, S.; Sharma, P.K. Potential Transdermal Delivery of Herbal Drug Via Ethosomal System for the Treatment of Various. World J. Pharm. Res. 2020, 9, 250–267. [Google Scholar] [CrossRef]
- Cortesi, R.; Ravani, L.; Zaid, A.N.; Menegatti, E.; Romagnoli, R.; Drechsler, M.; Esposito, E.; Cortesi, R. Ethosomes for the Delivery of Anti-HSV-1 Molecules: Preparation, Characterization and in Vitro Activity. Pharmazie 2010, 65, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Partoazar, A.; Kianvash, N.; Darvishi, M.H.; Nasoohi, S.; Rezayat, S.M.; Bahador, A. Ethosomal Curcumin Promoted Wound Healing and Reduced Bacterial Flora in Second Degree Burn in Rat. Drug Res. 2016, 66, 660–665. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Lu, C.T.; Zhang, Y.; Xiao, J.; Zhao, Y.P.; Tian, J.L.; Xu, Y.Y.; Feng, Z.G.; Xu, C.Y. Selection of High Efficient Transdermal Lipid Vesicle for Curcumin Skin Delivery. Int. J. Pharm. 2013, 454, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Patel, N.; Madan, P.; Lin, S. Formulation and Rheological Evaluation of Ethosome-Loaded Carbopol Hydrogel for Transdermal Application. Drug Dev. Ind. Pharm. 2016, 42, 1315–1324. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Mihai, L.G.; Scheau, A.E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef]
- Ilie, M.A.; Caruntu, C.; Lixandru, D.; Tampa, M.; Georgescu, S.R.; Constantin, M.M.; Constantin, C.; Neagu, M.; Zurac, S.A.; Boda, D. In Vivo Confocal Laser Scanning Microscopy Imaging of Skin Inflammation: Clinical Applications and Research Directions (Review). Exp. Ther. Med. 2019, 17, 1004–1011. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Caruntu, C.; Dumitru, C.; Surcel, M.; Zurac, S. Inflammation: A Key Process in Skin Tumorigenesis (Review). Oncol. Lett. 2019, 17, 4068–4084. [Google Scholar] [CrossRef]
- Kazemi, M.; Mombeiny, R.; Tavakol, S.; Keyhanvar, P.; Mousavizadeh, K. Combination Therapy of Nanoethosomal Piroxicam Formulation along with Iontophoresis as an Anti-Inflammatory Transdermal Delivery System for Wound Healing. Int. Wound J. 2019, 16, 1144–1152. [Google Scholar] [CrossRef]
- Fu, X.; Shi, Y.; Wang, H.; Zhao, X.; Sun, Q.; Huang, Y.; Qi, T.; Lin, G. Ethosomal Gel for Improving Transdermal Delivery of Thymosin β-4. Int. J. Nanomed. 2019, 14, 9275–9284. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H. The Influence of the Crystallinity of Lipid Nanoparticles on Their Occlusive Properties. Int. J. Pharm. 2002, 242, 377–379. [Google Scholar] [CrossRef]
- Khalil, M.H.; Marcelletti, J.F.; Katz, L.R.; Katz, D.H.; Pope, L.E. Topical Application of Docosanol-or Stearic Acid-Containing Creams Reduces Severity of Phenol Burn Wounds in Mice. Contact Dermat. 2000, 43, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid Nanoparticles (SLN, NLC) in Cosmetic and Pharmaceutical Dermal Products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid Lipid Nanoparticles: A Modern Formulation Approach in Drug Delivery System. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Daneshmand, S.; Golmohammadzadeh, S.; Jaafari, M.R.; Movaffagh, J.; Rezaee, M.; Sahebkar, A.; Malaekeh-Nikouei, B. Encapsulation Challenges, the Substantial Issue in Solid Lipid Nanoparticles Characterization. J. Cell. Biochem. 2018, 119, 4251–4264. [Google Scholar] [CrossRef] [PubMed]
- Musicanti, C.; Gasco, P. Solid Lipid Nanoparticles—SLN. In Encyclopedia of Nanotechnology; Springer: Dordrecht, The Netherlands, 2012; pp. 2471–2487. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Mneimneh, A.T. Formulation and Applications of Lipid-Based Nanovehicles: Spotlight on Self-Emulsifying Systems. Adv. Pharm. Bull. 2021, 11, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.D.; Lu, X.; Jay, M.; Dziubla, T.D. Optimization of the Lyophilization Process for Long-Term Stability of Solidlipid Nanoparticles. Drug Dev. Ind. Pharm. 2012, 38, 1270–1279. [Google Scholar] [CrossRef]
- Dingler, A.; Gohla, S. Production of Solid Lipid Nanoparticles (SLN): Scaling up Feasibilities. J. Microencapsul. 2002, 19, 11–16. [Google Scholar] [CrossRef]
- Patel, K.K.; Surekha, D.B.; Tripathi, M.; Anjum, M.M.; Muthu, M.S.; Tilak, R.; Agrawal, A.K.; Singh, S. Antibiofilm Potential of Silver Sulfadiazine-Loaded Nanoparticle Formulations: A Study on the Effect of DNase-I on Microbial Biofilm and Wound Healing Activity. Mol. Pharm. 2019, 16, 3916–3925. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Pecorelli, A.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Cervellati, F.; Nastruzzi, C.; Cortesi, R.; Valacchi, G. Production and Characterization of Nanoparticle Based Hyaluronate Gel Containing Retinyl Palmitate for Wound Healing. Curr. Drug Deliv. 2018, 15, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Moglad, H.E.; Fatima, F.; Muqtader, A.M.; Devanathad, V.; Khalid Anw, M.; Aldawsa, M. Development of Topical Antibacterial Gel Loaded with Cefadroxil Solid Lipid Nanoparticles: In Vivo Wound Healing Activity and Epithelialization Study. Int. J. Pharmacol. 2020, 16, 298–309. [Google Scholar] [CrossRef]
- Poon, V.K.M.; Burd, A. In Vitro Cytotoxity of Silver: Implication for Clinical Wound Care. Burns 2004, 30, 140–147. [Google Scholar] [CrossRef]
- Ghaffari, S.; Alihosseini, F.; Rezayat Sorkhabadi, S.M.; Bidgoli, S.A.; Mousavi, S.E.; Haghighat, S.; Nasab, A.A.; Kianvash, N. Nanotechnology in Wound Healing; Semisolid Dosage Forms Containing Curcumin-Ampicillin Solid Lipid Nanoparticles, in-Vitro, Ex-Vivo and in-Vivo Characteristics. Adv. Pharm. Bull. 2018, 8, 395–400. [Google Scholar] [CrossRef]
- Puri, D.; Mishra, A.; Singh, A.P.; Gaur, P.K.; Singh, M.; Yasir, M. Formulation Development of Topical Preparation Containing Nanoparticles of Povidone-Iodine for Wound Healing. Assay Drug Dev. Technol. 2021, 19, 115–123. [Google Scholar] [CrossRef]
- Küchler, S.; Wolf, N.B.; Heilmann, S.; Weindl, G.; Helfmann, J.; Yahya, M.M.; Stein, C.; Schäfer-Korting, M. 3D-Wound Healing Model: Influence of Morphine and Solid Lipid Nanoparticles. J. Biotechnol. 2010, 148, 24–30. [Google Scholar] [CrossRef]
- Shrikhande, G.; Khaodhiar, L.; Scali, S.; Lima, C.; Hubbard, M.; Dudley, K.; Ganda, O.; Ferran, C.; Veves, A. Valsartan Improves Resting Skin Blood Flow in Type 2 Diabetic Patients and Reduces Poly(Adenosine Diphosphate-Ribose) Polymerase Activation. J. Vasc. Surg. 2006, 43, 760–770. [Google Scholar] [CrossRef][Green Version]
- Abadir, P.; Hosseini, S.; Faghih, M.; Ansari, A.; Lay, F.; Smith, B.; Beselman, A.; Vuong, D.; Berger, A.; Tian, J.; et al. Topical Reformulation of Valsartan for Treatment of Chronic Diabetic Wounds. J. Investig. Dermatol. 2018, 138, 434–443. [Google Scholar] [CrossRef]
- El-Salamouni, N.S.; Gowayed, M.A.; Seiffein, N.L.; Abdel- Moneim, R.A.; Kamel, M.A.; Labib, G.S. Valsartan Solid Lipid Nanoparticles Integrated Hydrogel: A Challenging Repurposed Use in the Treatment of Diabetic Foot Ulcer, in-Vitro/in-Vivo Experimental Study. Int. J. Pharm. 2021, 592, 120091. [Google Scholar] [CrossRef]
- Jain, P.; Rahi, P.; Pandey, V.; Asati, S.; Soni, V. Nanostructure Lipid Carriers: A Modish Contrivance to Overcome the Ultraviolet Effects. Egypt. J. Basic Appl. Sci. 2017, 4, 89–100. [Google Scholar] [CrossRef]
- Khan, A.A.; Mudassir, J.; Akhtar, S.; Murugaiyah, V.; Darwis, Y. Freeze-Dried Lopinavir-Loaded Nanostructured Lipid Carriers for Enhanced Cellular Uptake and Bioavailability: Statistical Optimization, in Vitro and in Vivo Evaluations. Pharmaceutics 2019, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Karakash, I.; Vasileska, J.; Shalabalija, D.; Mihailova, L.; Dodov, G.; Raicki, R.S.; Simonoska Crcarevska, M. Freeze-Drying of Nanostructured Lipid Carriers Loaded with Salvia off. Extract for Alzheimer’s Disease Treatment. Maced. Pharm. Bull. 2020, 66, 219–220. [Google Scholar] [CrossRef]
- Xia, Q.; Kong, R. Freeze-Drying and Characterization of Vitamin A Palmitate-Loaded Nanostructured Lipid Carriers (NLC). Mater. Sci. Forum 2011, 694, 365–369. [Google Scholar] [CrossRef]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of Nanostructured Lipid Carriers: Understanding the Types, Designs, and Parameters in the Process of Formulations. J. Nanopart. Res. 2020, 22, 1–29. [Google Scholar] [CrossRef]
- Mishra, S.; Kesharwani, R.; Tiwari, A.K.; Patel, D.K. Improvement of Drug Penetration through the Skin by Using Nanostructured Lipid Carriers ( NLC ). Int. J. Pharm. Pharm. Res. 2016, 6, 1–16. [Google Scholar]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Gainza, G.; Pastor, M.; Aguirre, J.J.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. A Novel Strategy for the Treatment of Chronic Wounds Based on the Topical Administration of RhEGF-Loaded Lipid Nanoparticles: In Vitro Bioactivity and in Vivo Effectiveness in Healing-Impaired Db/Db Mice. J. Control. Release 2014, 185, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Khezri, K.; Farahpour, M.R.; Mounesi Rad, S. Efficacy of Mentha Pulegium Essential Oil Encapsulated into Nanostructured Lipid Carriers as an in Vitro Antibacterial and Infected Wound Healing Agent. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124414. [Google Scholar] [CrossRef]
- Nicolae, I.; Daniela Ene, C.; Georgescu, S.R.; Tampa, M.; Matei, C.; Ceausu, E. Effects of UV Radiation and Oxidative DNA Adduct 8-Hydroxy-2’-Deoxiguanosine on the Skin Diseases. Rev. Chim Buchar. 2014, 65, 1036–1041. [Google Scholar]
- Tampa, M.; Sarbu, M.-I.; Mitran, M.-I.; Mitran, C.-I.; Matei, C.; Georgescu, S.-R. The Pathophysiological Mechanisms and the Quest for Biomarkers in Psoriasis, a Stress-Related Skin Disease. Dis. Markers 2018, 2018, 5823684. [Google Scholar] [CrossRef]
- Tampa, M.; Nicolae, I.; Ene, C.D.; Sarbu, I.; Matei, C.; Georgescu, S.R. Vitamin C and Thiobarbituric Acid Reactive Substances (TBARS) in Psoriasis Vulgaris Related to Psoriasis Area Severity Index (PASI). Rev. Chim. 2017, 68, 43–47. [Google Scholar] [CrossRef]
- Georgescu, S.; Daniela, C.; Tampa, M.; Matei, C.; Benea, V.; Nicolae, I. Oxidative Stress-Related Markers and Alopecia Areata Through Latex Turbidimetric Immunoassay Method. Mater. Plast. 2016, 53, 522–526. [Google Scholar]
- Căruntu, C.; Negrei, C.; Boda, D.; Constantin, C.; Căruntu, A.; Neagu, M. Biotechnological Advances for Diagnosis of Peripheral Diabetic Neuropathy. Rom. Biotechnol. Lett. 2014, 19, 9846–9858. [Google Scholar]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-Encapsulated Nanoparticles as Innovative Antimicrobial and Wound Healing Agent. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Duvnjak Romić, M.; Špoljarić, D.; Šegvić Klarić, M.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Melatonin Loaded Lipid Enriched Chitosan Microspheres—Hybrid Dressing for Moderate Exuding Wounds. J. Drug Deliv. Sci. Technol. 2019, 52, 431–439. [Google Scholar] [CrossRef]
- Romić, M.D.; Klarić, M.Š.; Lovrić, J.; Pepić, I.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Melatonin-Loaded Chitosan/Pluronic® F127 Microspheres as in Situ Forming Hydrogel: An Innovative Antimicrobial Wound Dressing. Eur. J. Pharm. Biopharm. 2016, 107, 67–79. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of Nanoparticles from Natural Lipids for Topical Delivery of Thymol: Investigation of Its Anti-Inflammatory Properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef]
- Bleasdale, B.; Finnegan, S.; Murray, K.; Kelly, S.; Percival, S.L. The Use of Silicone Adhesives for Scar Reduction. Adv. Wound Care 2015, 4, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Patisiran: First Global Approval. Drugs 2018, 78, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Herter, E.K.; Xu Landén, N. Non-Coding RNAs: New Players in Skin Wound Healing. Adv. Wound Care 2017, 6, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Ashton, S.; Song, Y.H.; Nolan, J.; Cadogan, E.; Murray, J.; Odedra, R.; Foster, J.; Hall, P.A.; Low, S.; Taylor, P.; et al. Aurora Kinase Inhibitor Nanoparticles Target Tumors with Favorable Therapeutic Index in Vivo. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ma, L.; Long, X.; Wang, X. LncRNA Expression Profiles and Validation in Keloid and Normal Skin Tissue. Int. J. Oncol. 2015, 47, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Chan, Y.C.; Sen, C.K. MicroRNAs in Skin and Wound Healing. Physiol. Genom. 2011, 43, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Zhang, J.; Wang, M.; Lanting, L.; Yuan, H.; Rossi, J.J.; Natarajan, R. MicroRNA-192 in Diabetic Kidney Glomeruli and Its Function in TGF-β-Induced Collagen Expression via Inhibition of E-Box Repressors. Proc. Natl. Acad. Sci. USA 2007, 104, 3432–3437. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, E.J.; Dunne, N.; McCarthy, H.O. MicroRNA as Therapeutic Targets for Chronic Wound Healing. Mol. Ther. Nucleic Acids 2017, 8, 46–55. [Google Scholar] [CrossRef]
- Mao, Z.; Zhou, X.; Gao, C. Influence of Structure and Properties of Colloidal Biomaterials on Cellular Uptake and Cell Functions. Biomater. Sci. 2013, 2013, 896–911. [Google Scholar] [CrossRef]
- Ghatak, S.; Li, J.; Chan, Y.C.; Gnyawali, S.C.; Steen, E.; Yung, B.C.; Khanna, S.; Roy, S.; Lee, R.J.; Sen, C.K. AntihypoxamiR Functionalized Gramicidin Lipid Nanoparticles Rescue against Ischemic Memory Improving Cutaneous Wound Healing. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1827–1831. [Google Scholar] [CrossRef]
- Li, J.; Ghatak, S.; El Masry, M.S.; Das, A.; Liu, Y.; Roy, S.; Lee, R.J.; Sen, C.K. Topical Lyophilized Targeted Lipid Nanoparticles in the Restoration of Skin Barrier Function Following Burn Wound. Mol. Ther. 2018, 26, 2178–2188. [Google Scholar] [CrossRef]
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation Using an Adhesive Hydrogel Loaded with MiRNA-Laden Nanoparticles Promotes Wound Healing. Small 2019, 15, 1–15. [Google Scholar] [CrossRef]
- Tezgel, Ö.; DiStasio, N.; Laghezza-Masci, V.; Taddei, A.R.; Szarpak-Jankowska, A.; Auzély-Velty, R.; Navarro, F.P.; Texier, I. Collagen Scaffold-Mediated Delivery of NLC/SiRNA as Wound Healing Materials. J. Drug Deliv. Sci. Technol. 2020, 55, 101421. [Google Scholar] [CrossRef]
- Randeria, P.S.; Seeger, M.A.; Wang, X.Q.; Wilson, H.; Shipp, D.; Mirkin, C.A.; Paller, A.S. SiRNA-Based Spherical Nucleic Acids Reverse Impaired Wound Healing in Diabetic Mice by Ganglioside GM3 Synthase Knockdown. Proc. Natl. Acad. Sci. USA 2015, 112, 5573–5578. [Google Scholar] [CrossRef] [PubMed]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid-Polymer Hybrid Nanoparticles as a New Generation Therapeutic Delivery Platform: A Review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, H.; Yu, M.; Liao, Z.; Wang, X.; Zhang, F.; Ji, W.; Wu, B.; Han, J.; Zhang, H.; et al. Paclitaxel Loaded Folic Acid Targeted Nanoparticles of Mixed Lipid-Shell and Polymer-Core: In Vitro and in Vivo Evaluation. Eur. J. Pharm. Biopharm. 2012, 81, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Rhee, J.W.; Drum, C.L.; Bronson, R.T.; Golomb, G.; Langer, R.; Farokhzad, O.C. In Vivo Prevention of Arterial Restenosis with Paclitaxel-Encapsulated Targeted Lipid-Polymeric Nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 19347–19352. [Google Scholar] [CrossRef]
- Desai, P.R.; Marepally, S.; Patel, A.R.; Voshavar, C.; Chaudhuri, A.; Singh, M. Topical Delivery of Anti-TNFα SiRNA and Capsaicin via Novel Lipid-Polymer Hybrid Nanoparticles Efficiently Inhibits Skin Inflammation in Vivo. J. Control. Release 2013, 170, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Sharma, G.; Singh, B.; Chhibber, S.; Katare, O.P. Nano-Engineered Lipid-Polymer Hybrid Nanoparticles of Fusidic Acid: An Investigative Study on Dermatokinetics Profile and MRSA-Infected Burn Wound Model. Drug Deliv. Transl. Res. 2019, 9, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Sharma, G.; Singh, B.; Chhibber, S.; Patil, A.B.; Katare, O.P. Chitosan-Tailored Lipidic Nanoconstructs of Fusidic Acid as Promising Vehicle for Wound Infections: An Explorative Study. Int. J. Biol. Macromol. 2018, 115, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.; Kushwaha, K.; Yadav, R.B.; Agrawal, U. Hybrid Nanoparticles for the Topical Delivery of Norfloxacin for the Effective Treatment of Bacterial Infection Produced after Burn. J. Microencapsul. 2017, 34, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Utreja, P. Vesicular Nanocarrier Based Treatment of Skin Fungal Infections: Potential and Emerging Trends in Nanoscale Pharmacotherapy. Asian J. Pharm. Sci. 2019, 14, 117–129. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef] [PubMed]
| Drug Loaded in Vesicular Systems | Properties PS = Particle Size (nm) EE = Encapsulation Efficiency (%) ZP = Zeta Potential (mV) | Experimental Design | Administration | Effects | Refs. | |
|---|---|---|---|---|---|---|
| LIPOSOMES | ||||||
| Madecassoside | PS = 151 ZP = −54 EE = 70.14 | In vivo | Franz diffusion cell using full-thickness dorsal skin excised from Sprague Dawley rats | 0.4 mL of madecassoside liposomes was applied on the skin tissue | Double emulsion liposomes loaded with madecassoside possessed better EE, smaller size, remarkable stability and a better capacity to deliver the active agent at the target site | [49] |
| In vivo | Sprague Dawley rats with second-degree burns | Topically, applied once daily for 12 days | Better therapeutic wound healing effect Significantly improved wound closure | |||
| Rosmarinus officinalis essential oil and Salvia triloba essential oil | PS = 203.9 ± 14.13 ZP = −30 ± 7.6 EE = 65 PS = 229.6 ± 15.45 ZP = −30 ± 3.9 EE = 57 | In vivo | Selectively permeable bag membrane (cut-off 3–5 KD) | 200μL essential oils dissolved in 1 mL of methanol | Linear kinetic of essential oils release, reaching ~40% in 1 h and ~100% in 3 h | [81] |
| 2,2-diphenyl-1-picrylhydrazil (DPPH) test | Essential oils methanol solutions of 5 μL/mL liposome suspensions | Increased antioxidant activity Preservation of the antioxidant properties High anti-lipid peroxidation activity | ||||
| Soybean lipoxygenase inhibition assay | 5 mg/mL and 1 mg/mL of essential oils and liposomes | Moderate anti-inflammatory activity | ||||
| Disk inhibition zone (DIZ) on appropriate agar plates | Paper disks with 5 μL of pure essential oil or essential oil liposomes | Increased diameter of inhibition compared with unformulated EOs Strong inhibitory effect against Klebsiella pneumoniae | ||||
| Epidermal growth factor (EGF) | PS = 4.44 ± 0.03 ZP = 4.44 ± 0.03 EE = 58.1 | In vivo | Franz type of diffusion cells | 1 mL of EGF liposome formulation with a concentration of 2 Lg/mL | Increased stability of EGF in liposome formulation | [50] |
| In vivo | Female Sprague Dawley rats with second-degree burned areas | Topically once daily for 14 days | Fast and increased healing effect Increased synthesis of collagens and epidermal thickness | |||
| TRANSFERSOMES | ||||||
| Highly skin-permeable growth factors combined with hyaluronic acid (LMWP-fused GF) | PS = 107 ± 0.757 ZP = 56.5 ± 1.13 EE = 81.9 ± 4.63 | In vivo | Excised hairless mouse skin | 500 μL of LMWP-fused GF and hyaluronic acid combination compared with native growth factors and hyaluronic acid | Enhanced skin permeation | [64] |
| CCD-986sk cells | 100 ng/mL LMWP-fused GF | Increased cell proliferation | ||||
| In vivo | Diabetic female C57/BL6 mice Biopsy punch | Topically once daily for 11 days | Increased fibroblast proliferation Enhanced wound healing activity in diabetic wounds | |||
| ETHOSOMES | ||||||
| Silver sulfadiazine | PS = 206.7 ± 1.18 ZP = −67.3 ± 0.45 EE = 92.03 ± 0.79 | In vivo | Dialysis bag diffusion technique | 0.5 mL of formulation containing silver sulfadiazine | Reduced drug release correlated with the increased amount of lecithin | [82] |
| Bacterial growth inhibition | Gram-positive bacteria: Staphylococcus aureus, Staphylococcus epidermidis, Bacillus cereus, Bacillus subtilis and Enterococcus faecalis Gram-negative bacteria: Pseudomonas aeruginosa, Klebsiella pneumonia | Significantly decreased the colony count of bacteria compared with commercial cream | ||||
| In vivo | Male adult Wistar rats Second-degree burn wounds | Topical gel once daily for 21 days | Faster wound healing progress Wounds completely healed with no scar on the 15th day of treatment | |||
| Drug Loaded in Lipid Nanoparticles | Properties PS = Particle Size (nm) ZP = Zeta Potential (mV) EE = Encapsulation Efficiency (%) | Experimental Design | Administration | Effects | Refs. | |
|---|---|---|---|---|---|---|
| Solid Lipid Nanoparticles (SLNs) | ||||||
| Tetrahydro curcumin (THC) | PS = 96.6 EE% = 65.95 ± 0.14% | In vivo | Dialysis membrane with pore size 2.4 nm, molecular weight cut off 12–14 Kda | THC-SLNs dispersion (0.25 mL), THC-SLNs gel (500 mg), free THC in gel (500 mg), all containing 940 μg of THC | Higher release of THC incorporated in SLNs | [83] |
| Ex vivo | Pig ear skin and jacketed Franz glass diffusion cell | THC-SLNs dispersion (0.25 mL), THC-SLNs hydrogel (500 mg), THC gel (500 mg), all containing 940 μg of THC | Higher skin permeability Enhanced penetration as a result of the increased solubility and small particle size of THC-SLNs | |||
| In vivo | Male lacca mice Excisional wound | Topical hydrogel treatment, started from the second day until the 14th day | Increased anti-inflammatory activity Angiogenic potential Enhanced collagenous deposition at the wound site | |||
| All trans-retinoic acid (ATRA) | PS = 83.0 ± 6 ZP = −19 ± 1 EE = 98 ± 12 | In vivo | Franz diffusion cells | 500 μL solution of ATRA (200 μg/mL) or chitosan films | SLN-ATRA and chitosan films revealed a controlled drug release, essential to reduce local adverse effects of ATRA | [55] |
| In vivo | Diabetic C57BL/6 male mice Excisional wound | Topically for 14 days | Accelerated wound healing Improved collagen deposition (especially type III collagen) Reduced scar and leukocyte infiltration Decreased infiltration of neutrophils and macrophages in the wound No local adverse reactions | |||
| Chamomile oil | PS = 542.1 ± 27.5 ZP = −35.9 ± 0.602 | In vivo | Adult male Wistar rats Excisional wound | Topically | Accelerated wound-healing process Increased amount of collagen deposition Significantly increased level of TGF–Beta1 Stimulated the proliferation of fibroblasts and keratinocytes | [84] |
| LL37 and Serpin A1 | PS of LL37-SLNs = 232.2 ± 7.8 PS of A1-SLNs = 210 ± 5.6 EE > 80 | In vivo | BJ fibroblasts Primary human epidermal keratinocytes | 3 mg/mL LL37-A1-SLNs 5 mg/mL blank SLNs 5 μg/m LLL37 only 20 μg/mL A1 only | Faster cell migration and increased wound closure for LL37 and Serpin A1 incorporated in SLNs Decreased collagen type I production | [85] |
| Evaluation of the antibacterial activity | 2 mg/mL LL37 and Serpin A1 encapsulated in SLNs 5 μg/mL LL37 20 μg/mL Serpin A1 | The combination LL37 and Serpin A1 had a synergistic antibacterial activity, especially against S. aureus and E. coli | ||||
| Ex vivo | Female New Zealand white rabbits: inner pinna delipidized skin Franz diffusion cell | 10 mg/mL LL37-A1-SLNs | Increased permeability across the impaired skin Higher deposition of the drugs in the target area Protected the peptides from degradation | |||
| Nanostructured lipid carriers (NLCs) | ||||||
| 20(S)-protopanaxadiol | PS = 111.4 ± 5.9 ZP = −33.2 ± 5.5 EE = 97.9 ± 1.51 | In vivo | Raw 264.7 cells | 10 μM | Anti-inflammatory potential | [56] |
| HUVEC cells | 2.5 μM | Increased angiogenesis activity | ||||
| In vivo | Male mice (Lepr db/JNju, db/db) with hyperglycemia Excisional wound | Topically Every 2 days in 15-μL or 15-mg doses | Decreased inflammatory infiltration in the inflammatory stage of wound healing Increased angiogenesis during the proliferation phase Regulation collagen deposition in the remodeling phase Wounds closing without scars | |||
| Phenytoin | PS = 178.2 ± 4.53 | In vivo | Modified Franz diffusion cell with slight modification using dyalisis membrane | Hydrogel containing free drug and Phenytoin-NLC hydrogel | Phenytoin-NLC hydrogel has shown a lower release rate than free phenytoin | [58] |
| In vivo | Prospective double-blinded, randomized, controlled study 27 patients with diabetes and neuropathic foot ulceration | Topically Hydrogel Twice daily for 8 weeks | Phenytoin-NLC hydrogel was more effective in wound closure when compared to free phenytoin Reduced healing time No adverse reactions | |||
| LL37 | PS = 273.6 ± 27.64 ZP = −31.63 ± 1.94 EE = 96.40 ± 0.41 | In vivo | Human foreskin Fibroblasts: RAW 264.7 cells | 5000 ng/mL | Encapsulation of LL37 in NCLs did not affect its bioactivity | [86] |
| Evaluation of the antimicrobial activity | 20 μg/mL | Increased antimicrobial activity against E. coli | ||||
| In vivo | Balb/c mice male db/db mice Punch biopsy wound | Topically, spread over the wound bed on days 1 and 4 after wound induction | Significantly higher grade of reepithelization Accelerated anti-inflammatory effect Increased collagen deposition | |||
| Eucalyptus oil | PS = 220 to 300 ZP = −22.07 ± 0.29 EE = ~100 | In vivo | Normal human dermal fibroblasts from juvenile foreskin | 200 μL nanoparticles suspensions | Increased cell proliferation | [52] |
| Evaluation of the antimicrobial activity | Nanoparticles and eucalyptus oil dispersed in lecithin solution | Increased antibacterial effect on Streptococcus pyogenes compared with free drug and similar antimicrobial effect on Staphylococcus Aureus compared with free drug | ||||
| In vivo | Wistar Male rats Burn injuries followed by biopsy punch at 24 h | Topically daily for 18 days | Decreased lesion area at 4 days of treatment Promoted wound healing | |||
| Peppermint essential oil | PS = 40–250 ZP = −10 to −15 EE = 93.2 ± 1.2 | In vivo | Evaluation of the antimicrobial activity | 100 μL peppermint oil/peppermint oil loaded in NLCs | Same effect on most bacterial strains between the encapsulated drug and the free drug | [87] |
| In vivo | Mice Punch biopsy | Topically Gel formulation Applied once daily | Significantly diminished the infected wound Increased wound healing rate Increased fibroblast proliferation and collagen synthesis | |||
| Epidermal Growth Factor (EGF) and Curcumin | PS = 331.8 nm ZP = −6.64 ± 0.51 EE = 81.1- 99.4 | In vivo | NIH 3T3 fibroblasts and HaCaT keratinocytes | EGF–Cur-NLC (10 ng/mL EGF and 100 ng/mL curcumin) | Enhanced proliferation of fibroblasts and keratinocytes | [57] |
| In vivo | Male Sprague Dawley diabetic rats Biopsy punch | Topically | Increased cell proliferation Accelerated wound closure Decreased risk of bacterial infection | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, A.-M.; Caruntu, C.; Tampa, M.; Georgescu, S.R.; Matei, C.; Constantin, M.M.; Constantin, T.V.; Calina, D.; Ciubotaru, D.A.; Badarau, I.A.; et al. Applications of Nanosized-Lipid-Based Drug Delivery Systems in Wound Care. Appl. Sci. 2021, 11, 4915. https://doi.org/10.3390/app11114915
Matei A-M, Caruntu C, Tampa M, Georgescu SR, Matei C, Constantin MM, Constantin TV, Calina D, Ciubotaru DA, Badarau IA, et al. Applications of Nanosized-Lipid-Based Drug Delivery Systems in Wound Care. Applied Sciences. 2021; 11(11):4915. https://doi.org/10.3390/app11114915
Chicago/Turabian StyleMatei, Andreea-Mariana, Constantin Caruntu, Mircea Tampa, Simona Roxana Georgescu, Clara Matei, Maria Magdalena Constantin, Traian Vasile Constantin, Daniela Calina, Diana Alina Ciubotaru, Ioana Anca Badarau, and et al. 2021. "Applications of Nanosized-Lipid-Based Drug Delivery Systems in Wound Care" Applied Sciences 11, no. 11: 4915. https://doi.org/10.3390/app11114915
APA StyleMatei, A.-M., Caruntu, C., Tampa, M., Georgescu, S. R., Matei, C., Constantin, M. M., Constantin, T. V., Calina, D., Ciubotaru, D. A., Badarau, I. A., Scheau, C., & Caruntu, A. (2021). Applications of Nanosized-Lipid-Based Drug Delivery Systems in Wound Care. Applied Sciences, 11(11), 4915. https://doi.org/10.3390/app11114915













