Arcopilus aureus MaC7A as a New Source of Resveratrol: Assessment of Amino Acid Precursors, Volatiles, and Fungal Enzymes for Boosting Resveratrol Production in Batch Cultures
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Arcopilus aureus MaC7A
2.2. Chemicals
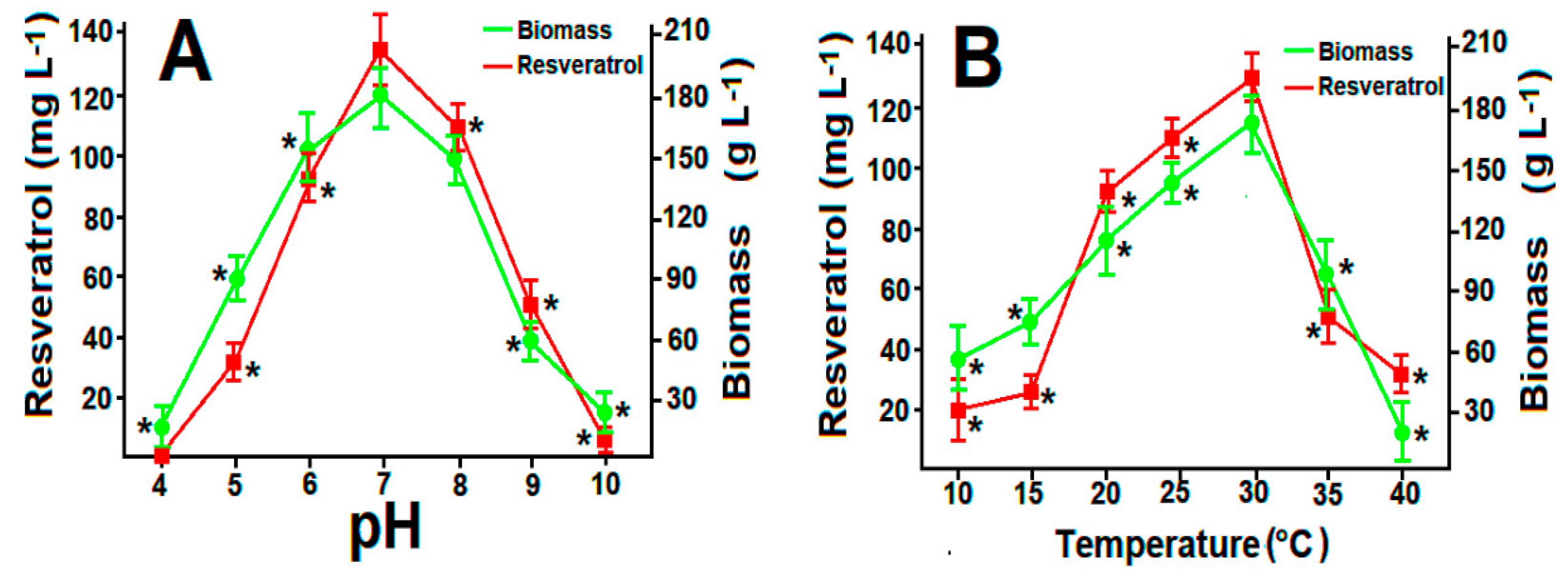
2.3. Fungal Viability and Effect of pH and Temperature on Resveatrol Production
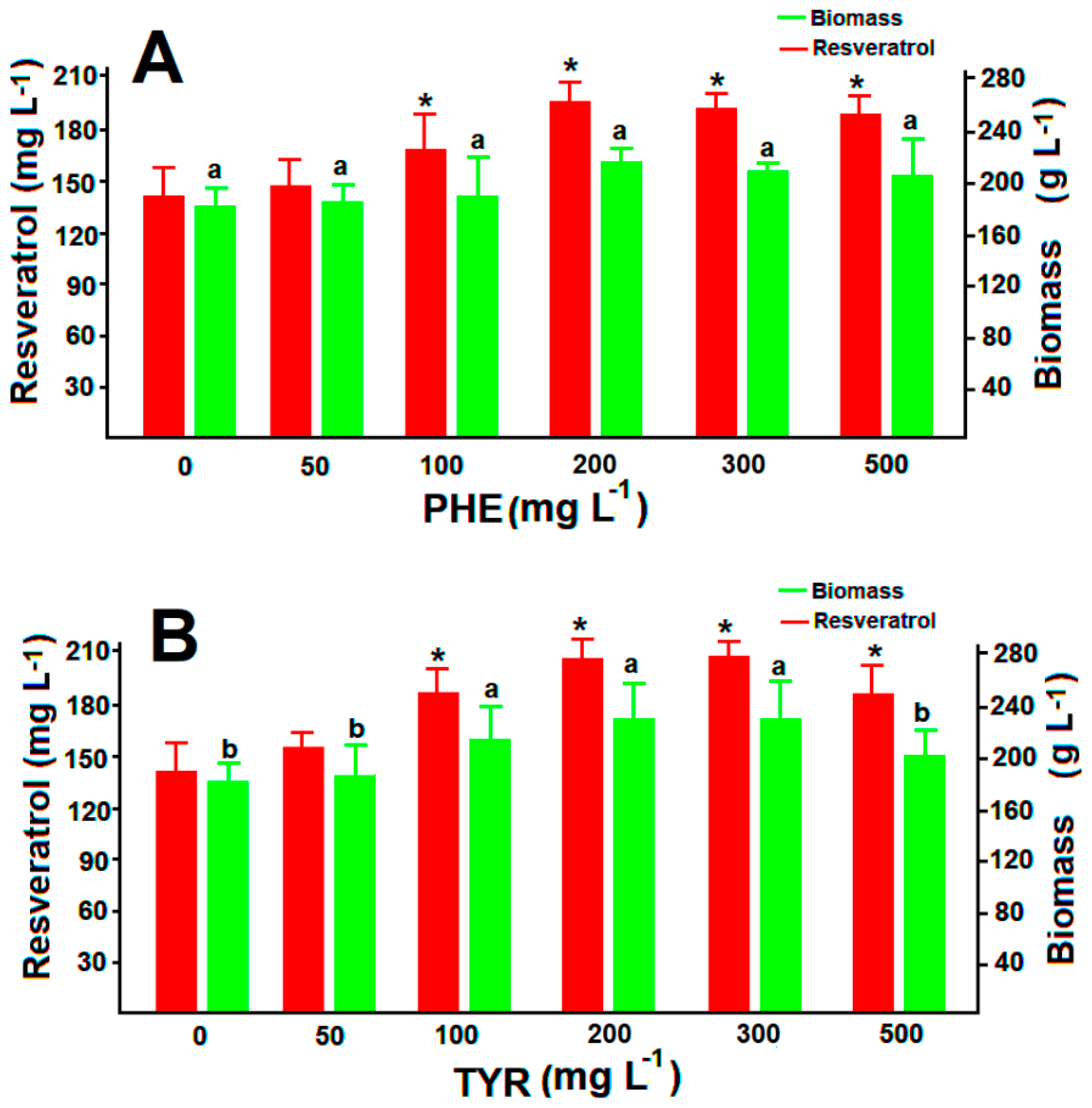
2.4. Feeding Experiments with Amino Acid Precursors of Resveratrol
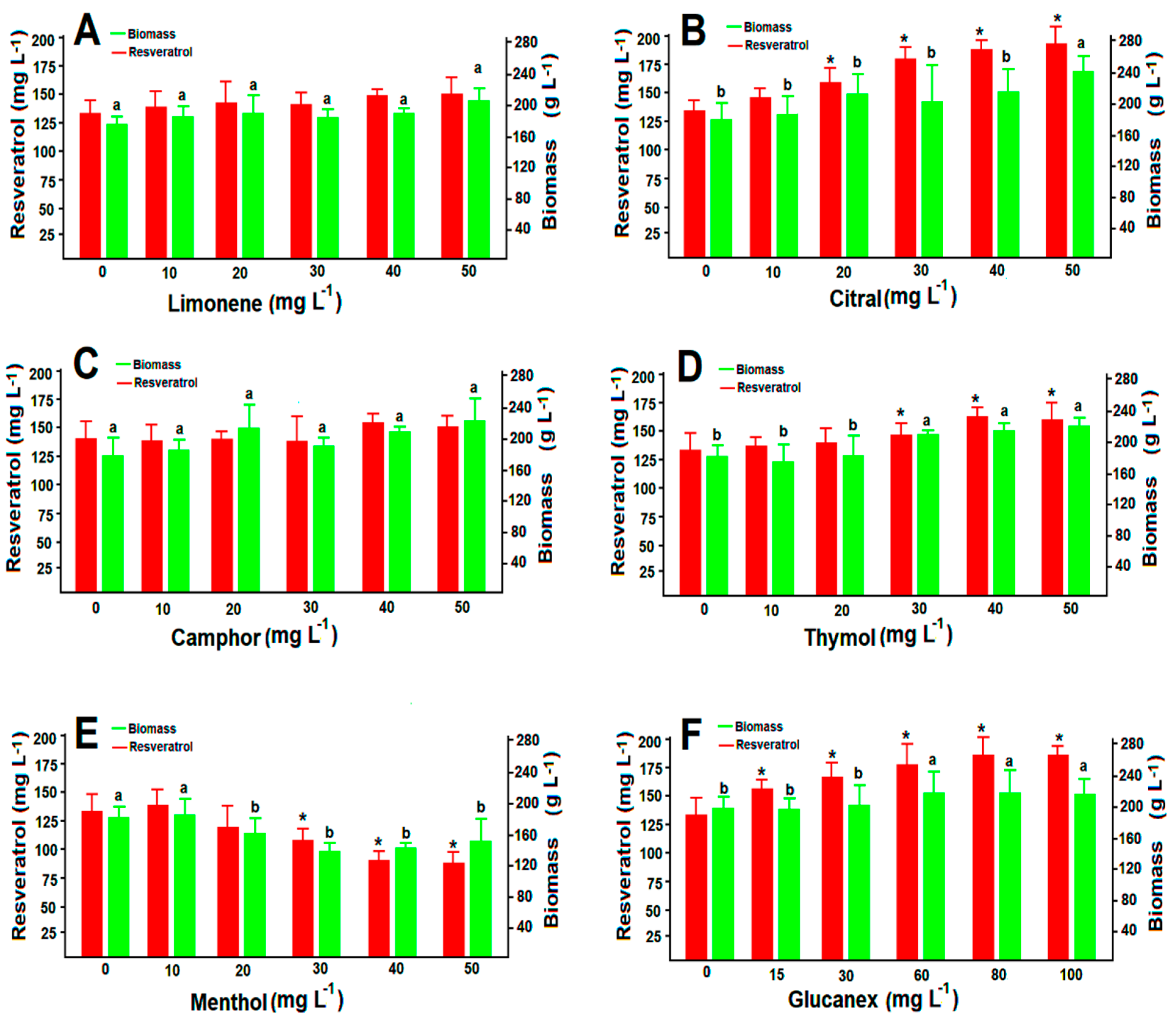
2.5. Feeding Experiments with Volatiles and Glucanex
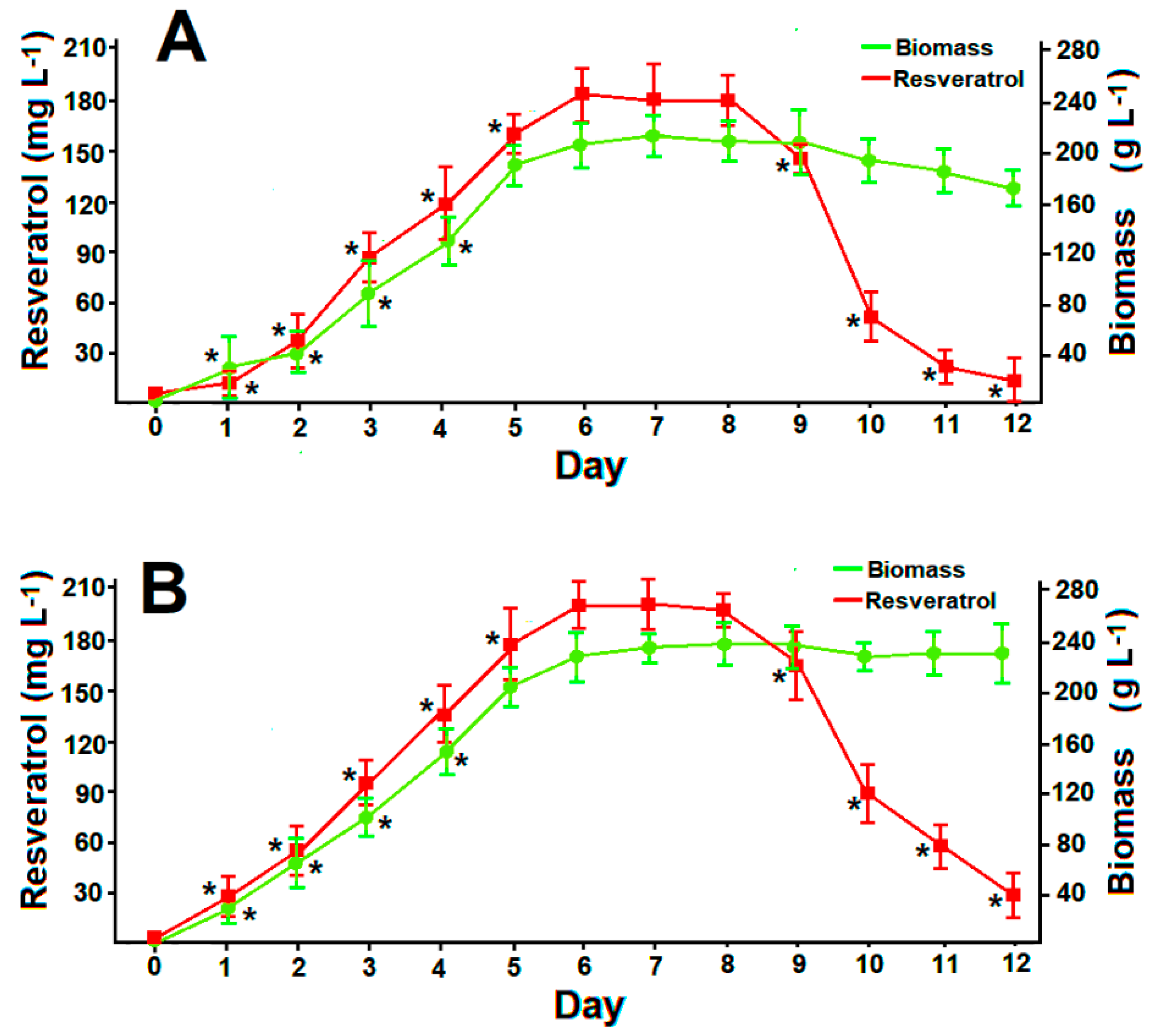
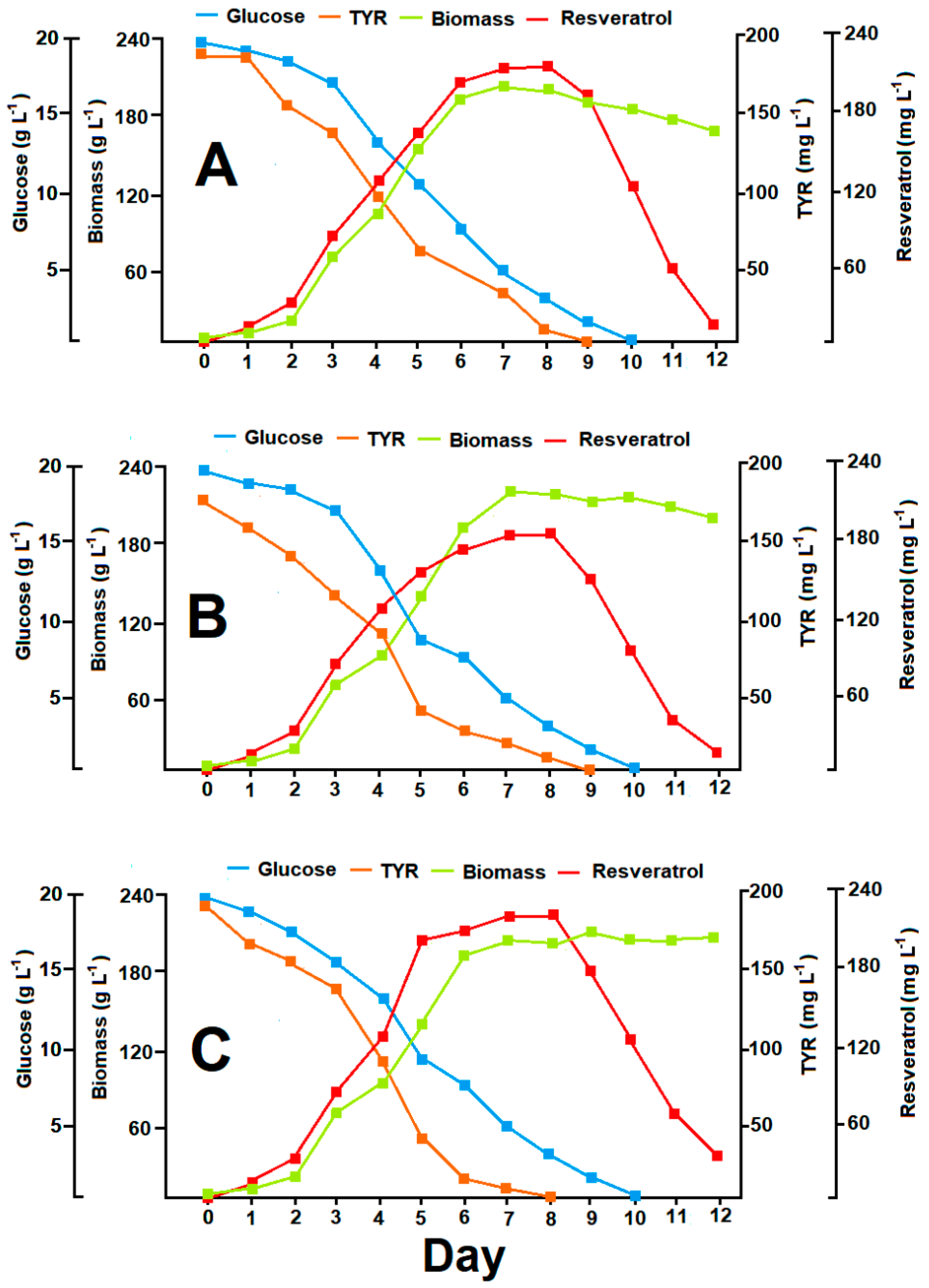
2.6. Opitimized Batch Cultures
2.7. Extraction and Analysis of Resveratrol
2.8. Statistical Analysis
3. Results
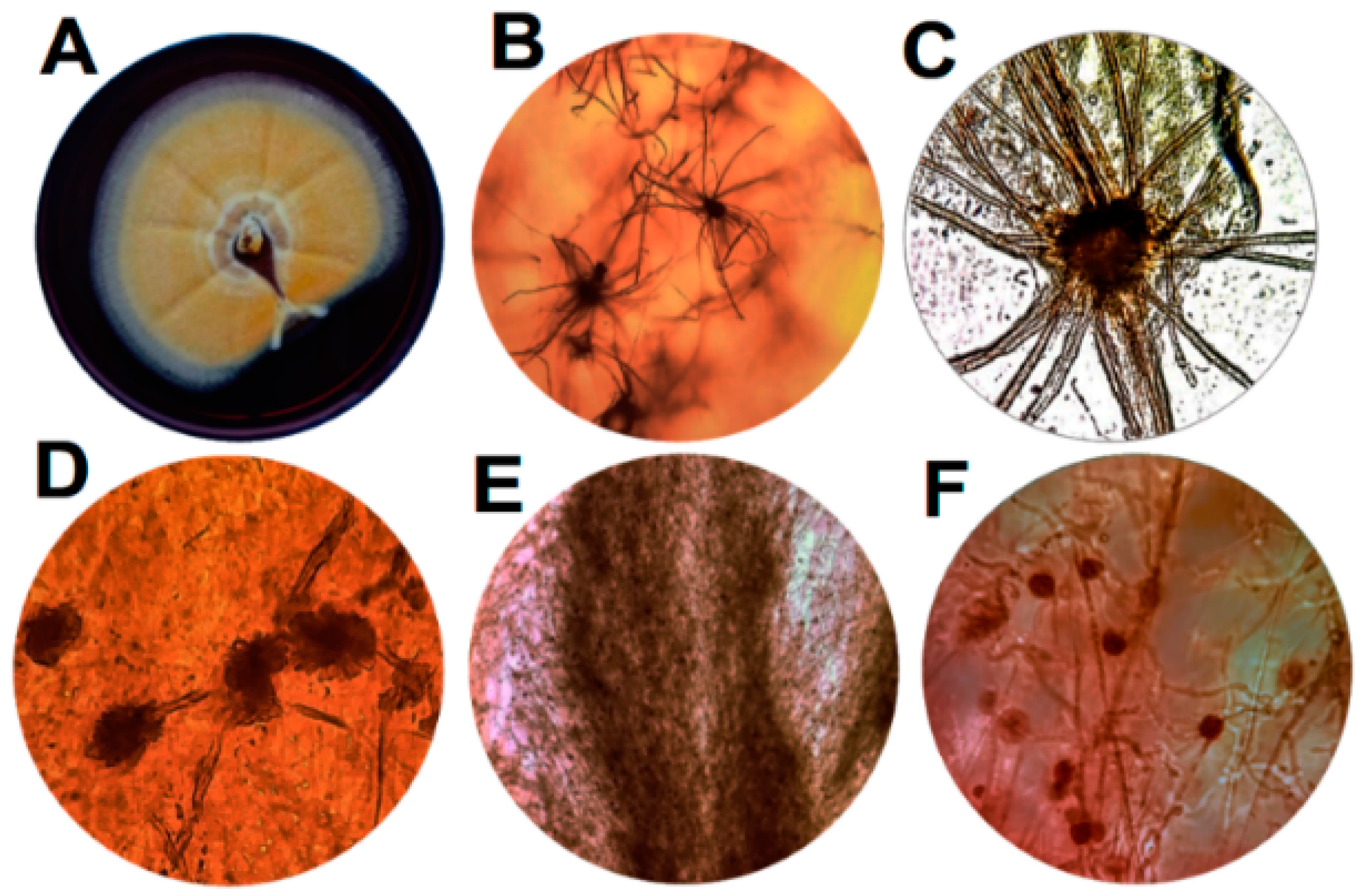
3.1. Morphological, Microscopic and Molecular Features of Arcopilus aereus MaC7A
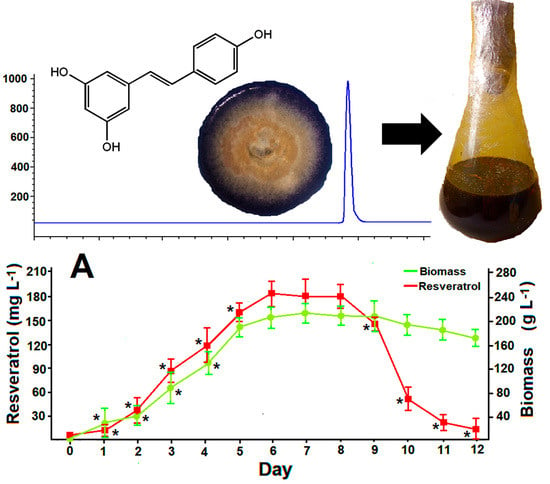
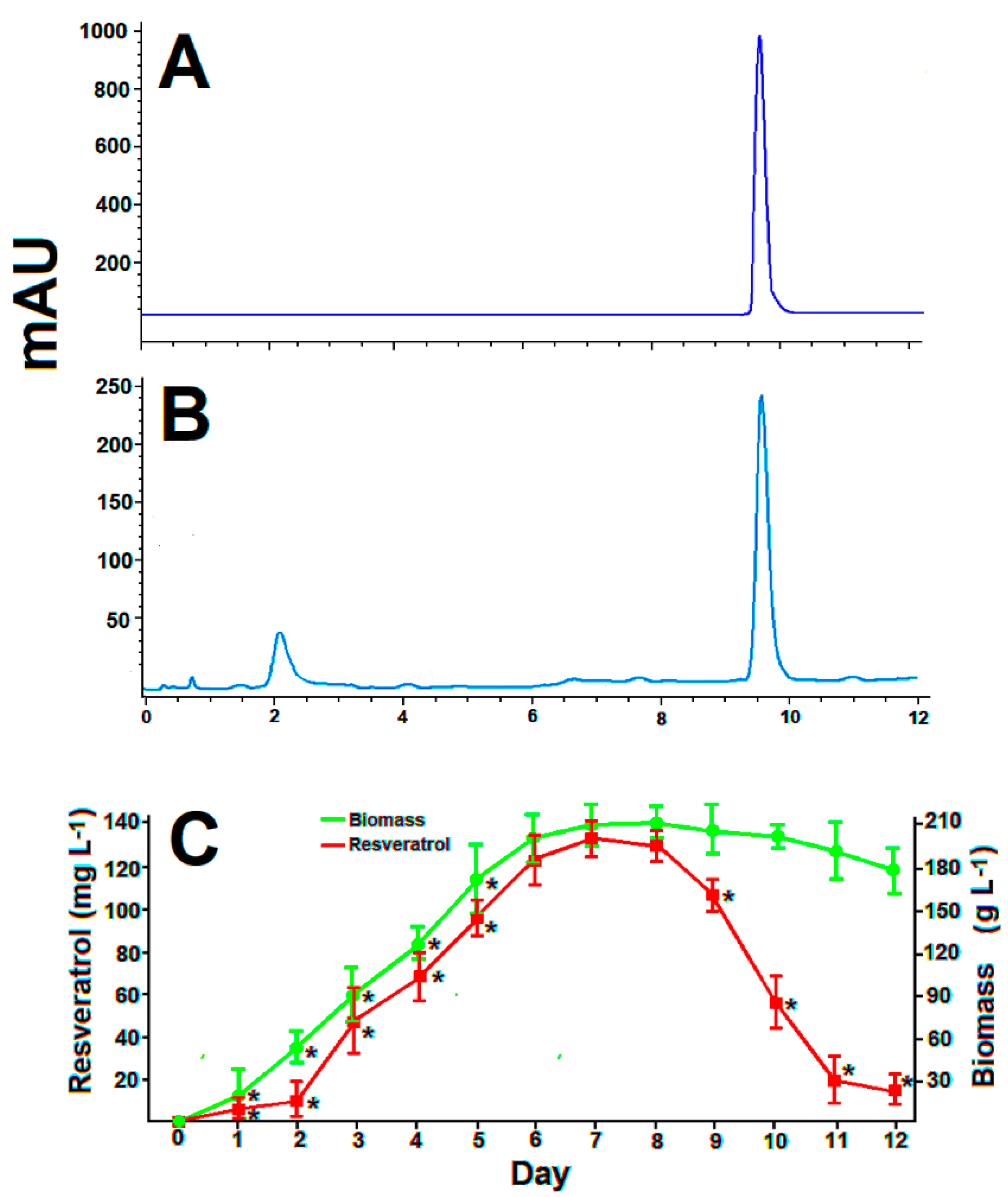
3.2. Standard batch Cultures of Arcopilus aureus MaC7A
3.3. Susceptibility of A. aureus MaC7A to Volatiles and Glucanex
3.4. Resveratrol Production in Feed Batch Cultures with Amino Acidid Precursors
3.5. Resveratrol Production under the Pressure of Volatiles and Glucanex
3.6. Resveratrol Production in Optimized Batch Cultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Villa-Ruano, N.; Rivera, A.; Rubio-Rosas, E.; Landeta-Cortés, G.; Varela-Caselis, J.L.; Romero-Arenas, O. Comparative activity of six recombinant stilbene synthases in yeast for resveratrol production. Appl. Sci. 2020, 10, 4847. [Google Scholar] [CrossRef]
- Marinella, M.A. Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19. Int. J. Clin. Pract. 2020, 74, e13535. [Google Scholar] [CrossRef]
- Chang, X.; Heene, E.; Qiao, F.; Nick, P. The phytoalexin resveratrol regulates the initiation of hypersensitive cell death in Vitis cell. PLoS ONE 2011, 6, e26405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.P.; Yu, S.Y.; Chen, C.J.; Li, H.; Wu, Y.L.; Li, H.H. Cloning a peanut resveratrol synthase gene and its expression in purple sweet potato. Plant Cell Rep. 2012, 31, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhao, S.; Li, Z.; Wang, Q.; Yao, F.; Yang, L.; Pan, J.; Liu, J. Evaluating the Effect of expressing a peanut resveratrol synthase gene in rice. PLoS ONE 2015, 10, e0136013. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, Y.; Wang, X.; Zhong, J.; Lin, Z. High Content of resveratrol in lettuce transformed with a stilbene synthase gene of Parthenocissus henryana. J. Agric. Food Chem. 2006, 54, 8082–8085. [Google Scholar] [CrossRef]
- Thapa, S.B.; Pandey, R.P.; Park, Y.; Sohng, J.K. Biotechnological advances in resveratrol production and its chemical diversity. Molecules 2019, 24, 2571. [Google Scholar] [CrossRef] [Green Version]
- Jeandet, P.; Delaunois, B.; Aziz, A.; Donnez, D.; Vasserot, Y.; Cordelier, S.; Courot, E. Metabolic engineering of yeast and plants for the production of the biologically active hydroxystilbene, resveratrol. J. Biomed. Biotechnol. 2012, 579089. [Google Scholar] [CrossRef]
- Li, M.; Kildegaard, K.R.; Chen, Y.; Rodriguez, A.; Borodina, I.; Nielsen, J. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab. Eng. 2015, 32, 1–11. [Google Scholar] [CrossRef]
- Li, M.; Schneider, K.; Kristensen, M.; Borodina, I.; Nielsen, J. Engineering yeast for highlevel production of stilbenoid antioxidants. Sci. Rep. 2015, 6, 36827. [Google Scholar] [CrossRef] [Green Version]
- Dwibedi, V.; Saxena, S. Arcopilus aureus, a resveratrol-producing endophyte from Vitis vinifera. Appl. Biochem. Biotechnol. 2018, 186, 476–495. [Google Scholar] [CrossRef]
- Dwibedi, V.; Saxena, S. Diversity and phylogeny of resveratrol-producing culturable endophytic fungi from Vitis species in India. 3 Biotech 2019, 9, 182. [Google Scholar] [CrossRef]
- Dwibedi, V.; Kalia, S.; Saxena, S. Isolation and enhancement of resveratrol production in Xylaria psidii by exploring the phenomenon of epigenetics: Using DNA methyltransferases and histone deacetylase as epigenetic modifiers. Mol. Biol. Rep. 2019, 46, 4123–4137. [Google Scholar] [CrossRef] [PubMed]
- Dwibedi, V.; Rath, S.K.; Prakash, R.; Saxena, S. Response surface statistical optimization of fermentation parameters for resveratrol production by the endophytic fungus Arcopilus aureus and its tyrosinase inhibitory activity. Biotechnol. Lett. 2021, 43, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, H.S.; Nguyen, T.T.T.; Lee, H.B. Characterization of three species of sordariomycetes isolated from freshwater and soil samples in Korea. Mycobiology 2018, 47, 20–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Jiménez, M.N.; Zavaleta-Mancera, H.A.; Rebollar-Alviter, A.; AguilarRincón, V.H.; García-de-los-Santos, G.; Vaquera-Huerta, H.; Silva-Rojas, H.V. Phylogenetics and histology provide insight into damping-off infections of ‘Poblano’ pepper seedlings caused by Fusarium wilt in greenhouses. Mycol. Prog. 2018, 17, 1237–1249. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA gene for phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 2nd ed.; Innis, M.A., Gelfand, D.H., Sninsky, J.S., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Chadha, S.; Kale, P. Simple fluorescence-based high throughput cell viability assay for filamentous fungi. Lett. Appl. Microbiol. 2015, 61, 238–244. [Google Scholar] [CrossRef]
- Lefebvre, D.; Gabriel, V.; Vayssier, Y.; Fontagné-Faucher, C. Simultaneous HPLC determination of sugars, organic acids and ethanol in sourdough process. LWT Food Sci Technol. 2002, 355, 407–414. [Google Scholar] [CrossRef]
- Fabiani, A.; Versari, A.; Parpinello, G.P.; Castellari, M.; Galass, S. High-performance liquid chromatographic analysis of free amino acids in fruit juices using derivatization with 9-fluorenylmethyl-chloroformate. J. Chromatogr. Sci. 2002, 40, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Glavnik, V.; Simonovska, B.; Albreht, A.; Vovk, I. TLC and HPLC screening of p-coumaric acid, trans-resveratrol, and pterostilbene in bacterial cultures, food supplements, and wine. J. Planar Chromatogr. 2012, 25, 251–258. [Google Scholar] [CrossRef]
- Bowden, N.A.; Sanders, J.P.M.; Bruins, M.E. Solubility of the proteinogenic α-amino acids in water, ethanol, and ethanol−water mixtures. J. Chem. Eng. Data 2018, 63, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Boruta, T. Uncovering the repertoire of fungal secondary metabolites: From Fleming’s laboratory to the International Space Station. Bioengineered 2018, 9, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farh, M.E.A.; Jeon, J. Roles of fungal volatiles from perspective of distinct lifestyles in filamentous fungi. Plant Pathol. J. 2020, 36, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mareia, G.I.K.; Rasoula, M.A.A.; Abdelgaleil, S.A.M. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pest. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Ait-Lahsen, H.; Soler, A.; Rey, M.; De La Cruz, J.; Monte, E.; Llobell, A. An antifungal exo--1,3-glucanase (AGN13.1) from the biocontrol fungus Trichoderma harzianum. Appl. Environ. Microbiol. 2001, 67, 5833–5839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Agent | MIC (mg L−1) * |
|---|---|
| Limonene | 267. 7± 0.54 b |
| Citral | 130.82 ± 0.68 d |
| Camphor | 389.30 ± 0.79 a |
| Menthol | 92.19 ± 0.41 e |
| Thymol | 261.8 ± 1.32 b |
| Glucanex | 201.5 ± 0.59 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa-Ruano, N.; Morales-Mora, L.Á.; Varela-Caselis, J.L.; Rivera, A.; Valencia de Ita, M.d.l.Á.; Romero-Arenas, O. Arcopilus aureus MaC7A as a New Source of Resveratrol: Assessment of Amino Acid Precursors, Volatiles, and Fungal Enzymes for Boosting Resveratrol Production in Batch Cultures. Appl. Sci. 2021, 11, 4583. https://doi.org/10.3390/app11104583
Villa-Ruano N, Morales-Mora LÁ, Varela-Caselis JL, Rivera A, Valencia de Ita MdlÁ, Romero-Arenas O. Arcopilus aureus MaC7A as a New Source of Resveratrol: Assessment of Amino Acid Precursors, Volatiles, and Fungal Enzymes for Boosting Resveratrol Production in Batch Cultures. Applied Sciences. 2021; 11(10):4583. https://doi.org/10.3390/app11104583
Chicago/Turabian StyleVilla-Ruano, Nemesio, Luis Ángel Morales-Mora, Jenaro Leocadio Varela-Caselis, Antonio Rivera, María de los Ángeles Valencia de Ita, and Omar Romero-Arenas. 2021. "Arcopilus aureus MaC7A as a New Source of Resveratrol: Assessment of Amino Acid Precursors, Volatiles, and Fungal Enzymes for Boosting Resveratrol Production in Batch Cultures" Applied Sciences 11, no. 10: 4583. https://doi.org/10.3390/app11104583
APA StyleVilla-Ruano, N., Morales-Mora, L. Á., Varela-Caselis, J. L., Rivera, A., Valencia de Ita, M. d. l. Á., & Romero-Arenas, O. (2021). Arcopilus aureus MaC7A as a New Source of Resveratrol: Assessment of Amino Acid Precursors, Volatiles, and Fungal Enzymes for Boosting Resveratrol Production in Batch Cultures. Applied Sciences, 11(10), 4583. https://doi.org/10.3390/app11104583