Abstract
The purpose of this study was to isolate functional Bacillus strains from Korean fermented soybeans and to evaluate their potential as probiotics. The L-asparaginase activity of MKHJ 1-1 was the highest among 162 Bacillus strains. This strain showed nonhemolysis and did not produce β-glucuronidase. Among the nine target bacteria, MKHJ 1-1 inhibited the growth of Escherichia coli, Pseudomonas aeruginosa, Shigella sonnei, Shigella flexneri, Klebsiella pneumoniae, Staphylococcus aureus, and Bacillus cereus. 16S rRNA gene sequence analysis resulted in MKHJ 1-1 identified as Bacillus subtilis subsp. stercoris D7XPN1. As a result of measuring the survival rate in 0.1% pepsin solution (pH 2.5) and 0.3% bile salt solution for 3 h, MKHJ 1-1 exhibited high acid resistance and was able to grow in the presence of bile salt. MKHJ 1-1 showed outstanding autoaggregation ability after 24 h. In addition, its coaggregation with pathogens was strong. Therefore, MKHJ 1-1 is a potential probiotic with L-asparaginase activity and without L-glutaminase activity, suggesting that it could be a new resource for use in the food and pharmaceutical industry.
1. Introduction
Probiotics are defined as live microorganisms, which, when administered in adequate amounts, provide a health benefit to the host [1]. The majority of commercially available probiotic strains are Lactobacillus spp. and Bifidobacterium spp. [2]. In addition, several Bacillus species have probiotic potential. Cutting [3] has reported that the use of Bacillus species as probiotic dietary supplements has been rapidly expanding as the number of studies of Bacillus species on probiotic effects has increased. It is demonstrated that Bacillus exerts beneficial probiotic effects, including the secretion of antimicrobials [4], stimulation of the immune system [5], and overall enhancement of gut microflora [6]. Bacillus spp. have the advantage of being able to survive in foods that require harsh processing conditions such as high temperature and high pressure [7]. Bacillus spp. used as probiotics in humans include Bacillus coagulans, Bacillus subtilis, Bacillus licheniformis, Bacillus cereus, Bacillus cereus var. toyoi, Bacillus natto (subtilis), Bacillus clausii, Bacillus pumilus, Bacillus amyloliquefaciens, and Bacillus polyfermenticus [3,8].
L-asparaginase (E.C. 3.5.1.1) is an amidohydrolase enzyme that hydrolyzes L-asparagine to L-aspartic acid and ammonia [9]. In the pharmaceutical industry, L-asparaginase is also used for the treatment of acute lymphocytic leukemia (ALL), Hodgkin’s disease, acute myeloid leukemia, chronic lymphocytic leukemia, lymphosarcoma, reticulosarcoma, and melanosarcoma [10]. The anticancer mechanism of L-asparaginase inhibits tumor cell growth by causing the depletion of extracellular L-asparagine. Tumor cells grow depending on extracellular asparagine because they do not produce intracellular asparagine or have a reduced activity of asparagine synthetase [11,12]. However, L-asparaginase, which is used to treat cancer, has various side effects such as fever, chills, vomiting, weight loss, pancreatitis due to hepatocellular dysfunction, and death during treatment of ALL [13]. This is due to its intrinsic toxicity associated with the presence of L-glutaminase activity. L-glutaminase is important for nitrogen transport in the blood, and prolonged depletion of amino acids during L-asparaginase treatment causes severe biochemical disorders in the body [14,15].
L-asparaginase enzymes have been already found in several bacteria, Bacillus species being one of the most abundant ones due to its resistance to extreme environments. Ameen et al. (2020) [16] have reported that the production L-glutaminase-free L-asparaginase activity makes B. subtilis superior to the commercially available asparaginases that show glutaminase activity causing diverse severe health problems to patients during anti-cancer therapy.
Thus, this study aimed to isolate Bacillus strains possessing L-asparaginase activity and free of L-glutaminase activity from Korean fermented soybean foods and to assess the probiotic characteristics of Bacillus strains.
2. Materials and Methods
2.1. Isolation of Strains Producing L-Asparaginase and Free of L-Glutaminase Activity
A total of 268 strains were isolated from Korean fermented soybean foods such as chunggukjang (fermented paste made from whole soybeans, known as a type of quick doenjang), deonjang (fermented soybean paste), gochujang (fermented red chili paste), and soy sauce (fermented condiment made by soaking naturally fermented soybean brick), which were collected from a home and local market, using Nutrient agar (NA; Becton, Dickinson and Co., Sparks, MD, USA). Representative colonies were randomly selected from NA plates and screened for survivability in artificial gastric acid and bile salt solution [17]. Colonies with L- asparaginase and without L-glutaminase activity were confirmed using Modified Czapek Dox (MCD) medium [18]. The selected colonies were inoculated into the MCD medium containing L-asparagine or L-glutamine at 30 °C for 24 h. After incubation, pink colonies from the MCD medium containing L-asparagine and colonies with no color change from the MCD medium containing L-glutamine were selected.
2.2. L-Asparaginase Activity
The L-asparaginase activity of the selected strains was assessed according to the modified Worthington Enzyme Manual [19]. The selected strains were inoculated into asparaginase activity optimal medium [20] and incubated at 30 °C for 24 h. The culture broth was centrifuged at 12,501× g for 5 min. The culture supernatants were used as crude enzyme samples. Then, 45 μL of the culture supernatant was incubated with 650 μL of 10 mM L-asparagine in 50 mM Tris-HCl buffer (pH 8.5) at 37 °C for 1 h. To terminate the enzyme reaction, 45 μL of 1.5 M trichloroacetic acid solution was added and centrifuged at 12,501× g for 8 min. Then, 250 μL of its supernatant was reacted with 3.5 mL distilled water and 500 μL of Nessler’s reagent (Kanto Chemical Co., Inc., Tokyo, Japan) at room temperature for 10 min. After the reaction, the mixture was detected at 410 nm using the Cary 60 UV/Vis spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA, USA). Ammonia solution was used for the preparation of a standard curve. One unit of L-asparaginase activity was determined as the amount of enzyme that produced 1 μmol/mL of ammonia per 1 min under the assay conditions at 37 °C.
2.3. Hemolysis
The selected strains were evaluated for hemolysis according to a modified method described by Ritter et al. (2018) [21]. The culture of the selected strains incubated at 30 °C for 24 h was streaked onto tryptic soy agar (TSA; Bacto™; Becton, Dickinson and Co., Sparks, MD, USA) containing 5% sheep blood (Kisan Biotech Co., Ltd., Seoul, Korea) using a sterile cotton swab and incubated at 37 °C for 24 h. Bacillus cereus KCTC 1012 was used as a positive control for hemolysis.
2.4. β-Glucuronidase Activity Assay
β-glucuronidase activity was performed according to a modified method described by Shokryazdan et al. (2016) [22]. The selected strains were cultivated in NB for 24 h at 30 °C, and then centrifuged at 12,501× g for 5 min. The culture supernatant was used to provide crude enzyme samples. Briefly, 0.4 mL of 2 mM ρ-nitrophenyl-β-D-glucuronide (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), 0.1 mL of phosphate buffer (pH 7.0), and 0.2 mL of culture supernatant were incubated at 37 °C for 30 min. The reaction was stopped by adding 0.6 mL of 0.5 N NaOH. The β-glucuronidase activity of the supernatant was determined by measuring the absorbance at 405 nm using the Cary 60 UV/Vis spectrophotometer. ρ-Nitrophenol was used for the preparation of a standard curve. One unit of β-glucuronidase was determined as the amount of enzyme that can release 1 μmol/L ρ-nitrophenol per 1 min under the assay conditions at 37 °C. Escherichia coli KCTC 1682 producing β-glucuronidase was used as a positive control.
2.5. Antimicrobial Activity
The antimicrobial activity spectra of the selected strains were determined on the nine bacteria: E. coli KCTC 1682, Staphylococcus aureus KCTC 3881, Listeria monocytogenes KCTC 3710, and Listeria innocua KCTC 3586 were cultured on tryptic soy broth (TSB; Becton, Dickinson and Co., Sparks, MD, USA) and Shigella sonnei KCTC 2518, Shigella flexneri KCTC 22192, Klebsiella pneumoniae KCTC 2208, Pseudomonas aeruginosa KCTC 1750, and B. cereus KCTC 1012 were cultured on nutrient broth (NB; Becton, Dickinson and Co., Sparks, MD, USA) at 37 °C for 18 h. The target bacteria adjusted to OD600 = 0.1 to match 7 log CFU/mL were streaked onto the medium using sterile cotton swab. A 6 mm paper disc (Advantec, Toyo Roshi Kaisha Ltd., Tokyo, Japan) was placed on the medium on which inoculated target bacteria and 20 μL of each culture of the selected strains were spotted for 48 h. After 24 h incubation at 37 °C, the diameters of the inhibition zone were measured.
2.6. Identification of the Isolated Strain
Biochemical analysis, such as Gram staining, the endospore forming test, and the catalase test, was performed. The selected strain was identified by 16S rRNA gene sequence analysis performed by Solgent Co., Ltd. (Daejeon, Korea). The nucleotide sequence comparisons were conducted using the EzBioCloud database (https://www.ezbiocloud.net) (accessed on 1 October 2019). The selected strains were cultured in NB at 30 °C for 24 h and stored at −80 °C, with 40% sterile glycerol stock, until needed.
2.7. Tolerance to Artificial Gastric Acid and Bile Salt
Tolerance to artificial gastric acid and bile salt was measured according to the modified methods described by Lee et al. (2017) [17] and Lee et al. (2012) [23]. A 24 h culture of the selected strain was inoculated (10%) into 0.85% sterile saline solution containing 0.1% pepsin (pH 2.5) at 37 °C for 1, 2, and 3 h in a water bath (WB-22). Reaction solutions were spread onto NA plates and incubated at 37 °C for 24 h.
Bile salt solution was prepared by adding of 0.3% bile salt to buffer (12.4 g/L K2HPO4, 11.37 g/L trisodium citrate dehydrate, 7.6 g/L KH2PO4, and 6 g/L (NH4)2SO4, pH 6.9). A 24 h culture of the selected strain was inoculated (10%) into bile salt solution and incubated at 37 °C for 1, 2, and 3 h in a water bath (WB-22). The reaction solutions were spread onto NA plates and incubated at 37 °C for 24 h. The relative survival rate (RSR) was calculated as the percentage of Bacillus sp. colonies grown on the NA plate compared with the initial bacterial concentration.
2.8. Cell Hydrophobicity
Cell hydrophobicity was analyzed using a modified version of the method described by Nithya and Halami (2013) [24]. The selected strain was cultivated in TSB for 24 h at 30 °C, and then centrifuged at 9425× g for 5 min. Cells were washed twice and adjusted to OD600 of 0.5 with phosphate buffered saline (PBS; 8 g/L NaCl, 0.2 g/L KCl, 1.44 g/L Na2HPO4, and 0.24 g/L KH2PO4, pH 7.4). This absorbance was used as A0. A volume of 2 mL solvent, such as hexadecane (Daejung Chemicals and Metals Co., Ltd., Siheung, Korea), chloroform (Samchun Pure Chemical Co., Ltd., Pyeong-taek, Korea), and ethyl acetate (Duksan Pure Chemical Co., Ltd., Ansan, Korea), was added to 2 mL bacterial suspension and vortexed for 5 min. The mixtures were separated into two phases, and the solvents were removed after 30 min at room temperature. Its absorbance was measured at 600 nm and used as A1. The cell hydrophobicity percentage was calculated according to the formula:
A0 = absorbance before mixing with hydrocarbons; A1 = absorbance after mixing with hydrocarbons.
2.9. Autoaggregation and Coaggregation
Autoaggregation was assessed using the modified method described by Kang et al. (2017) [25] to confirm the adhesion ability of the cell to the intestinal wall. The selected strain was cultivated in TSB for 24 h at 30 °C and then centrifuged at 9425× g for 5 min. The cells were washed twice and adjusted to OD600 of 0.3 with PBS (pH 7.4). The suspension was left to stand at room temperature and monitored at different time intervals (4, 5, and 24 h). Then, the absorbance of the suspension was measured at 600 nm (At). The autoaggregation percentage was calculated according to the formula:
At = absorbance after 4, 5, and 24 h at 600 nm; A0 = absorbance of 0 h at 600 nm.
The coaggregation analysis was performed according to Bao et al. (2010) [26] and Jeon et al. (2017) [27]. In the coaggregation analysis, the method for preparing the bacterial suspension was the same as that for the autoaggregation assay. Then, 2 mL bacterial suspension of probiotic strain (selected strain) and 2 mL pathogenic strains were mixed. The mixtures were incubated at room temperature without agitation. The absorbance at 600 nm of the mixtures described above was monitored during incubation for 4, 5, and 24 h. The coaggregation was calculated according to the formula:
Apat, Aprobio = the absorbance of the pathogen and the probiotic strain at 0 h, respectively; Amix = the absorbance of the mixed culture at different times.
2.10. Statistical Analysis
Data are expressed as mean ± standard deviation of triplicates. A one-way ANOVA and Tukey’s test were performed to evaluate significant difference (p < 0.05) using Minitab 16.0 software (Minitab Inc., State College, PA, USA).
3. Results and Discussion
3.1. Isolation of Strains Producing L-Asparaginase and Free of L-Glutaminase Activity
A total of 268 Bacillus strains from 32 Korean fermented soybean foods survived from acid and bile salt solution. To screen the strains that produce L-asparaginase and those that are free of L-glutaminase, these strains were allowed to grow in the MCD medium containing L-asparagine or L-glutamine, respectively, as a sole nitrogen source. The pink zone around the bacterial colony indicates pH alteration, which originated from ammonia accumulation in the MCD medium. The 162 strains showed the presence of a pink colony in the MCD medium containing L-asparagine, which exhibits L-asparaginase activity. These strains showed no color change in the MCD medium containing L-glutamine, implying the absence of L-glutaminase activity.
3.2. L-Asparaginase Activity
The 14 strains with L-asparaginase activity above 0.60 U/mL were selected. Among the strains, MKHJ 1-1 and 2-2 showed significantly higher L-asparaginase activity than other strains (Table 1, p < 0.05). Asparaginase therapy is used worldwide, but some problems arise when this enzyme is used for the treatment of leukemia and other malignant tumors. This is caused by the intrinsic L-glutaminase activity of L-asparaginase [28]. Therefore, MKHJ1-1 and 2-2 with high L-asparaginase activity and without L-glutaminase activity seems to be suitable for use as a therapeutic agent for L-asparaginase.

Table 1.
L-asparaginase and L-glutaminase activity of selected strains.
3.3. Hemolysis and β-Glucuronidase Activity
MKHJ 1-1, 1-2, 2-2, 13-2, 17-1, and 19-2 strains were found to exhibit γ-hemolysis (Table 2). Hemolytic properties can be divided into α-hemolysis (which oxidizes hemoglobin to methemoglobin without destroying the erythrocyte membrane), β-hemolysis (which destroys the erythrocyte membrane, causing jaundice and anemia), and γ-hemolysis (without hemolysis). α-Hemolysis causes the incomplete lysis of erythrocytes, resulting in a green-hued zone around the bacterial colonies, and β- hemolysis causes the complete lysis of erythrocytes, resulting in a clear zone around the bacterial colonies [29]. β-Hemolysis is considered harmful, whereas a-hemolysis and γ-hemolysis are considered safe [27].

Table 2.
Hemolysis and β-glucuronidase activity of selected strains.
Probiotics should not produce harmful enzymes such as β-glucuronidase, which is the enzyme that causes colorectal cancer. β-glucuronidase catalyzes the hydrolysis the ρ-nitrophenyl-β-D-glucuronide to the β-glucuronic acid and nitrophenol and it produces a yellow color [30]. In this study, β-glucuronidase enzyme activity was measured quantitatively, using ρ-nitrophenyl-β-D-glucuronide as a substrate, after by visually confirming color change. When observed visually, all the selected strains, except E. coli, used as a positive control, were colorless. The β-glucuronidase activity of the selected strains was significantly lower than that of E. coli (0.81 ± 0.16 U/mL). In the hemolysis and β-glucuronidase activity results, only 6 (MKHJ 1-1, 1-2, 2-2, 13-2, 17-1, and 19-2) among the 14 strains were verified as safe.
3.4. Antimicrobial Activity
Table 3 presents the antimicrobial activity of the selected strains with L-asparaginase and free of L-glutaminase activity. Bacillus MKHJ 1-1, 13-2, and 19-2 showed broad antimicrobial spectra against target bacteria. Among the selected strains, in contrast, 2-2 did not have antimicrobial activity. Some Bacillus spp. are known to play an important role that involves the inhibition of pathogens in the intestinal tract by producing enzymes that break down cell walls (such as chitinase, glucanase, and protease), lipopeptide, biosurfactants, and bacteriocin. Among them, the lipopeptides produced by Bacillus spp. include surfactin, iturin, and fengycin [31]. Iturin is only detected in restricted species, such as B. subtilis, B. amyloliquefaciens, and B. pumilus, whereas surfactin and fengycin are found in most Bacillus spp. [32]. The antimicrobial activity of the selected strains may be due to the interaction of the protease, lipopeptide, and bacteriocin. When the harmful intestinal bacteria increase, MKHJ 1-1, 13-2, and 19-2 can be expected to stabilize the intestinal flora and inhibit the harmful bacteria. Based on the results of L-asparaginase, hemolysis, β-glucuronidase, and antimicrobial activity, MKHJ 1-1 were finally selected.

Table 3.
Antimicrobial activity of Bacillus strains against pathogenic bacteria.
3.5. Identification of the Isolated Strain
MKHJ 1-1 was Gram-positive, rod shaped, endospore forming, and catalase positive (data not shown). 16S rRNA sequencing of MKHJ 1-1 revealed 99.84% identity to Bacillus subtilis subsp. stercoris D7XPN1 (data not shown). MKHJ 1-1 was named Bacillus subtilis MKHJ 1-1.
3.6. Tolerance to Artificial Gastric Acid and Bile Salt
In order for the probiotic strains to function in the intestine, they must survive during both ingestion and in the harsh environments of the gastrointestinal tract, including the acidic condition of the stomach and bile salts [24]. As shown in Table 4, MKHJ 1-1 showed ≥85% survivability at pH 2.5. It was found to have an excellent survival rate of 97.45% in artificial gastric acid after 3 h. Bile tolerance studies were performed using 0.3% bile salt, a concentration similar to that of human bile juice. MKHJ 1-1 had 42.39% tolerance in the presence of 0.3% bile salt. MKHJ 1-1 had higher acid resistance compared to B. polyfermenticus KU3 and B. polyfermenticus SCD (RSR of 57.25% and 64.83% after 2 h, respectively, at pH 2.5) reported by Lee et al. (2015) [33]. At the same time, it exhibits resistance to bile salt. Thus, MKHJ 1-1 is expected to have adequate stability as a probiotic.

Table 4.
Relative survival rate (RSR) of MKHJ 1-1.
3.7. Cell Hydrophobicity
Adhesion to hexadecane (nonpolar solvent) indicates the hydrophobic/hydrophilic surface properties of bacteria. Affinity for chloroform (polar acid solvent) and ethyl acetate (polar basic solvent) accounts for the electron donor and electron acceptor properties of the bacterial cell surface, respectively [34]. MKHJ 1-1 presented hydrophobicity values of 31.70% for hexadecane and higher affinity for basic solvents, such as ethyl acetate, than for acidic solvents, such as chloroform (Figure 1). These results indicate that MKHJ 1-1 has strong electron acceptors and weak electron donors. Many studies on the physical chemistry of microbial cell surfaces have shown that the presence of (glycol) protein material on the cell surface increases hydrophobicity, whereas the presence of polysaccharides is associated with hydrophilicity [35]. Therefore, it is assumed that the selected strain was composed of polysaccharides rather than protein material. However, according to Thirabunyanon and Thongwittaya’s (2012) [36] report, Bacillus strains with 17% to 57% hydrophobicity in hexadecane were 2.8 to 4.9 log CFU/well attached in Caco-2 cells. It is expected that MKHJ 1-1 can attach to intestinal epithelial cells during ingestion.
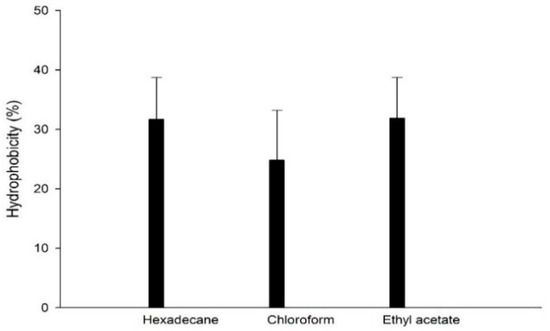
Figure 1.
Cell hydrophobicity of MKHJ 1-1 against various solvents.
3.8. Autoaggregation and Coaggregation
In most cases, aggregation ability is associated with cell adhesion properties. Bacterial aggregation between microorganisms of the same strain (autoaggregation) or genetically different strains (coaggregation) is of great importance in the human gut [26].
MKHJ 1-1 showed autoaggregation ability with 91.32 ± 0.74% after 24 h (Table 5). The autoaggregation percentages of B. clausii ATCC 700160 and B. subtilis P223 were reported to be 93.42 ± 0.86% and 86.03 ± 2.46% after 24 h incubation, respectively [27]. MKHJ 1-1 was found to have outstanding autoaggregation ability compared to previously reported Bacillus strains. A strain with high autoaggregation ability is known to have high cell adhesion [35]. Thus, MKHJ 1-1 is expected to have high cell adhesion.

Table 5.
Autoaggregation and coaggregation ability of MKHJ 1-1.
Coaggregation ability was tested using E. coli KCTC 1682 and S. aureus KCTC 3881. After 24 h, coaggregation of MKHJ 1-1 (61.72%) was similar to the results of Jeon’s study (2017) [27], in which the coaggregation percentages of B. subtilis P223 with S. aureus ATCC 6538 and E. coli ATCC 25922 were 68.96% and 70.19%, respectively. Coaggregation with a potential pathogen has demonstrated that probiotic strain can inhibit the growth of pathogens in the gastrointestinal and urogenital tracts by producing antimicrobial substances [37].
Overall, MKHJ 1-1 exhibited desirable autoaggregation and coaggregation ability as a potential probiotic strain.
4. Conclusions
In conclusion, MKHJ 1-1, which is isolated from Gochujang, produces L-asparaginase and is free of L-glutaminase. It also possesses probiotic characteristics, such as excellent resistance to acid and bile salt, nonhemolysis, non-β-glucuronidase activity, broad antimicrobial spectra, and aggregation ability. It is assumed that MKHJ 1-1 can be used as a probiotic and a potential resource for the production of L-asparaginase without the presence of L-glutaminase activity in the food industry.
Author Contributions
Formal analysis, data curation, and writing—original draft, H.L.; investigation and editing, S.O.; funding acquisition and writing—review and editing, S.Y.; conceptualization, supervision, and writing—review and editing, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This article is a part of Hyeji Lim’s master’s thesis at the Department of Food Science and Nutrition, Dankook University, Korea, under the supervision of Misook Kim.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO/WHO. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- Patel, A.K.; Ahire, J.J.; Pawar, S.P.; Chaudhari, B.L.; Chincholkar, S.B. Comparative accounts of probiotic characteristics of Bacillus spp. isolated from food wastes. Food Res. Int. 2009, 42, 505–510. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Urdaci, M.C.; Bressollier, P.; Pinchuk, I. Bacillus clausii probiotic strains antimicrobial and immunomodulatory activities. J. Clin. Gastroenterol. 2004, 38, S86–S90. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Racedo, S.M.; Ripert, G.; Housez, B.; Cazaubiel, M.; Maudet, C.; Jüsten, P.; Marteau, P.; Urdaci, M.C. Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: A randomized, double-blind placebo-controlled study. Immun. Ageing 2015, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Varin, T.V.; Anhê, F.F.; Dubé, P.; Roy, D.; Pilon, G.; Marette, A.; Levy, É.; Jacquot, C.; Urdaci, M.; et al. Modulatory effects of a cranberry extract co-supplementation with Bacillus subtilis CU1 probiotic on phenolic compounds bioavailability and gut microbiota composition in high-fat diet-fed mice. PharmaNutrition 2015, 3, 89–100. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhang, L.; Liu, W.; Zhang, Y.; Zhang, X.; Sun, T. In vitro assessment of probiotic properties of Bacillus isolated from naturally fermented congee from Inner Mongolia of China. World J. Microbiol. Biotechnol. 2010, 26, 1369–1377. [Google Scholar] [CrossRef]
- Suva, M.; Sureja, V.; Kheni, D. Novel insight on probiotic Bacillus subtilis: Mechanism of action and clinical applications. J. Curr. Res. Sci. Med. 2016, 2, 65. [Google Scholar] [CrossRef]
- Mihooliya, K.N.; Nandal, J.; Swami, L.; Verma, H.; Chopra, L.; Sahoo, D.K. A new pH indicator dye-based method for rapid and efficient screening of l -asparaginase producing microorganisms. Enzym. Microb. Technol. 2017, 107, 72–81. [Google Scholar] [CrossRef]
- Sharma, A.; Husain, I. Evaluation of Antitumor Activity of Glutaminase-Free Periplasmic Asparaginase from Indigenous Bacterial Isolates as Candidates for Cancer Therapy. In Proceedings of the National Academy of Sciences, India Section B: Biological Sciences; J.B. Metzler: Stuttgart, Germany, 2015; Volume 87, pp. 997–1004. [Google Scholar]
- Lorenzi, P.L.; Reinhold, W.C.; Rudelius, M.; Gunsior, M.; Shankavaram, U.; Bussey, K.J.; Scherf, U.; Eichler, G.S.; Martin, S.E.; Chin, K.; et al. Asparagine synthetase as a causal, predictive biomarker for l-asparaginase activity in ovarian cancer cells. Mol. Cancer 2006, 5, 2613–2623. [Google Scholar] [CrossRef]
- Shin, S.R. Comparative Analysis of Antitumor Effect Using Tumor-Targeting Salmonella and L-Asparaginase with Different AC-Tivity; Chonnam National University: Gwangju, Korea, 2014. [Google Scholar]
- Aishwarya, S.S.; Iyappan, S.; Lakshmi, K.V.; Rajnish, K.N. In silico analysis, molecular cloning, expression and characterization of l-asparaginase gene from Lactobacillus reuteri DSM 20016. 3Biotech 2017, 7. [Google Scholar] [CrossRef]
- Derst, C.; Henseling, J.; Röhm, K.-H. Engineering the substrate specificity of Escherichia coli asparaginase. II. Selective reduction of glutaminase activity by amino acid replacements at position 248. Protein Sci. 2000, 9, 2009–2017. [Google Scholar] [CrossRef]
- Kravchenko, O.V.; Kislitsin, Y.A.; Popov, A.N.; Nikonov, S.V.; Kuranova, I.P. Three-dimensional structures of L-asparaginase from Erwinia carotovora complexed with aspartate and glutamate. Acta Cryst. Sect. D 2008, 64, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; Alshehri, W.A.; Al-Enazi, N.M.; Almansob, A. L-Asparaginase activity analysis, ansZ gene identification and an-ticancer activity of a new Bacillus subtilis isolated from sponges of the Red Sea. Biosci. Biotechnol. Biochem. 2020, 84, 2576–2584. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Jin, Y.-I.; Jeong, J.-C.; Chang, Y.H.; Lee, Y.; Jeong, Y.; Kim, M. Probiotic characteristics of Bacillus strains isolated from Korean traditional soy sauce. LWT 2017, 79, 518–524. [Google Scholar] [CrossRef]
- Gulati, R.; Saxena, R.K.; Gupta, R. A rapid plate assay for screening L-asparaginase producing micro-organisms. Lett. Appl. Microbiol. 1997, 24, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Worthington, V. Worthington Enzyme Manual: Enzymes and Related Biochemicals; Worthington Biochemical Corp: Lakewood, NJ, USA, 1993; p. 399. [Google Scholar]
- Park, S.Y. Production of Thermostable L-Asparaginase from Bacillus methylotrophicus MKSY2013; Dankook University: Yongin, Korea, 2015. [Google Scholar]
- Ritter, A.C.; Correa, A.P.F.; Veras, F.F.; Brandelli, A. Characterization of Bacillus subtilis available as probiotics. J. Microbiol. Res. 2018, 8, 23–32. [Google Scholar]
- Shokryazdan, P.; Jahromi, M.F.; Liang, J.B.; Kalavathy, R.; Sieo, C.C.; Ho, Y.W. Safety Assessment of Two New Lactobacillus Strains as Probiotic for Human Using a Rat Model. PLoS ONE 2016, 11, e0159851. [Google Scholar] [CrossRef]
- Lee, J.; Park, I.; Choi, Y.; Cho, J. Bacillus strains as feed additives: In vitro evaluation of its potential probiotic properties. Rev. Colomb. Cienc. Pecu. 2012, 25, 577–585. [Google Scholar]
- Nithya, V.; Halami, P.M. Evaluation of the probiotic characteristics of Bacillus species isolated from different food sources. Ann. Microbiol. 2012, 63, 129–137. [Google Scholar] [CrossRef]
- Kang, C.-H.; Han, S.H.; Kim, Y.; Jeong, Y.; Paek, N.-S. Antibacterial Activity and Probiotic Properties of Lactic Acid Bacteria Isolated from Traditional Fermented Foods. KSBB J. 2017, 32, 199–205. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, Y.; Zhang, Y.; Liu, Y.; Wang, S.; Dong, X.; Wang, Y.; Zhang, H. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control 2010, 21, 695–701. [Google Scholar] [CrossRef]
- Jeon, H.-L.; Lee, N.-K.; Yang, S.-J.; Kim, W.-S.; Paik, H.-D. Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci. Biotechnol. 2017, 26, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.V.; Saran, S.; Kameswaran, K.; Kumar, V.; Saxena, R.K. Efficient production of L-asparaginase from Bacillus li-cheniformis with low-glutaminase activity: Optimization, scale up and acrylamide degradation studies. Bioresour. Technol. 2012, 125, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Park, S.K.; Kim, B.G.; Ryu, D.G.; Lim, E.S.; Kim, Y.M. Isolation and characterization of cholesterol-lowering lactic acid bacteria from kimchi. Korean J. Food Sci. Technol. 2017, 49, 377–382. [Google Scholar]
- Delisle, G.J.; Ley, A. Rapid detection of Escherichia coli in urine samples by a new chromogenic β-glucuronidase assay. J. Clin. Microbiol. 1989, 27, 778–779. [Google Scholar] [CrossRef]
- Pérez-García, A.; Romero, D.; de Vicente, A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Lee, N.-K.; Son, S.-H.; Jeon, E.B.; Jung, G.H.; Lee, J.-Y.; Paik, H.-D. The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J. Funct. Foods 2015, 14, 513–518. [Google Scholar] [CrossRef]
- Xu, H.; Jeong, H.S.; Lee, H.Y.; Ahn, J. Assessment of cell surface properties and adhesion potential of selected probiotic strains. Lett. Appl. Microbiol. 2009, 49, 434–442. [Google Scholar] [CrossRef]
- Kos, B.; Šušković, J.; Vuković, S.; Šimpraga, M.; Frece, J.; Matošić, S. Adhesion and aggregation ability of probiotic strain Lac-tobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef]
- Thirabunyanon, M.; Thongwittaya, N. Protection activity of a novel probiotic strain of Bacillus subtilis against Salmonella En-teritidis infection. Res. Vet. Sci. 2012, 93, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Botes, M.; Loos, B.; van Reenen, C.A.; Dicks, L.M.T. Adhesion of the probiotic strains Enterococcus mundtii ST4SA and Lac-tobacillus plantarum 423 to Caco-2 cells under conditions simulating the intestinal tract, and in the presence of antibiotics and anti-inflammatory medicaments. Arch. Microbiol. 2008, 190, 573–584. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).