Antibacterial Activity of TiO2 Nanoparticles Prepared by One-Step Laser Ablation in Liquid
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of TiO2 NPs
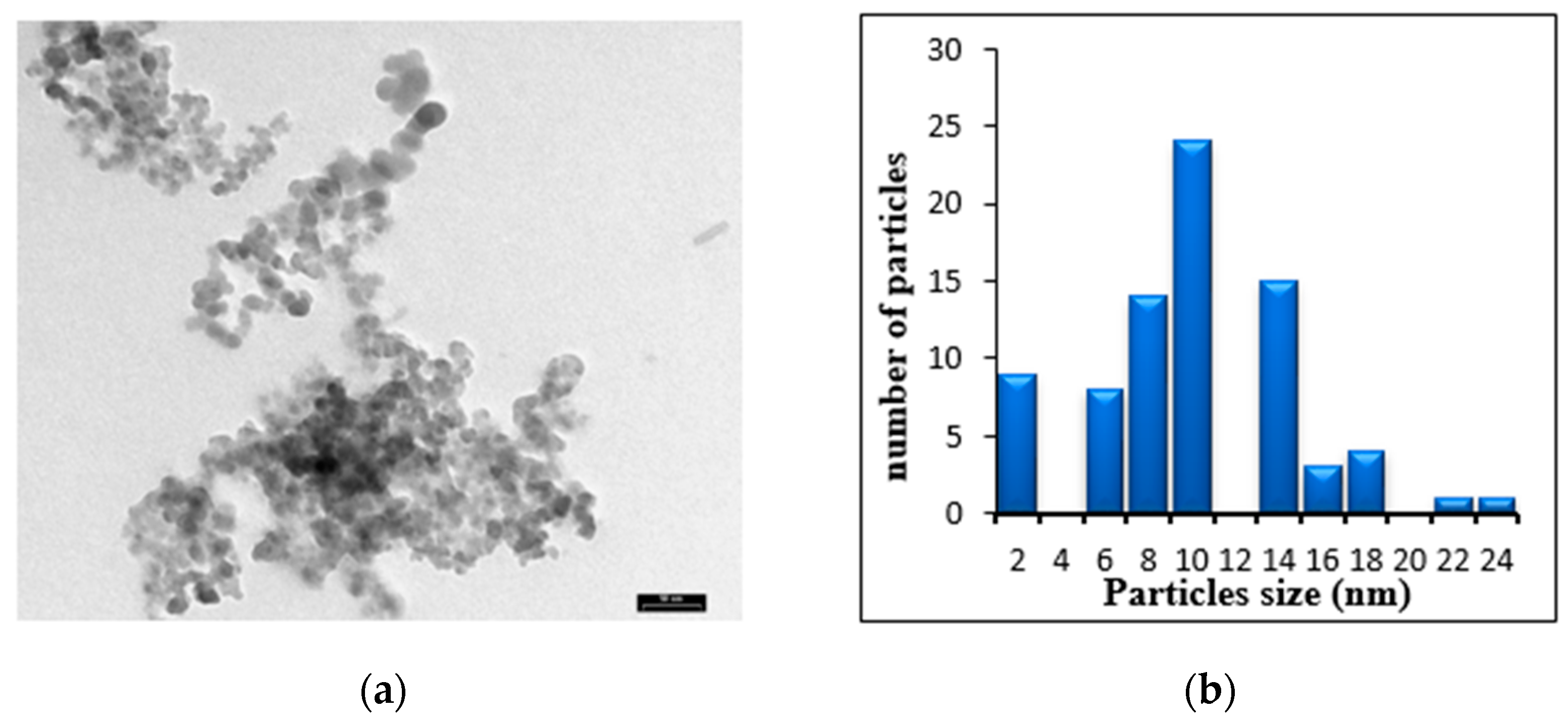
2.2. Characterization of TiO2 NPs
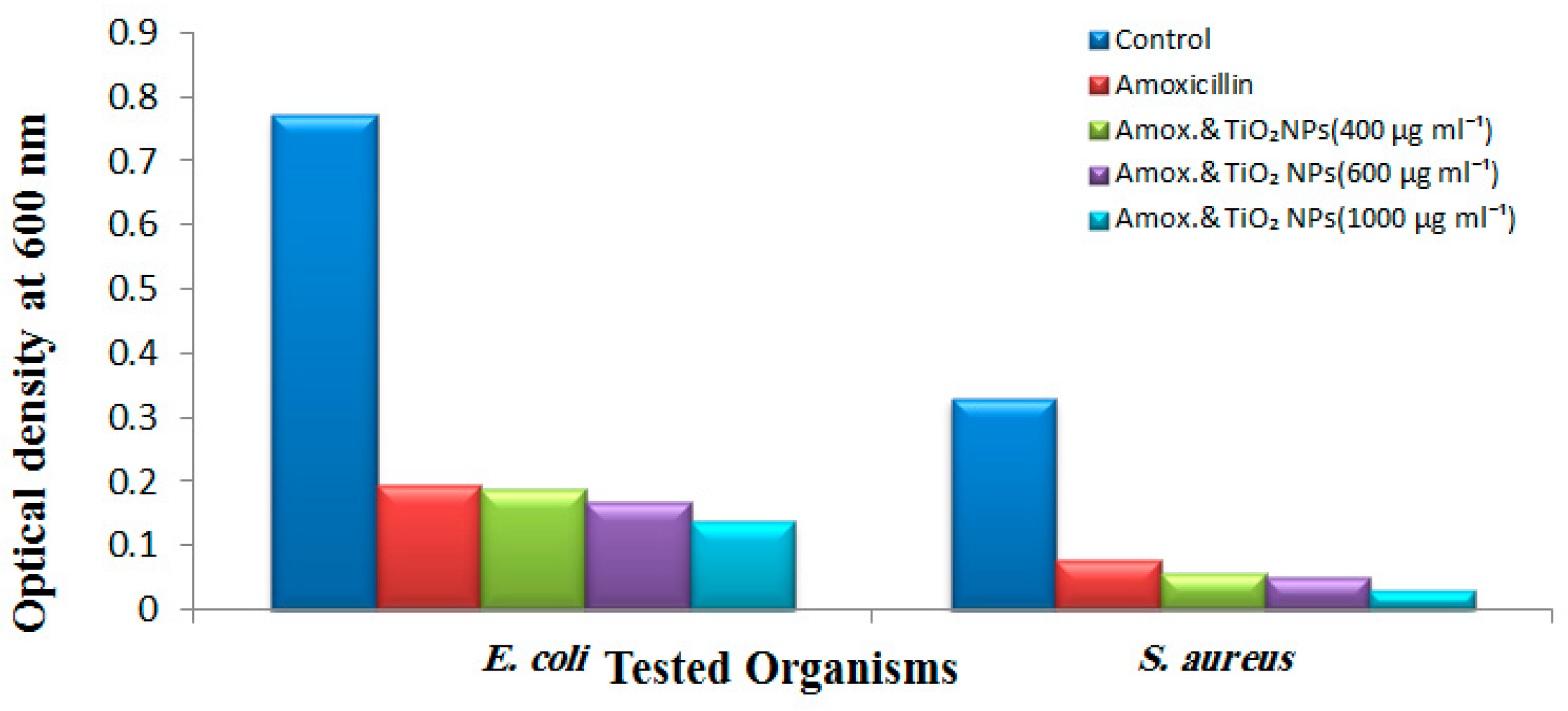
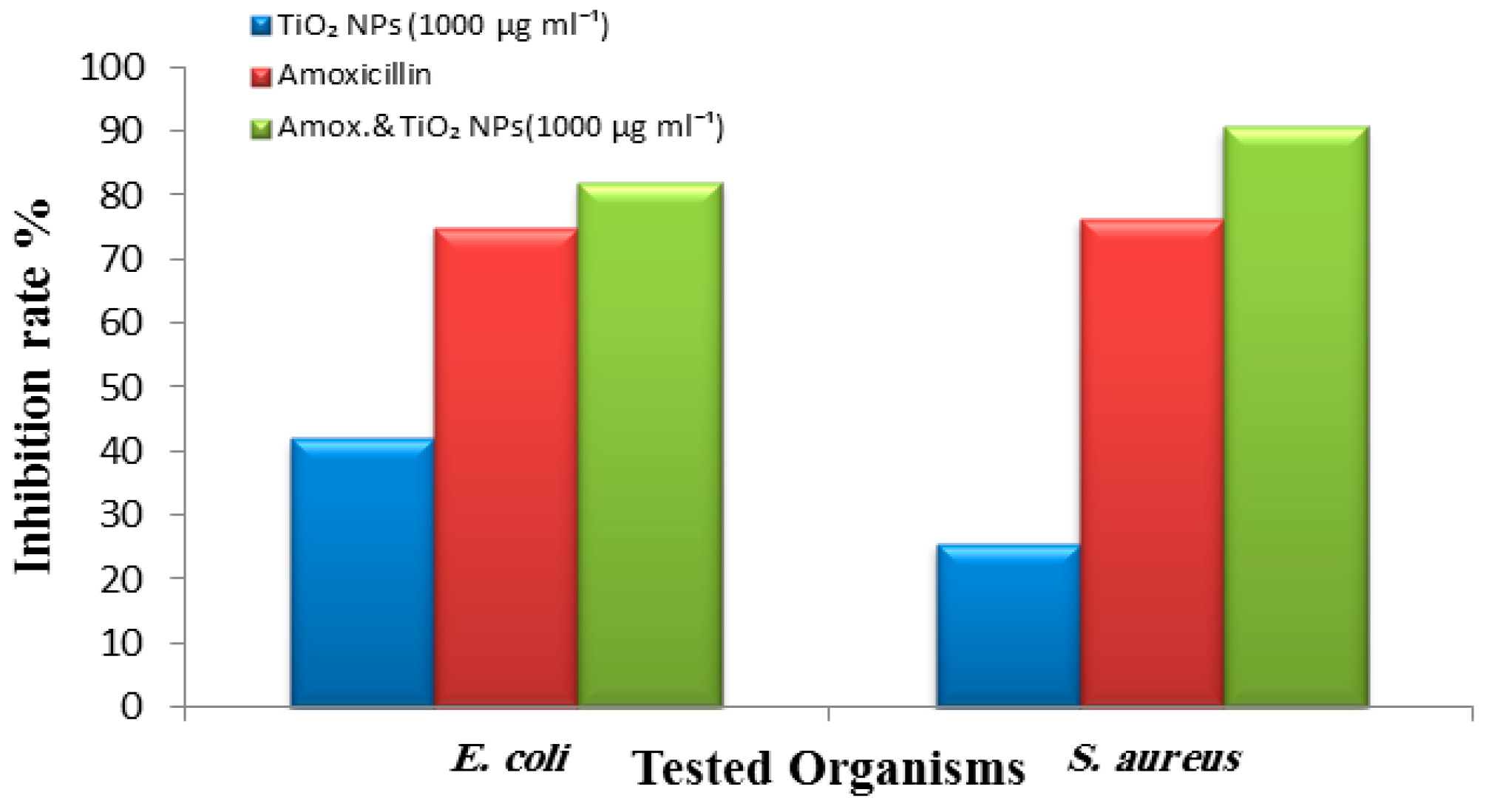
2.3. Antibacterial Activity of TiO2 NPs
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golightly, J.S.; Castleman, A. Analysis of titanium nanoparticles created by laser irradiation under liquid environments. J. Phys. Chem. B 2006, 110, 19979–19984. [Google Scholar] [CrossRef] [PubMed]
- Khashan, K.S.; Mohsin, M.H. Characterization of carbon nitride nanoparticles prepared by laser ablation in liquid for optoelectronic application. Surf. Rev. Lett. 2015, 22, 1550055. [Google Scholar] [CrossRef]
- Khashan, K.S.; Hadi, A.; Mahdi, M.; Hamid, M.K. Nanosecond pulse laser preparation of InZnO (IZO) nanoparticles NPs for high-performance photodetector. Appl. Phys. A 2019, 125, 51. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 2020, 20, 8856. [Google Scholar] [CrossRef]
- Saimon, J.A.; Madhat, S.N.; Khashan, K.S.; Hassan, A.I. Characterization of CdZnO/Si heterojunction photodiode prepared by pulsed laser deposition. Int. J. Mod. Phys. B 2018, 32, 1850341. [Google Scholar] [CrossRef]
- Hadi, A.A.; Badr, B.A.; Mahdi, R.O.; Khashan, K.S. Rapid laser fabrication of Nickel oxide nanoparticles for UV detector. Optik 2020, 219, 165019. [Google Scholar] [CrossRef]
- Barreca, F.; Acacia, N.; Barletta, E.; Spadaro, D.; Curro, G.; Neri, F. Small size TiO2 nanoparticles prepared by laser ablation in water. Appl. Surf. Sci. 2010, 256, 6408–6412. [Google Scholar] [CrossRef]
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper oxide nanoparticles: Synthesis, characterization and their antibacterial activity. J. Clust. Sci. 2011, 22, 121–129. [Google Scholar] [CrossRef]
- Albukhaty, S.; Al-Karagoly, H.; Dragh, M.A. Synthesis of zinc oxide nanoparticles and evaluated it’s activity against bacterial isolates. J. Biotech Res. 2020, 11, 47–53. [Google Scholar]
- Pan, Z.; Lee, W.; Slutsky, L.; Clark, R.A.; Pernodet, N.; Rafailovich, M.H. Adverse effects of titanium dioxide nanoparticles on human dermal fibroblasts and how to protect cells. Small 2009, 5, 511–520. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Memic, A. Size-dependent Antimicrobial Properties of CuO Nanoparticles against Gram-positive and -negative Bacterial Strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef]
- Podporska-Carroll, J.; Panaitescu, E.; Quilty, B.; Wang, L.; Menon, L.; Pillai, S.C. Antimicrobial properties of highly efficient photocatalytic TiO2 nanotubes. Appl. Catal. B Environ. 2015, 176, 70–75. [Google Scholar] [CrossRef]
- Tsuang, Y.H.; Sun, J.S.; Huang, Y.C.; Lu, C.H.; Chang, W.H.S.; Wang, C.C. Studies of photokilling of bacteria using titanium dioxide nanoparticles. Artif. Organs 2008, 32, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Albukhaty, S.; Al-Bayati, L.; Al-Karagoly, H.; Al-Musawi, S. Preparation and characterization of titanium dioxide nanoparticles and in vitro investigation of their cytotoxicity and antibacterial activity against Staphylococcus aureus and Escherichia coli. Anim. Biotechnol. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bahjat, H.H.; Ismail, R.A.; Sulaiman, G.M.; Jabir, M.S. Magnetic field-assisted laser ablation of titanium dioxide nanoparticles in water for anti-bacterial applications. J. Inorg. Organomet. Polym. Mater. 2021, 1–8. [Google Scholar] [CrossRef]
- Yang, G. Laser Ablation in Liquids: Principles and Applications in the Preparation of Nanomaterials; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Hamad, A.H.; Khashan, K.S.; Hadi, A.A.; Yang, D. Laser Ablation in Different Environments and Generation of Nanoparticles. In Applications of Laser Ablation–Thin Film Deposition, Nanomaterial Synthesis and Surface Modification; IntechOpen: London, UK, 2016; pp. 177–194. [Google Scholar]
- Acacia, N.; Barreca, F.; Barletta, E.; Spadaro, D.; Currò, G.; Neri, F. Laser ablation synthesis of indium oxide nanoparticles in water. Appl. Surf. Sci. 2010, 256, 6918–6922. [Google Scholar] [CrossRef]
- Khashan, K.S.; Sulaiman, G.M.; Mahdi, R. The effect of laser energy on the properties of carbon nanotube—Iron oxide nanoparticles composite prepared via pulsed laser ablation in liquid. Mater. Res. Express 2018, 5, 105004. [Google Scholar] [CrossRef]
- Hameed, R.; Khashan, K.S.; Sulaiman, G.M. Preparation and characterization of graphene sheet prepared by laser ablation in liquid. Mater. Today Proc. 2020, 20, 535–539. [Google Scholar] [CrossRef]
- Khashan, K.S.; Abdulameer, F.A.; Jabir, M.S.; Hadi, A.A.; Sulaiman, G.M. Anticancer activity and toxicity of carbon nanoparticles produced by pulsed laser ablation of graphite in water. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 35010. [Google Scholar] [CrossRef]
- Liu, P.-S.; Cai, W.-P.; Wan, L.-X.; Shi, M.-D.; Luo, X.-D.; Jing, W.-P. Fabrication and characteristics of rutile TiO2 nanoparticles induced by laser ablation. Trans. Nonferrous Met. Soc. China 2009, 19. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Romero, N.; Fischer, S.; Dookran, J.; Berger, A.; Doiron, A.L. Recent developments in the use of nanoparticles for treatment of biofilms. Nanotechnol. Rev. 2017, 6, 383–404. [Google Scholar] [CrossRef]
- Swarnkar, R.; Singh, S.; Gopal, R. Effect of aging on copper nanoparticles synthesized by pulsed laser ablation in water: Structural and optical characterizations. Bull. Mater. Sci. 2011, 34, 1363–1369. [Google Scholar] [CrossRef]
- Mahdy, S.A.; Raheed, Q.J.; Kalaichelvan, P. Antimicrobial activity of zero-valent iron nanoparticles. Int. J. Mod. Eng. Res. 2012, 2, 578–581. [Google Scholar]
- Nikolov, A.; Atanasov, P.; Milev, D.; Stoyanchov, T.; Deleva, A.; Peshev, Z. Synthesis and characterization of TiOx nanoparticles prepared by pulsed-laser ablation of Ti target in water. Appl. Surf. Sci. 2009, 255, 5351–5354. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Hong, S.M.; Lee, S.; Jung, H.J.; Yu, Y.; Shin, J.H.; Kwon, K.-Y.; Choi, M.Y. Simple preparation of anatase TiO2 nanoparticles via pulsed laser ablation in liquid. Bull. Korean Chem. Soc. 2013, 34, 279–282. [Google Scholar] [CrossRef]
- Mahfouz, R.; Aires, F.C.S.; Brenier, A.; Jacquier, B.; Bertolini, J. Synthesis and physico-chemical characteristics of nanosized particles produced by laser ablation of a nickel target in water. Appl. Surf. Sci. 2008, 254, 5181–5190. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman characteriztion of TiO2 nanoparticles coated with polyethyleneglycol as carrier for 2-methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Baladi, A.; Mamoory, R.S. Effect of Laser Wavelength and Ablation Time on Pulsed Laser Ablation Synthesis of Al Nanoparticles in Ethanol; World Scientific: Singapore, 2012; pp. 58–65. [Google Scholar]
- Rosická, D.; Šembera, J. Changes in the nanoparticle aggregation rate due to the additional effect of electrostatic and magnetic forces on mass transport coefficients. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Al-Nori, T.M. Antibacterial activity of silver and gold nanoparticles against Streptococcus, Staphylococcus aureus and E. coli. Al-Mustansiriyah J. Sci. 2012, 23, 45–54. [Google Scholar]
- Haghi, M.; Hekmatafshar, M.; Janipour, M.B.; Gholizadeh, S.S.; Faraz, M.; Sayyadifar, F.; Ghaedi, M. Antibacterial effect of TiO2 nanoparticles on pathogenic strain of E. coli. Int. J. Adv. Biotechnol. Res. 2012, 3, 621–624. [Google Scholar]
- Murthy, P.S.; Venugopalan, V.; Arunya, D.D.; Dhara, S.; Pandiyan, R.; Tyagi, A. Antibiofilm Activity of Nano Sized CuO. In Proceedings of the International Conference on Nanoscience, Engineering and Technology, Chennai, India, 28–30 November 2011; IEEE: Chennai, India, 2011; pp. 580–583. [Google Scholar]
- Rajawat, S.; Qureshi, M.S. Comparative study on bactericidal effect of silver nanoparticles, synthesized using green technology, in combination with antibiotics on Salmonella typhi. J. Biomater. Nanobiotechnol. 2012, 3, 480. [Google Scholar] [CrossRef][Green Version]
- de Dicastillo, C.L.; Correa, M.G.; Martínez, F.B.; Streitt, C.; Galotto, M.J. Antimicrobial Effect of Titanium Dioxide Nanoparticles. In Antimicrobial Resistance-A One Health Perspective; IntechOpen: London, UK, 2020. [Google Scholar]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Arora, B.; Murar, M.; Dhumale, V. Antimicrobial potential of TiO2 nanoparticles against MDR Pseudomonas aeruginosa. J. Exp. Nanosci. 2015, 10, 819–827. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Vu, V.T.; Nguyen, T.A.; Tran, V.K.; Nguyen-Tri, P. Antibacterial activity of TiO2-and ZnO-decorated with silver nanoparticles. J. Compos. Sci. 2019, 3, 61. [Google Scholar] [CrossRef]
- Savi, G.D.; Trombin, A.C.; da Silva Generoso, J.; Barichello, T.; Possato, J.C.; Ronconi, J.V.V.; da Silva Paula, M.M. Antibacterial acivity of gold and silver nanoparticles impregnated with antimicrobial agents. Saúde Pesqui. 2013, 6, 227–235. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef]


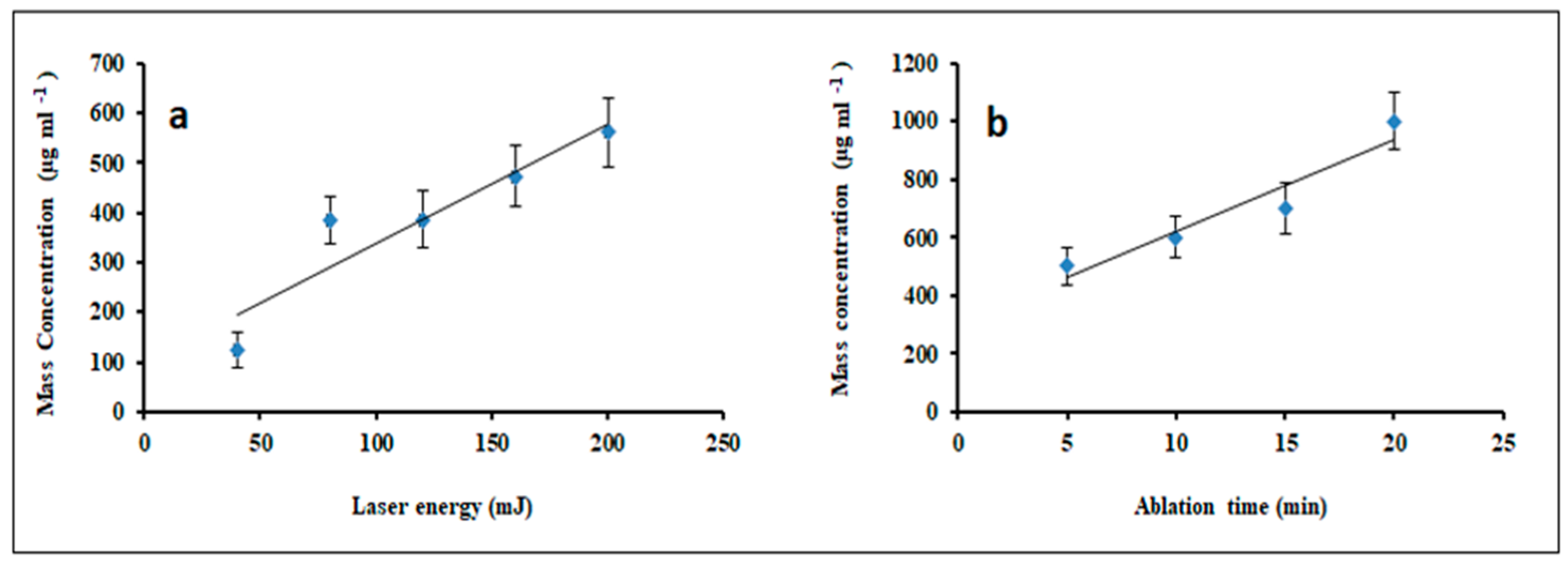
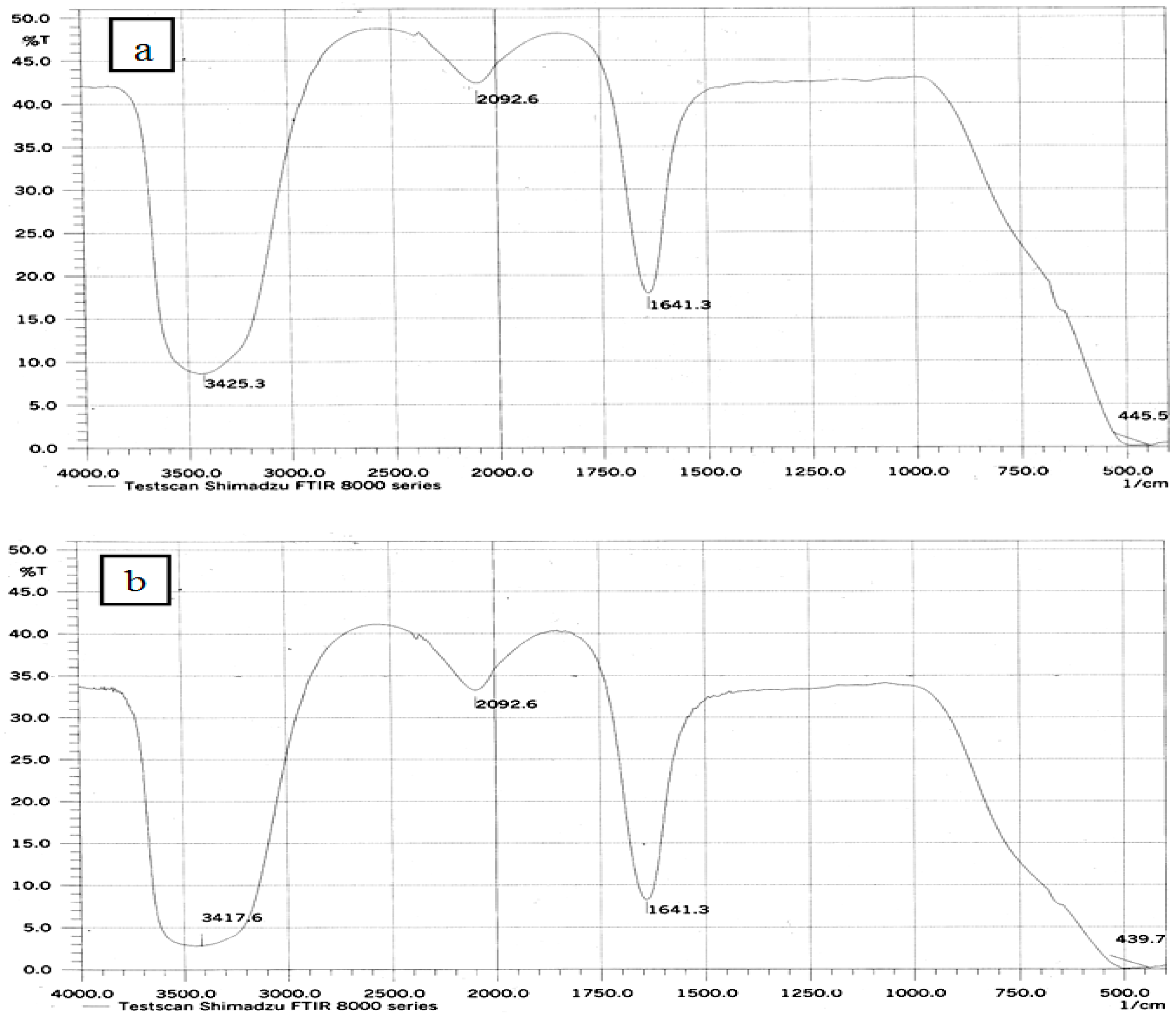
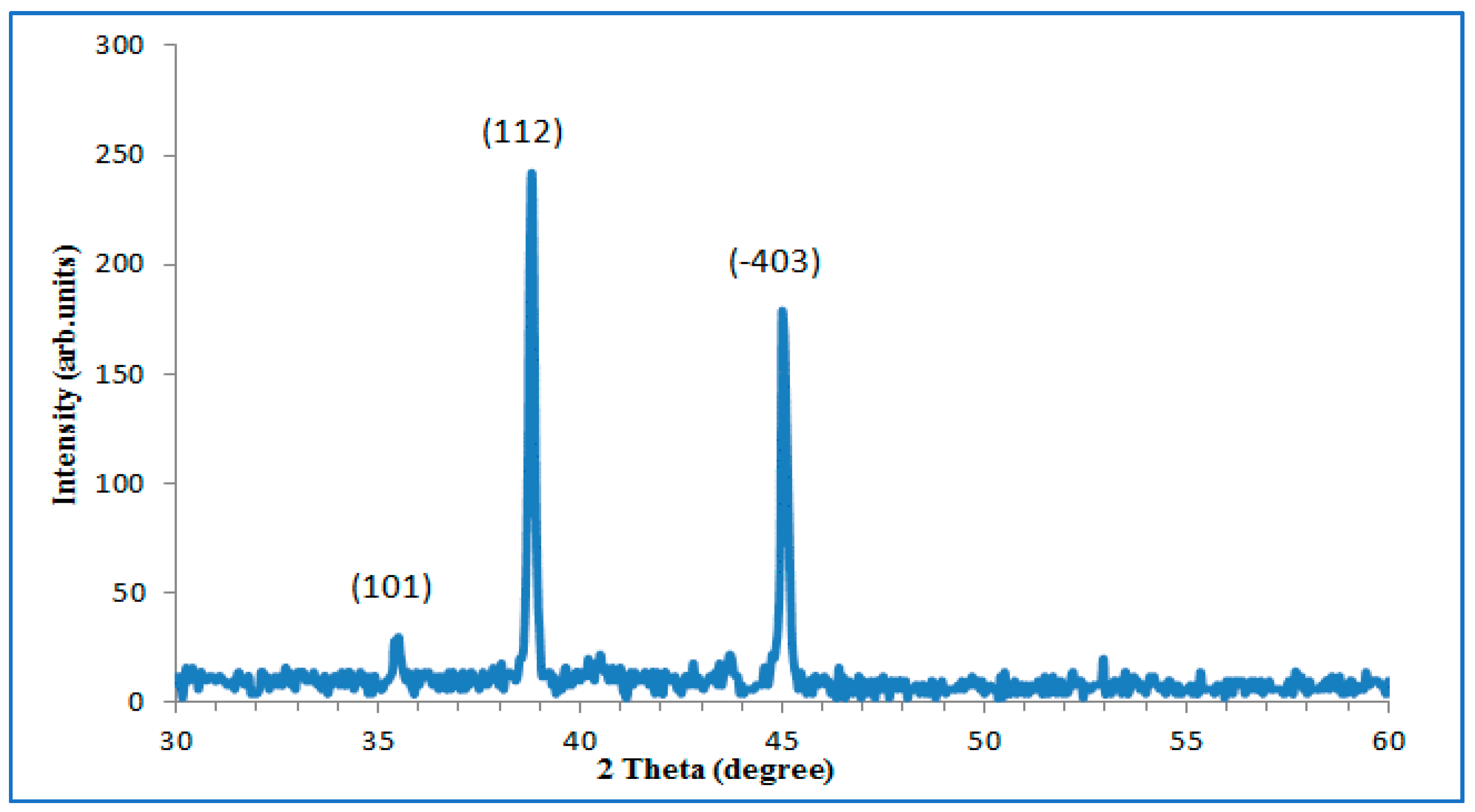
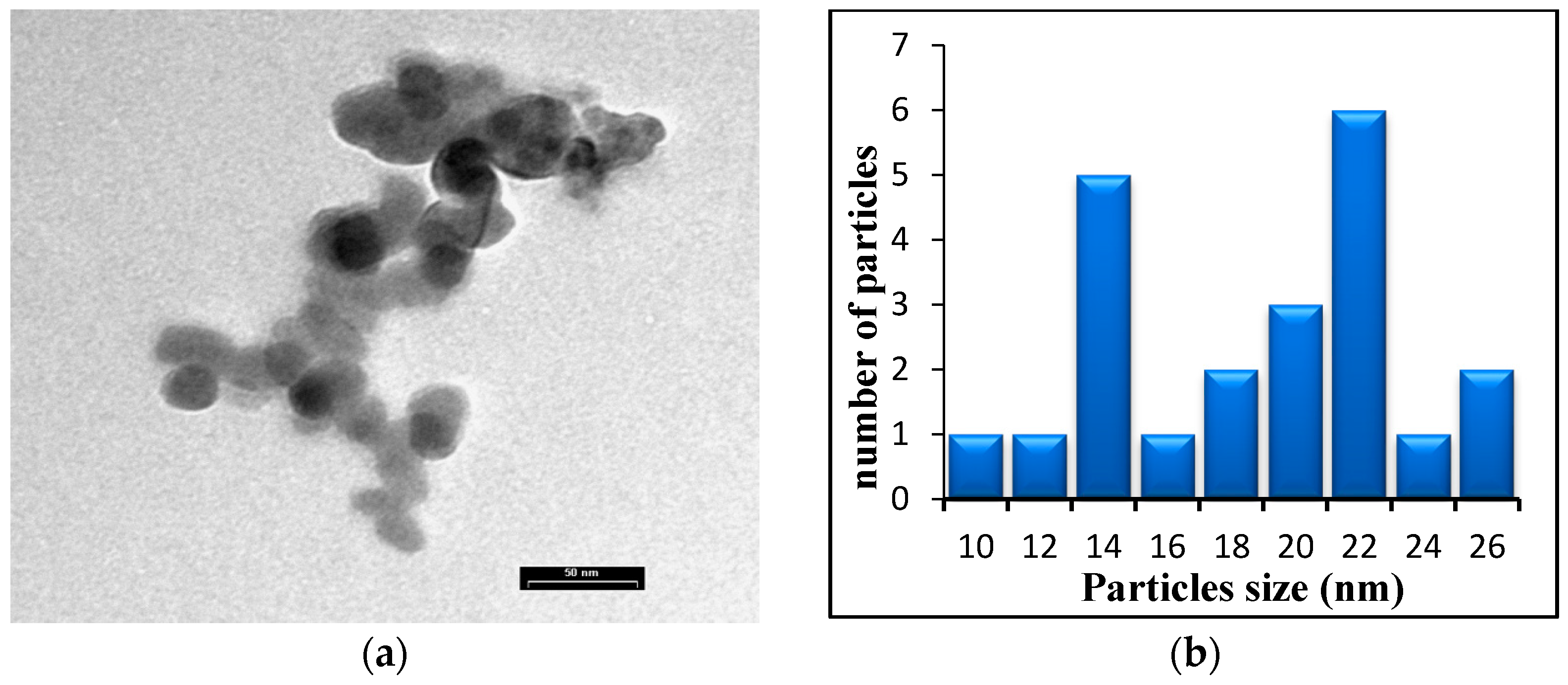



| Ablation Condition | 2θ (Degree) | Orientation (hkl) | G.S (nm) | FWHM (Degree) | Phase |
|---|---|---|---|---|---|
| 200 mJ 10 min | 35.4 | 101 | 41.67 | 0.2 | anatase TiO2 |
| 38.8 | 112 | 46.77 | 0.18 | anatase TiO2 | |
| 45 | −403 | 44.46 | 0.19 | β-TiO2 |
| Organisms | TiO2 NPs Along with Amoxicillin | IZ (mm) Mean ± S.D. |
|---|---|---|
| E. coli | Control (amoxicillin) | 8.0 ± 0.57 |
| 400 µg mL−1 | 10.3 ± 0.57 | |
| 600 µg mL−1 | 11.3 ± 0.57 | |
| 1000 µg mL−1 | 11.6 ± 0.57 | |
| S. aureus | Control (amoxicillin) | 9.3 ± 1.15 |
| 400 µg mL−1 | 12.3 ± 0.57 | |
| 600 µg mL−1 | 11.6 ± 1.15 | |
| 1000 µg mL−1 | 13.3 ± 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khashan, K.S.; Sulaiman, G.M.; Abdulameer, F.A.; Albukhaty, S.; Ibrahem, M.A.; Al-Muhimeed, T.; AlObaid, A.A. Antibacterial Activity of TiO2 Nanoparticles Prepared by One-Step Laser Ablation in Liquid. Appl. Sci. 2021, 11, 4623. https://doi.org/10.3390/app11104623
Khashan KS, Sulaiman GM, Abdulameer FA, Albukhaty S, Ibrahem MA, Al-Muhimeed T, AlObaid AA. Antibacterial Activity of TiO2 Nanoparticles Prepared by One-Step Laser Ablation in Liquid. Applied Sciences. 2021; 11(10):4623. https://doi.org/10.3390/app11104623
Chicago/Turabian StyleKhashan, Khawla S., Ghassan M. Sulaiman, Farah A. Abdulameer, Salim Albukhaty, Mohammed A. Ibrahem, Tahani Al-Muhimeed, and Abeer A. AlObaid. 2021. "Antibacterial Activity of TiO2 Nanoparticles Prepared by One-Step Laser Ablation in Liquid" Applied Sciences 11, no. 10: 4623. https://doi.org/10.3390/app11104623
APA StyleKhashan, K. S., Sulaiman, G. M., Abdulameer, F. A., Albukhaty, S., Ibrahem, M. A., Al-Muhimeed, T., & AlObaid, A. A. (2021). Antibacterial Activity of TiO2 Nanoparticles Prepared by One-Step Laser Ablation in Liquid. Applied Sciences, 11(10), 4623. https://doi.org/10.3390/app11104623








