Hydrodynamical and Hydrochemical Assessment of Pumped-Storage Hydropower (PSH) Using an Open Pit: The Case of Obourg Chalk Quarry in Belgium
Abstract
:1. Introduction
2. Materials and Methods
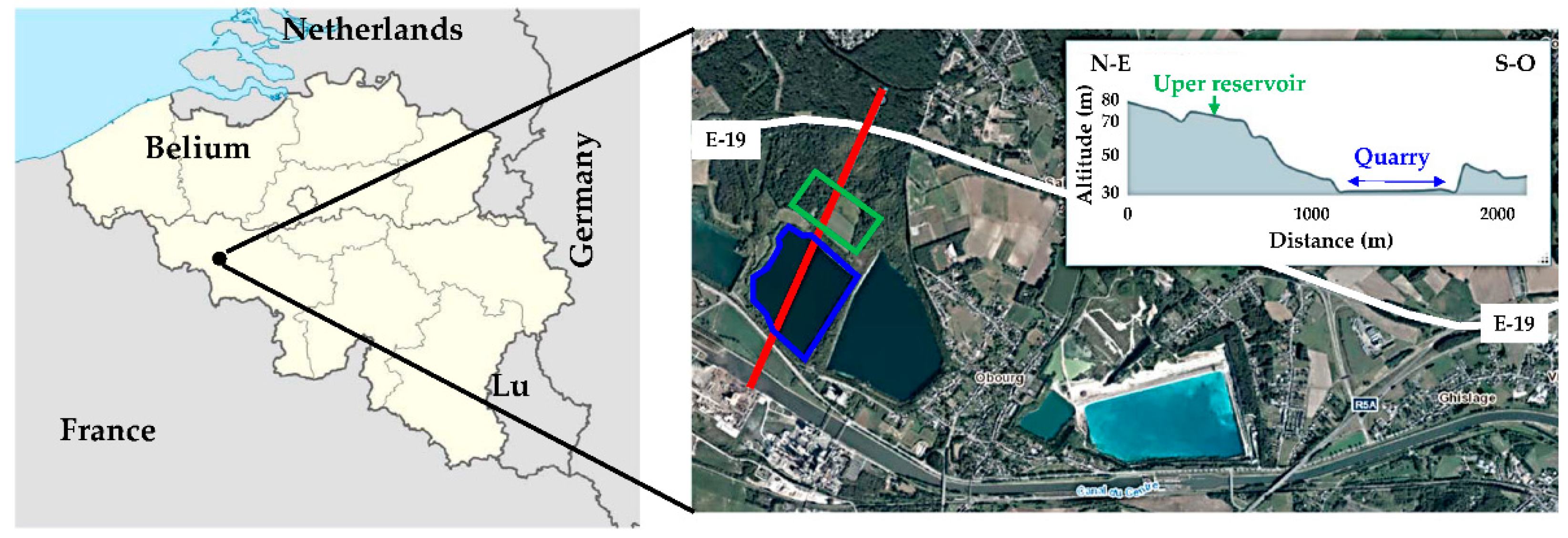
2.1. Study Site
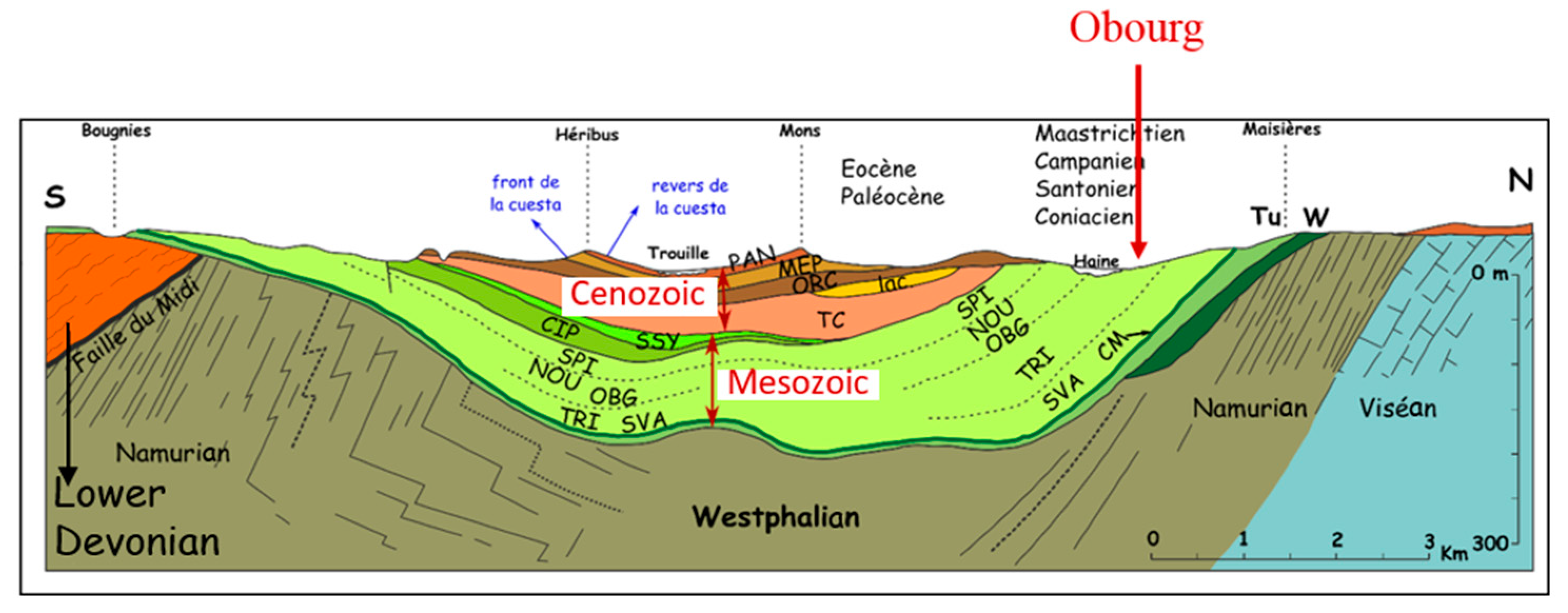
2.2. Geological and Hydrogeological Context
2.3. Hydrochemical Context
3. Model Development
3.1. Groundwater Flow Model
3.1.1. Model and Spatial Discretization
3.1.2. Boundary Conditions
3.1.3. Parametrization
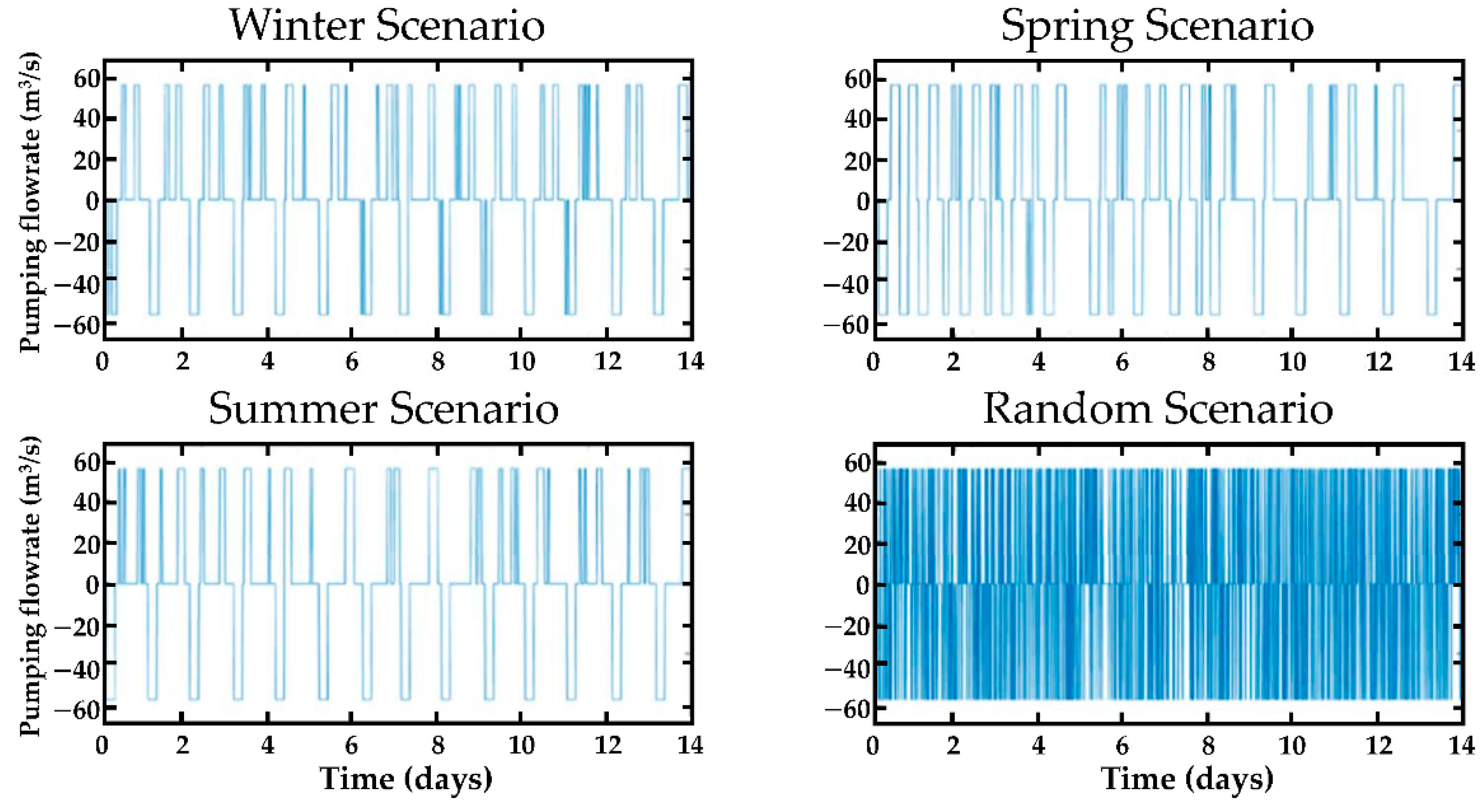
3.1.4. Simulated Pump–Storage Operations Scenarios
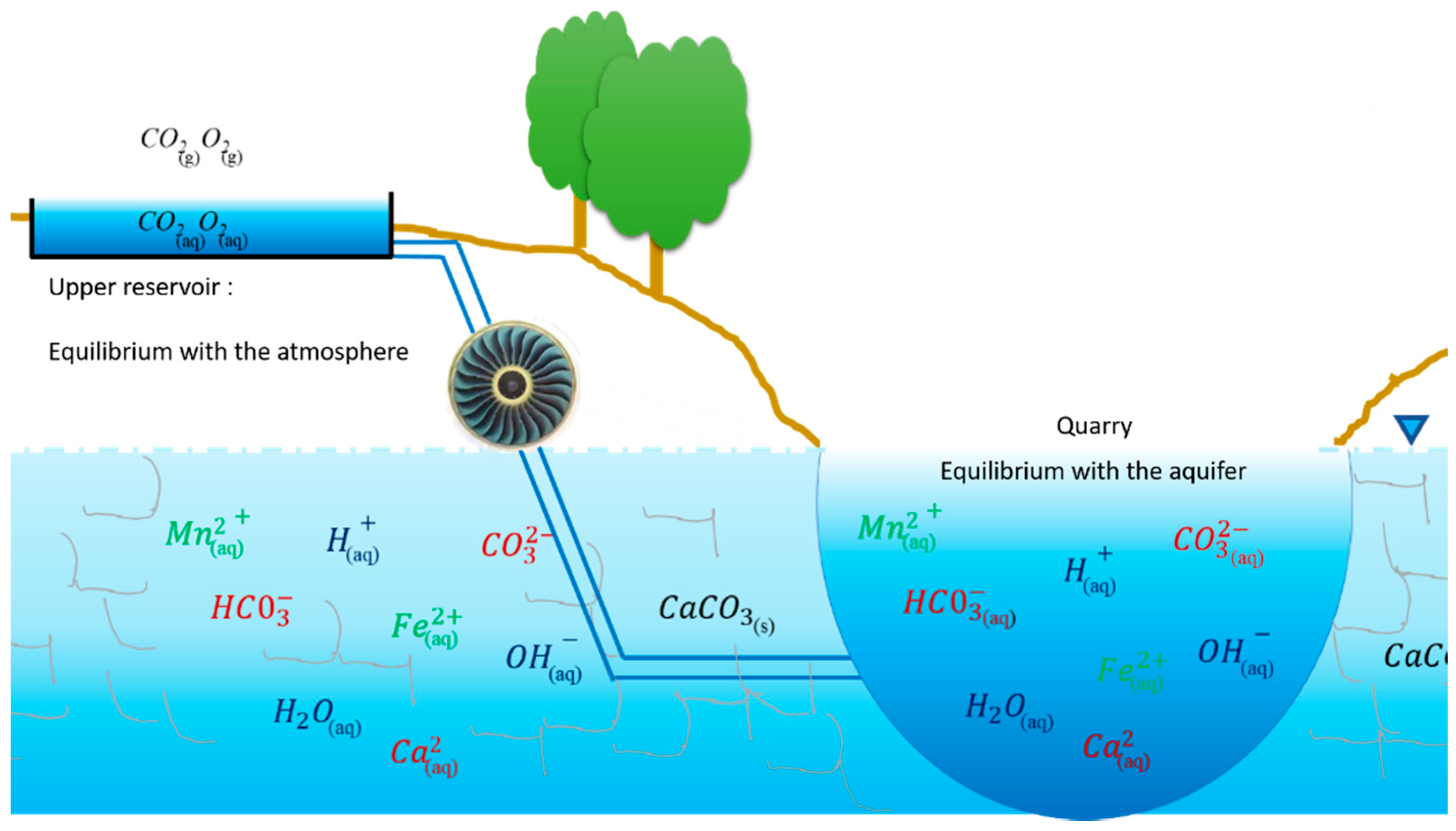
3.2. Groundwater Hydrochemical Model
3.2.1. Conceptual Model
3.2.2. Numerical Model and Boundary Conditions
3.2.3. Parameterization
4. Results
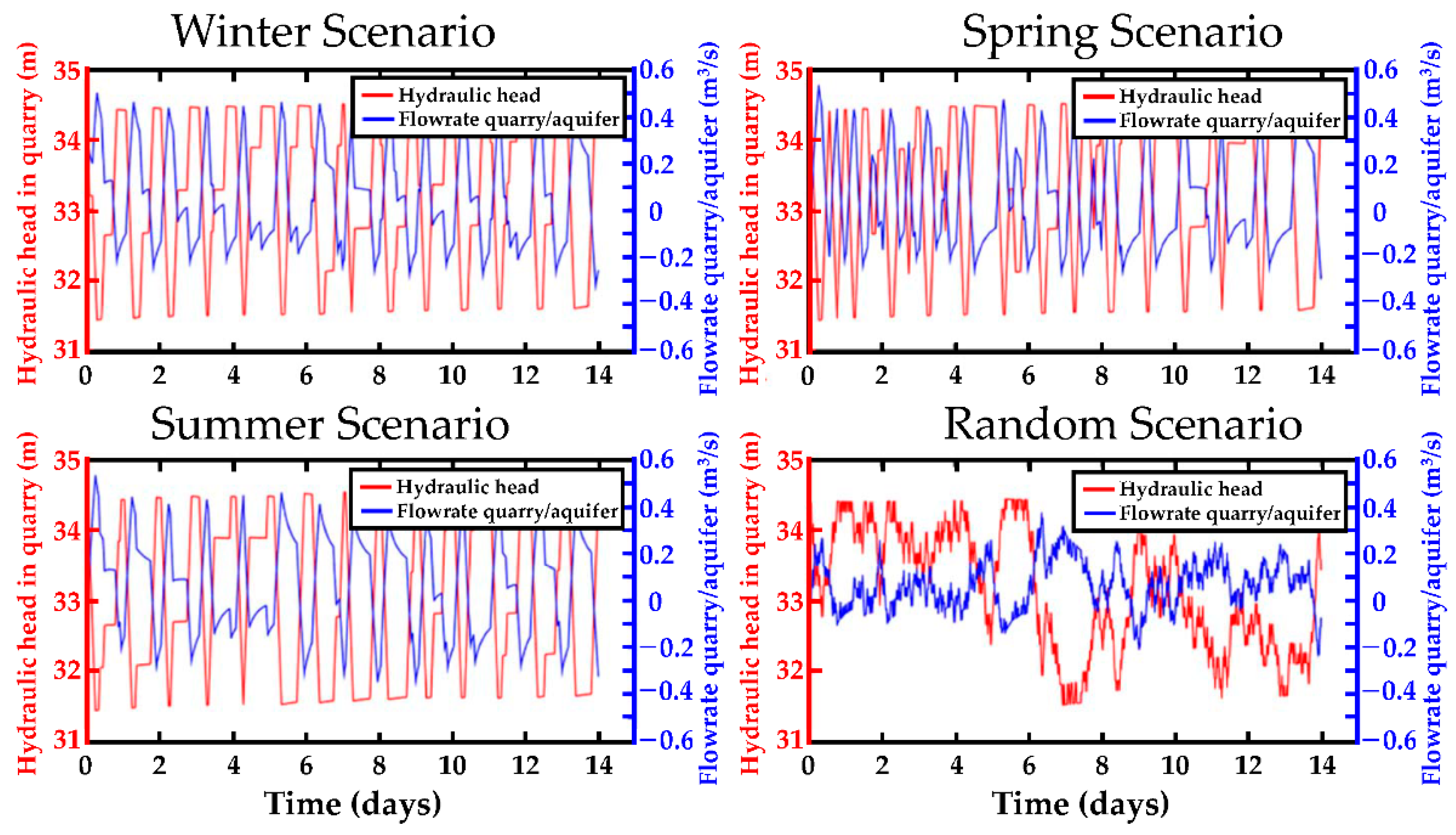
4.1. Hydraulic Head Fluctuations in the Quarry and Water Exchanges with the Aquifer
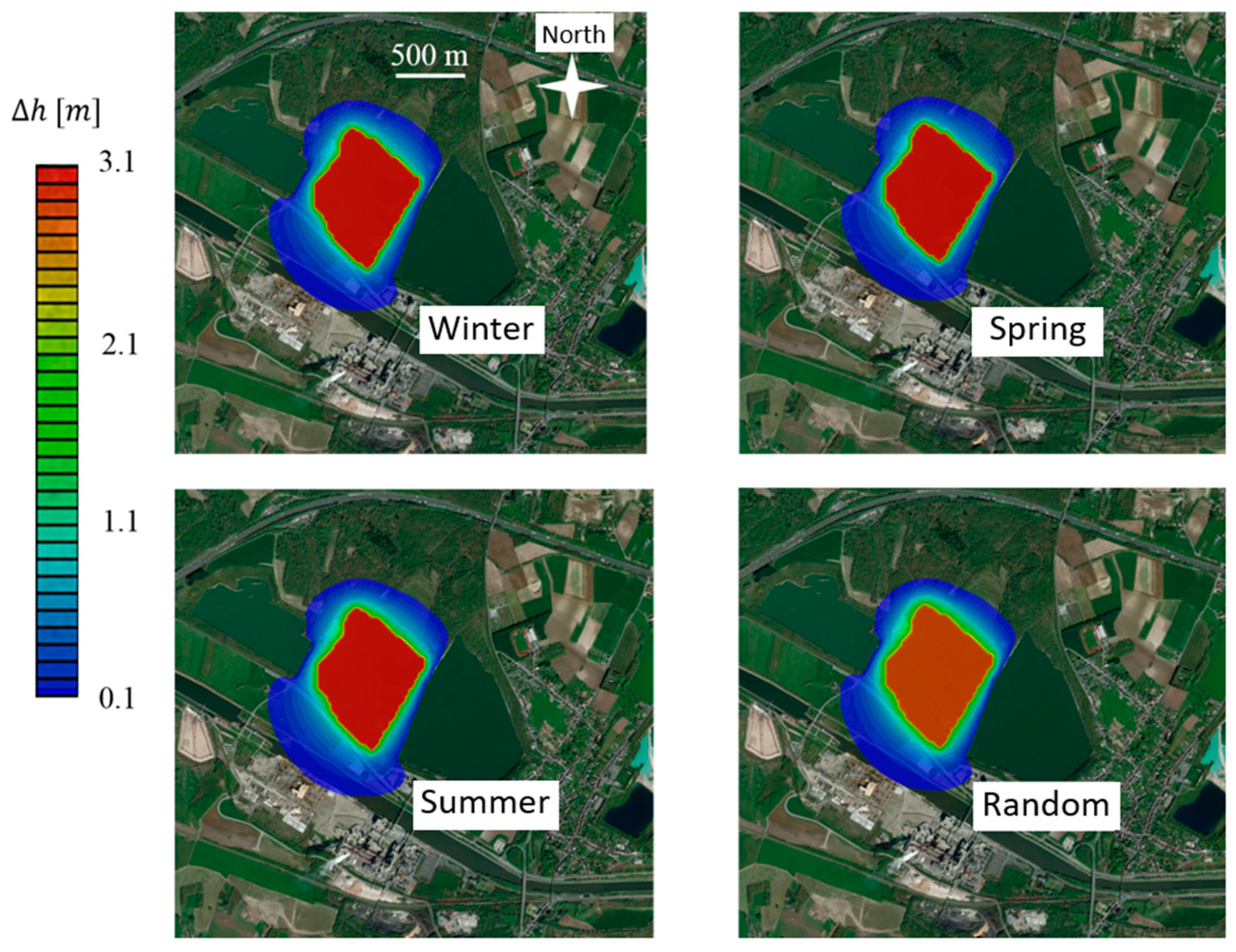
4.2. Piezometric Head Fluctuations in the Aquifer
4.3. Evolution of Water Chemistry in the Upper Reservoir
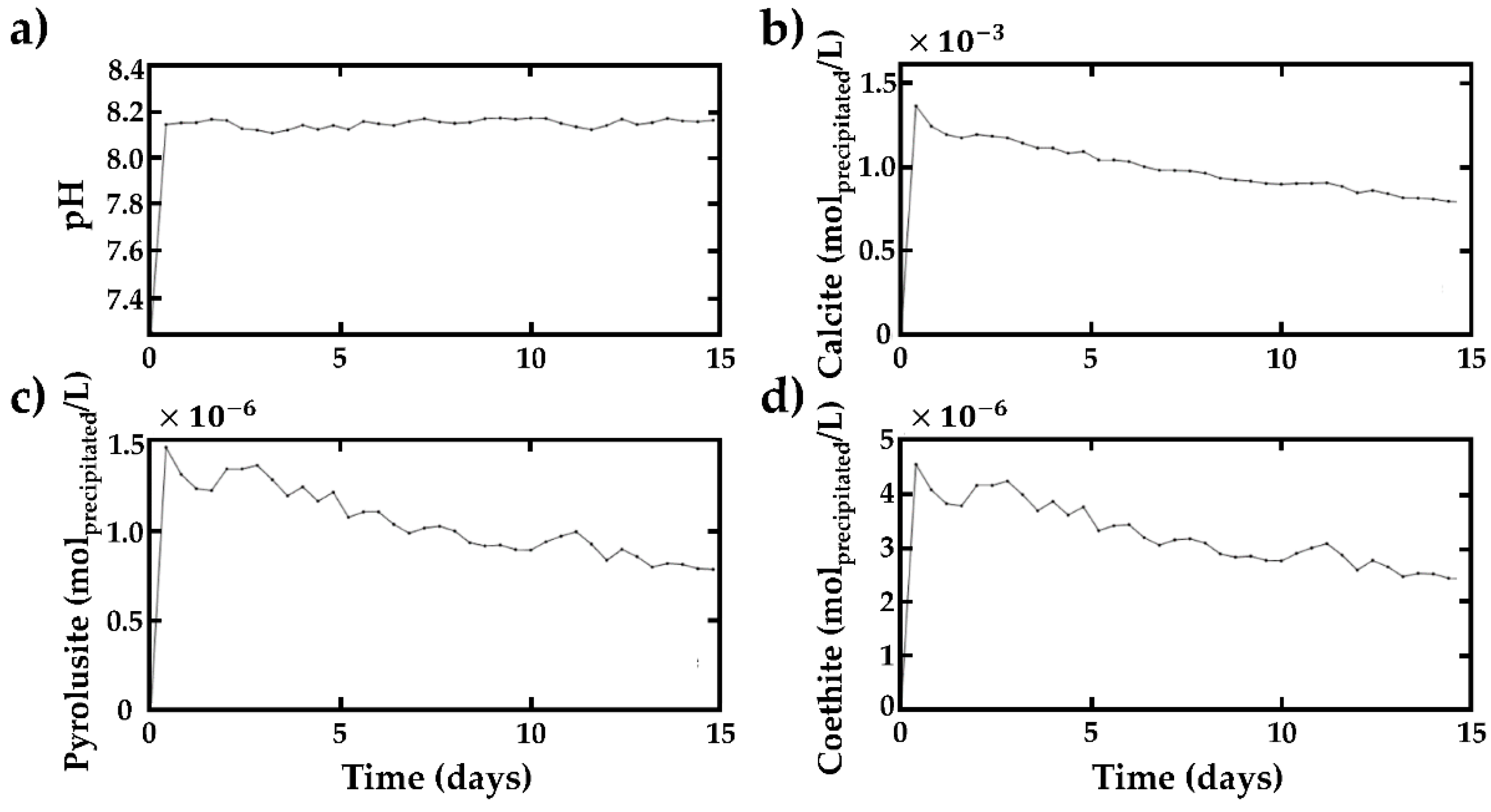
4.4. Hydrochemical Evolution of the Water in the Quarry
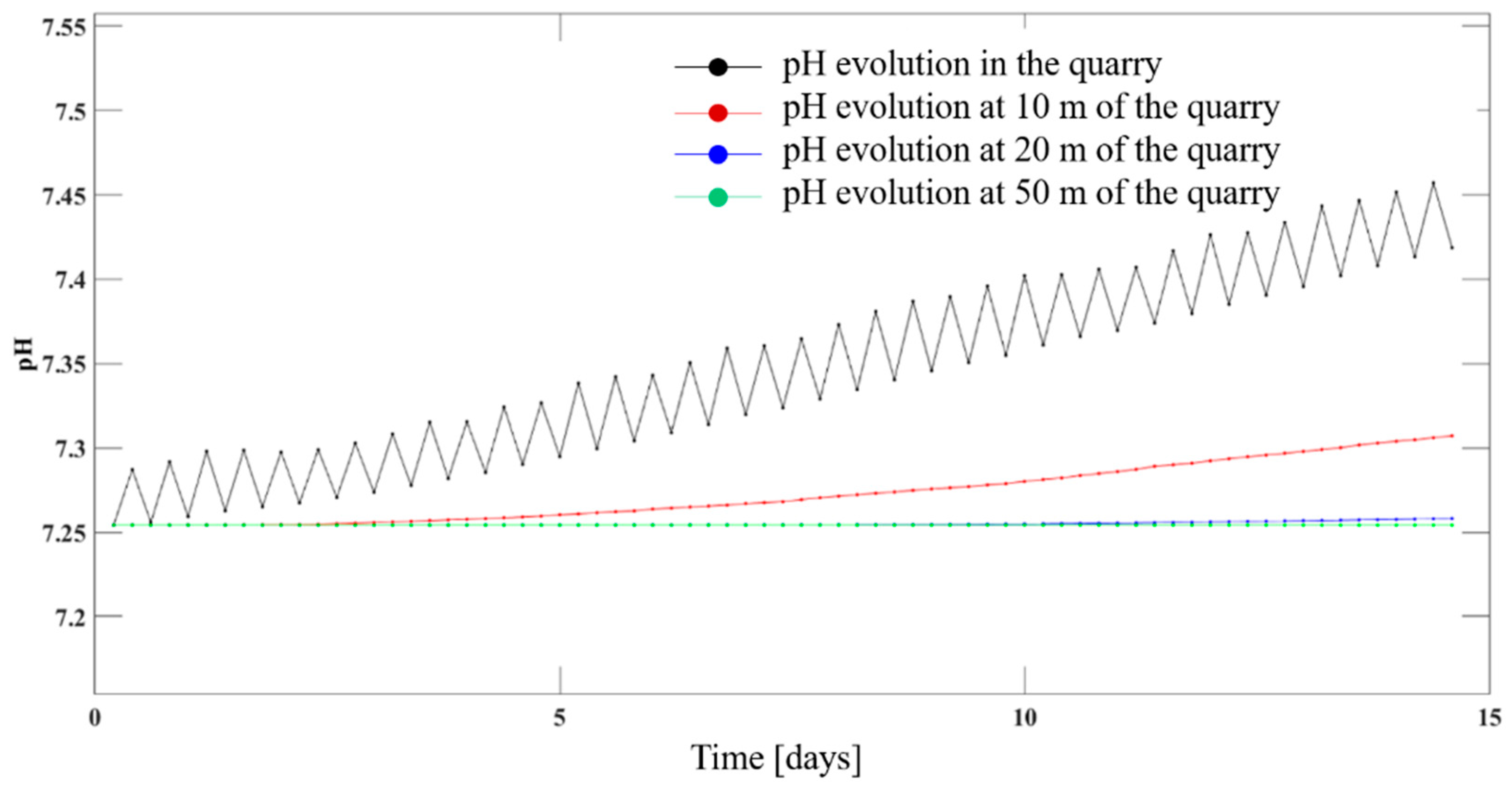
4.5. Hydrochemical Evolution of the Groundwater in the Chalk Aquifer
5. Conclusions
- Quarries cannot be considered as simple impervious reservoirs. They are in strong interaction with the surrounding aquifers. During PSH operations, water exchanges between the quarry and adjacent aquifers occur. The magnitude of the water exchanges depends on the hydrogeological context. This interaction has an impact on the fluctuation of the hydraulic head in the reservoir, on the difference in level between the upper and lower reservoirs, and on the efficiency of the system [8].
- The pump–discharge cycles in the reservoirs generate rapid and periodic fluctuations in the water level, which are propagated into the surrounding rock media. This can have an impact on other possible activities (e.g., groundwater abstraction station) in the vicinity. These load/discharge cycles in the rock media can also create potentially significant stability problems. These stability problems may, for example, occur at quarry walls, or in areas of altered rock in the form of localized collapses. These kinds of collapses are, for example, regularly observed around some limestone quarries where the groundwater table is lowered [35].
- Finally, it is also necessary to evaluate the hydrogeochemical behavior of the system during the PSH activities. From a hydrochemical perspective, carrying out pumping–discharge induces the aeration of the water. This aeration phenomenon can destabilize chemical balances between groundwater and minerals present in the rock. These cycles can therefore influence the chemical composition of the quarry water as well as that of the adjacent aquifer. It is important to study these possible hydrochemical modifications, both from the point of view of the exploited drinking water reservoir and of the water–rock interactions themselves.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menendez, J.; Ordónez, A.; Fernández-Oro, J.M.; Loredo, J.; Díaz-Aguado, M.B. Feasibility analysis of using mine water from abandoned coal mines in Spain for heating and cooling of buildings. Renew. Energy 2020, 146, 1166–1176. [Google Scholar] [CrossRef]
- Rehman, S.; Al-Hadhrami, L.M.; Alam, M. Pumped hydro energy storage system: A technological review. Renew. Sustain. Energy Rev. 2015, 44, 586–598. [Google Scholar] [CrossRef]
- Uddin, P.N.; Asce, M. Preliminary design of an underground reservoir for pumped storage. Geotech. Geol. Eng. 2003, 21, 331–355. [Google Scholar] [CrossRef]
- Erpicum, S.; Archambeau, P.; Dewals, B.; Pirotton, M.; Pujades, E.; Orban, P.; Dassargues, A.; Cerfontaine, B.; Charlier, R.; Poulain, A.; et al. Underground Pumped Hydro-Energy Storage in Wallonia (Belgium) Using Old Mines—Potential and Challenges. In Proceedings of the HYDRO2017 Conference, Sevilla, Spain, 9 October 2017. [Google Scholar]
- Madlener, R.; Specht, J. An Exploratory Economic Analysis of Underground Pumped-Storage Hydro Power Plants in Abandoned Coal Mines; Social Science Research Network: Rochester, NY, USA, 2013. [Google Scholar]
- Toubeau, J.-F.; Bottieau, J.; De Greve, Z.; Vallee, F.; Bruninx, K. Data-Driven Scheduling of Energy Storage in Day-Ahead Energy and Reserve Markets with Probabilistic Guarantees on Real-Time Delivery. IEEE Trans. Power Syst. 2020, 1. [Google Scholar] [CrossRef]
- Poulain, A.; de Dreuzy, J.-R.; Goderniaux, P. Pump Hydro Energy Storage systems (PHES) in groundwater flooded quarries. J. Hydrol. 2018, 559, 1002–1012. [Google Scholar] [CrossRef] [Green Version]
- Pujades, E.; Orban, P.; Bodeux, S.; Archambeau, P.; Erpicum, S.; Dassargues, A. Underground pumped storage hydropower plants using open pit mines: How do groundwater exchanges influence the efficiency? Appl. Energy 2017, 190, 135–146. [Google Scholar] [CrossRef]
- Pujades, E.; Jurado, A.; Orban, P.; Ayora, C.; Poulain, A.; Goderniaux, P.; Brouyère, S.; Dassargues, A. Hydrochemical changes induced by underground pumped storage hydropower and their associated impacts. J. Hydrol. 2018, 563, 927–941. [Google Scholar] [CrossRef] [Green Version]
- Xuan, P.T.; Van Pho, N.; Gas’Kova, O.L.; Bortnikova, S.B. Geochemistry of surface waters in the vicinity of open pit mines at the Cay Cham deposit, Thai Nguyen province, northern Vietnam. Geochem. Int. 2013, 51, 931–938. [Google Scholar] [CrossRef]
- Younger, P.L.; Banwart, S.A.; Hedin, R.S. Mine Water: Hydrology, Pollution, Remediation; Environmental Pollution; Springer: Dordrecht, The Netherlands, 2002; ISBN 978-1-4020-0137-6. [Google Scholar]
- España, J.S.; Pamo, E.L.; Pastor, E.S.; Andrés, J.R.; Rubí, J.A.M. The Removal of Dissolved Metals by Hydroxysulphate Precipitates during Oxidation and Neutralization of Acid Mine Waters, Iberian Pyrite Belt. Aquat. Geochem. 2006, 12, 269–298. [Google Scholar] [CrossRef]
- Severson, M.J. Preliminary Evaluation of Establishing an Underground Taconite Mine, to Be Used Later as a Lower Reservoir in a Pumped Hydro Energy Storage Facility, on the Mesabi Iron Range, Minnesota; University of Minnesota Duluth: Duluth, MI, USA, 2011. [Google Scholar]
- Habils, F.; Roland, S.; Rorive, A. Carte Hydrogéologique de Wallonie Au 1/25 000ème (Avec Notice Explicative); Planche Jurbise—Obourg N° 45/3-4; Service Public de Wallonie: Namur, Belgium, 2017. [Google Scholar]
- Hallet, V. Étude de la Contamination de la Nappe Aquifère de Hesbaye par les Nitrates: Hydrogéologie, Hydrochimie et Modélisation Mathématique des Écoulements et du Transport en Milieu Saturé (Contamination of the Hesbaye Aquifer by Nitrates: Hydrogeology, Hydrochemistry and Mathematical Modeling); University of Liège: Liège, Belgium, 1998. [Google Scholar]
- Brouyère, S. Etude et Modélisation du Transport et du Piégeage des Solutés en Milieu Souterrain Variablement Saturé. Evaluation des Paramètres Hydrodispersifs par la Réalisation et L’interprétation D’essais de Traçage In Situ; Université de Liège: Sart Tilman, Belgique, 2001. [Google Scholar]
- Dargne Carte Hydrogéologique de Wallonie [WWW Document]. Carte Hydrogéologique Wallonie. 2017. Available online: http://Environnement.Wallonie.Be/Cartosig/Cartehydrogeo/Application.Htm# (accessed on 29 January 2018).
- Gaviglio, P.; Bekri, S.; Vandycke, S.; Adler, P.; Schroeder, C.; Bergerat, F.; Darquennes, A.; Coulon, M. Faulting and deformation in chalk. J. Struct. Geol. 2009, 31, 194–207. [Google Scholar] [CrossRef]
- Goderniaux, P.; Beyek, A.; Tchotchom, A.; Poulian, A.; Wattier, M.-L.; Vandycke, S. Study of the heterogeneity of hydraulic properties in a chalk aquifer unit, using sequential pumping and tracing experiments with packer systems. In Engineering in Chalk: Proceedings of the Chalk 2018 Conference, London, UK, 17–18 September 2018; ICE Publishing: London, UK, 2018; pp. 675–680. ISBN 978-0-7277-6407-2. [Google Scholar]
- Hoffmann, R.; Goderniaux, P.; Jamin, P.; Chatton, E.; De La Bernardie, J.; Labasque, T.; Le Borgne, T.; Dassargues, A. Continuous dissolved gas tracing of fracture-matrix exchanges. Geophys. Res. Lett. 2020, 47, e2020GL088944. [Google Scholar] [CrossRef]
- Marlière, R. Carte Géologique et Texte Explicatif de La Feuille Jurbise—Obourg; Service Géologique de Belgique: Mons, Belgium, 1964. [Google Scholar]
- Mercado, A. The kinetics of mineral dissolution in carbonate aquifers as a tool for hydrological investigations, II. Hydrogeochemical models. J. Hydrol. 1977, 35, 365–384. [Google Scholar] [CrossRef]
- Edmunds, W.; Cook, J.; Darling, W.; Kinniburgh, D.; Miles, D.; Bath, A.; Morgan-Jones, M.; Andrews, J. Baseline geochemical conditions in the Chalk aquifer, Berkshire, U.K.: A basis for groundwater quality management. Appl. Geochem. 1987, 2, 251–274. [Google Scholar] [CrossRef]
- Edmunds, W.; Shand, P.; Hart, P.; Ward, R. The natural (baseline) quality of groundwater: A UK pilot study. Sci. Total. Environ. 2003, 310, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Gillon, M.; Crançon, P.; Aupiais, J. Modelling the baseline geochemistry of groundwater in a Chalk aquifer considering solid solutions for carbonate phases. Appl. Geochem. 2010, 25, 1564–1574. [Google Scholar] [CrossRef]
- Rorive, A. Determination Des Ressources Souterraines de La Nappe Du Crétacé de La Vallée de La Haine—Convention RW-IDEA, Rapport Final. 1983. [Google Scholar]
- Harbaugh, A.W. MODFLOW-2005: The U.S. Geological Survey modular ground-water model—The ground-water flow process. In Techniques and Methods; US Geological Survey: Reston, VI, USA, 2005; Volume 6. [Google Scholar]
- Parkhurst, D.L.; Kipp, K.L.; Engesgaard, P.; Charlton, S.R. PHAST—A program for simulating ground-water flow, solute transport, and multicomponent geochemical reactions. U.S. Geol. Surv. Tech. Methods 2005, 6, A8. [Google Scholar]
- Kipp, K.L. HST3D: A Computer Code for Simulation of Heat and Solute Transport in Three-Dimensional Ground-Water Flow Systems; Water-Resources Investigations Report; U.S. Geological Survey: Reston, VA, USA, 1987; Volume 86-4095.
- Parkhurst, D.; Appelo, T.; Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. US Geological Survey Techniques and Methods; US Geological Survey: Denver, CO, USA, 2013; Book 6, Chapter A43; 497p.
- Pujades, E.; Willems, T.; Bodeux, S.; Orban, P.; Dassargues, A. Underground pumped storage hydroelectricity using abandoned works (deep mines or open pits) and the impact on groundwater flow. Hydrogeol. J. 2016, 24, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Pujades, E.; Jurado, A.; Orban, P.; Dassargues, A. Parametric assessment of hydrochemical changes associated to underground pumped hydropower storage. Sci. Total. Environ. 2019, 659, 599–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimie et Pollutions des Eaux Souterraines ATTEIA Olivier. Available online: https://www.lavoisier.fr/livre/environnement/chimie-et-pollution-des-eaux-souterraines/atteia/descriptif-9782743020095 (accessed on 9 April 2021).
- Papier, S. Géomicrobiologie des Dépôts Bactériens Ferrugineux: Influence des Facteurs Biologiques et Environnementaux sur L’oxydation du fer en Milieu Continental; Université de Mons: Mons, Belgium, 2014. [Google Scholar]
- Kaufmann, O.; Quinif, Y. Geohazard map of cover-collapse sinkholes in the ‘Tournaisis’ area, southern Belgium. Eng. Geol. 2002, 65, 117–124. [Google Scholar] [CrossRef]


| Ion | Concentration (mg/L) |
|---|---|
| Ca2+ | 142.2 |
| 350.1 | |
| Mg2+ | 11.3 |
| Na+ | 26.2 |
| Cl- | 41.4 |
| SO42 | 111.5 |
| Mn2+ | 0.82 |
| Fe2+ | 2.62 |
| Alluvium | Chalk | Quarry | |
|---|---|---|---|
| Hydraulic conductivity [m/s] | 10−4 | 10−5 | 10−1 |
| Specific yield [%] | 30 | 16 | 100 |
| Min. Hydraulic Head [m] | Max. Hydraulic Head [m] | Max. Hydraulic Head Variations [m] | Max. Exchange Flowrate [m³. s−1] | |
|---|---|---|---|---|
| Winter | 31.44 | 34.59 | 3.14 | 0.53 |
| Spring | 31.43 | 34.55 | 3.12 | 0.50 |
| Summer | 31.43 | 34.61 | 3.18 | 0.53 |
| Random | 31.50 | 34.44 | 2.93 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulain, A.; Pujades, E.; Goderniaux, P. Hydrodynamical and Hydrochemical Assessment of Pumped-Storage Hydropower (PSH) Using an Open Pit: The Case of Obourg Chalk Quarry in Belgium. Appl. Sci. 2021, 11, 4913. https://doi.org/10.3390/app11114913
Poulain A, Pujades E, Goderniaux P. Hydrodynamical and Hydrochemical Assessment of Pumped-Storage Hydropower (PSH) Using an Open Pit: The Case of Obourg Chalk Quarry in Belgium. Applied Sciences. 2021; 11(11):4913. https://doi.org/10.3390/app11114913
Chicago/Turabian StylePoulain, Angélique, Estanislao Pujades, and Pascal Goderniaux. 2021. "Hydrodynamical and Hydrochemical Assessment of Pumped-Storage Hydropower (PSH) Using an Open Pit: The Case of Obourg Chalk Quarry in Belgium" Applied Sciences 11, no. 11: 4913. https://doi.org/10.3390/app11114913
APA StylePoulain, A., Pujades, E., & Goderniaux, P. (2021). Hydrodynamical and Hydrochemical Assessment of Pumped-Storage Hydropower (PSH) Using an Open Pit: The Case of Obourg Chalk Quarry in Belgium. Applied Sciences, 11(11), 4913. https://doi.org/10.3390/app11114913







