Isolation and Characterization of Probiotic Bacillus subtilis MKHJ 1-1 Possessing L-Asparaginase Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Strains Producing L-Asparaginase and Free of L-Glutaminase Activity
2.2. L-Asparaginase Activity
2.3. Hemolysis
2.4. β-Glucuronidase Activity Assay
2.5. Antimicrobial Activity
2.6. Identification of the Isolated Strain
2.7. Tolerance to Artificial Gastric Acid and Bile Salt
2.8. Cell Hydrophobicity
2.9. Autoaggregation and Coaggregation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Isolation of Strains Producing L-Asparaginase and Free of L-Glutaminase Activity
3.2. L-Asparaginase Activity
3.3. Hemolysis and β-Glucuronidase Activity
3.4. Antimicrobial Activity
3.5. Identification of the Isolated Strain
3.6. Tolerance to Artificial Gastric Acid and Bile Salt
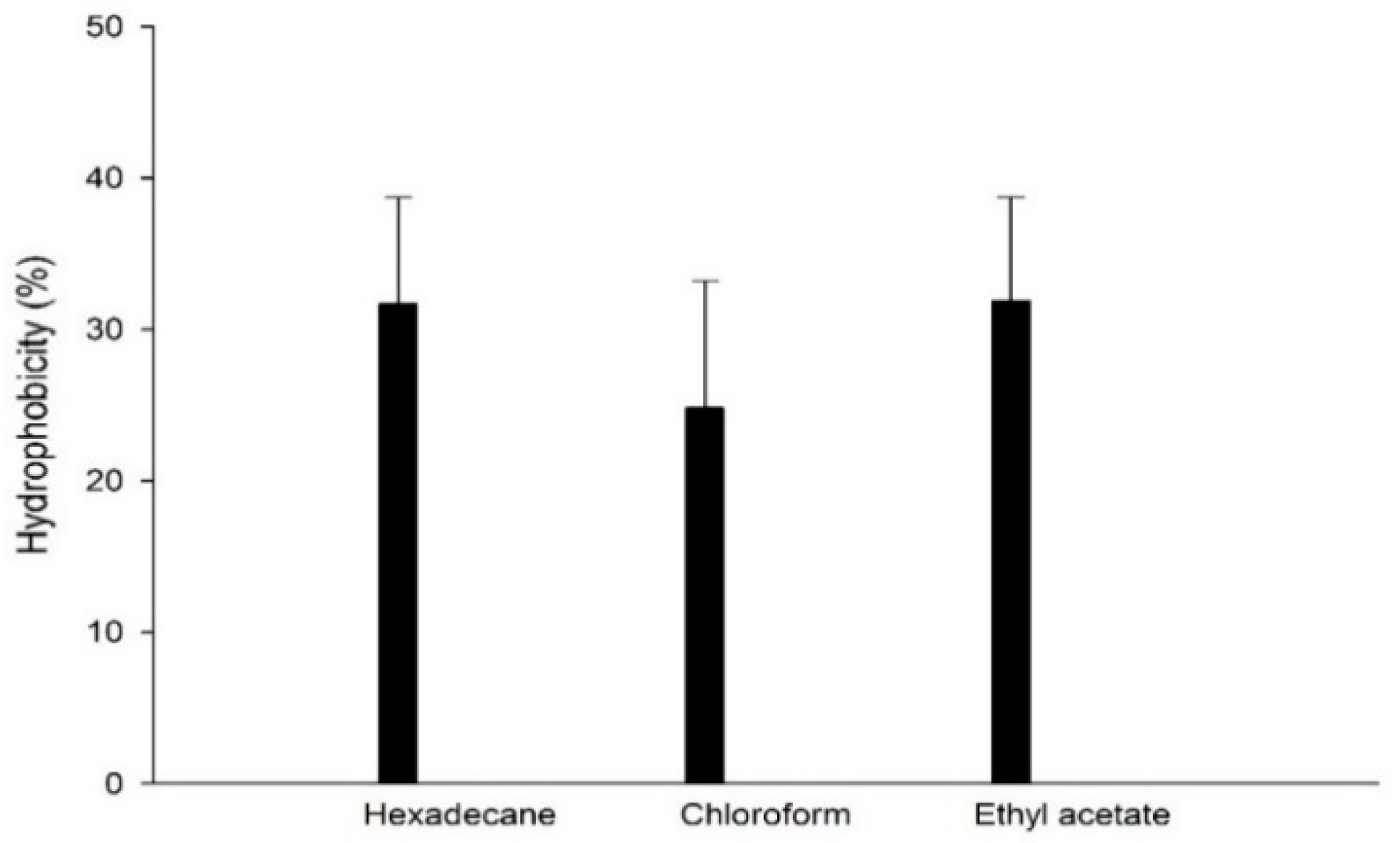
3.7. Cell Hydrophobicity
3.8. Autoaggregation and Coaggregation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- Patel, A.K.; Ahire, J.J.; Pawar, S.P.; Chaudhari, B.L.; Chincholkar, S.B. Comparative accounts of probiotic characteristics of Bacillus spp. isolated from food wastes. Food Res. Int. 2009, 42, 505–510. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Urdaci, M.C.; Bressollier, P.; Pinchuk, I. Bacillus clausii probiotic strains antimicrobial and immunomodulatory activities. J. Clin. Gastroenterol. 2004, 38, S86–S90. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Racedo, S.M.; Ripert, G.; Housez, B.; Cazaubiel, M.; Maudet, C.; Jüsten, P.; Marteau, P.; Urdaci, M.C. Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: A randomized, double-blind placebo-controlled study. Immun. Ageing 2015, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Varin, T.V.; Anhê, F.F.; Dubé, P.; Roy, D.; Pilon, G.; Marette, A.; Levy, É.; Jacquot, C.; Urdaci, M.; et al. Modulatory effects of a cranberry extract co-supplementation with Bacillus subtilis CU1 probiotic on phenolic compounds bioavailability and gut microbiota composition in high-fat diet-fed mice. PharmaNutrition 2015, 3, 89–100. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhang, L.; Liu, W.; Zhang, Y.; Zhang, X.; Sun, T. In vitro assessment of probiotic properties of Bacillus isolated from naturally fermented congee from Inner Mongolia of China. World J. Microbiol. Biotechnol. 2010, 26, 1369–1377. [Google Scholar] [CrossRef]
- Suva, M.; Sureja, V.; Kheni, D. Novel insight on probiotic Bacillus subtilis: Mechanism of action and clinical applications. J. Curr. Res. Sci. Med. 2016, 2, 65. [Google Scholar] [CrossRef]
- Mihooliya, K.N.; Nandal, J.; Swami, L.; Verma, H.; Chopra, L.; Sahoo, D.K. A new pH indicator dye-based method for rapid and efficient screening of l -asparaginase producing microorganisms. Enzym. Microb. Technol. 2017, 107, 72–81. [Google Scholar] [CrossRef]
- Sharma, A.; Husain, I. Evaluation of Antitumor Activity of Glutaminase-Free Periplasmic Asparaginase from Indigenous Bacterial Isolates as Candidates for Cancer Therapy. In Proceedings of the National Academy of Sciences, India Section B: Biological Sciences; J.B. Metzler: Stuttgart, Germany, 2015; Volume 87, pp. 997–1004. [Google Scholar]
- Lorenzi, P.L.; Reinhold, W.C.; Rudelius, M.; Gunsior, M.; Shankavaram, U.; Bussey, K.J.; Scherf, U.; Eichler, G.S.; Martin, S.E.; Chin, K.; et al. Asparagine synthetase as a causal, predictive biomarker for l-asparaginase activity in ovarian cancer cells. Mol. Cancer 2006, 5, 2613–2623. [Google Scholar] [CrossRef]
- Shin, S.R. Comparative Analysis of Antitumor Effect Using Tumor-Targeting Salmonella and L-Asparaginase with Different AC-Tivity; Chonnam National University: Gwangju, Korea, 2014. [Google Scholar]
- Aishwarya, S.S.; Iyappan, S.; Lakshmi, K.V.; Rajnish, K.N. In silico analysis, molecular cloning, expression and characterization of l-asparaginase gene from Lactobacillus reuteri DSM 20016. 3Biotech 2017, 7. [Google Scholar] [CrossRef]
- Derst, C.; Henseling, J.; Röhm, K.-H. Engineering the substrate specificity of Escherichia coli asparaginase. II. Selective reduction of glutaminase activity by amino acid replacements at position 248. Protein Sci. 2000, 9, 2009–2017. [Google Scholar] [CrossRef]
- Kravchenko, O.V.; Kislitsin, Y.A.; Popov, A.N.; Nikonov, S.V.; Kuranova, I.P. Three-dimensional structures of L-asparaginase from Erwinia carotovora complexed with aspartate and glutamate. Acta Cryst. Sect. D 2008, 64, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; Alshehri, W.A.; Al-Enazi, N.M.; Almansob, A. L-Asparaginase activity analysis, ansZ gene identification and an-ticancer activity of a new Bacillus subtilis isolated from sponges of the Red Sea. Biosci. Biotechnol. Biochem. 2020, 84, 2576–2584. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Jin, Y.-I.; Jeong, J.-C.; Chang, Y.H.; Lee, Y.; Jeong, Y.; Kim, M. Probiotic characteristics of Bacillus strains isolated from Korean traditional soy sauce. LWT 2017, 79, 518–524. [Google Scholar] [CrossRef]
- Gulati, R.; Saxena, R.K.; Gupta, R. A rapid plate assay for screening L-asparaginase producing micro-organisms. Lett. Appl. Microbiol. 1997, 24, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Worthington, V. Worthington Enzyme Manual: Enzymes and Related Biochemicals; Worthington Biochemical Corp: Lakewood, NJ, USA, 1993; p. 399. [Google Scholar]
- Park, S.Y. Production of Thermostable L-Asparaginase from Bacillus methylotrophicus MKSY2013; Dankook University: Yongin, Korea, 2015. [Google Scholar]
- Ritter, A.C.; Correa, A.P.F.; Veras, F.F.; Brandelli, A. Characterization of Bacillus subtilis available as probiotics. J. Microbiol. Res. 2018, 8, 23–32. [Google Scholar]
- Shokryazdan, P.; Jahromi, M.F.; Liang, J.B.; Kalavathy, R.; Sieo, C.C.; Ho, Y.W. Safety Assessment of Two New Lactobacillus Strains as Probiotic for Human Using a Rat Model. PLoS ONE 2016, 11, e0159851. [Google Scholar] [CrossRef]
- Lee, J.; Park, I.; Choi, Y.; Cho, J. Bacillus strains as feed additives: In vitro evaluation of its potential probiotic properties. Rev. Colomb. Cienc. Pecu. 2012, 25, 577–585. [Google Scholar]
- Nithya, V.; Halami, P.M. Evaluation of the probiotic characteristics of Bacillus species isolated from different food sources. Ann. Microbiol. 2012, 63, 129–137. [Google Scholar] [CrossRef]
- Kang, C.-H.; Han, S.H.; Kim, Y.; Jeong, Y.; Paek, N.-S. Antibacterial Activity and Probiotic Properties of Lactic Acid Bacteria Isolated from Traditional Fermented Foods. KSBB J. 2017, 32, 199–205. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, Y.; Zhang, Y.; Liu, Y.; Wang, S.; Dong, X.; Wang, Y.; Zhang, H. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control 2010, 21, 695–701. [Google Scholar] [CrossRef]
- Jeon, H.-L.; Lee, N.-K.; Yang, S.-J.; Kim, W.-S.; Paik, H.-D. Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci. Biotechnol. 2017, 26, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.V.; Saran, S.; Kameswaran, K.; Kumar, V.; Saxena, R.K. Efficient production of L-asparaginase from Bacillus li-cheniformis with low-glutaminase activity: Optimization, scale up and acrylamide degradation studies. Bioresour. Technol. 2012, 125, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Park, S.K.; Kim, B.G.; Ryu, D.G.; Lim, E.S.; Kim, Y.M. Isolation and characterization of cholesterol-lowering lactic acid bacteria from kimchi. Korean J. Food Sci. Technol. 2017, 49, 377–382. [Google Scholar]
- Delisle, G.J.; Ley, A. Rapid detection of Escherichia coli in urine samples by a new chromogenic β-glucuronidase assay. J. Clin. Microbiol. 1989, 27, 778–779. [Google Scholar] [CrossRef]
- Pérez-García, A.; Romero, D.; de Vicente, A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Lee, N.-K.; Son, S.-H.; Jeon, E.B.; Jung, G.H.; Lee, J.-Y.; Paik, H.-D. The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J. Funct. Foods 2015, 14, 513–518. [Google Scholar] [CrossRef]
- Xu, H.; Jeong, H.S.; Lee, H.Y.; Ahn, J. Assessment of cell surface properties and adhesion potential of selected probiotic strains. Lett. Appl. Microbiol. 2009, 49, 434–442. [Google Scholar] [CrossRef]
- Kos, B.; Šušković, J.; Vuković, S.; Šimpraga, M.; Frece, J.; Matošić, S. Adhesion and aggregation ability of probiotic strain Lac-tobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef]
- Thirabunyanon, M.; Thongwittaya, N. Protection activity of a novel probiotic strain of Bacillus subtilis against Salmonella En-teritidis infection. Res. Vet. Sci. 2012, 93, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Botes, M.; Loos, B.; van Reenen, C.A.; Dicks, L.M.T. Adhesion of the probiotic strains Enterococcus mundtii ST4SA and Lac-tobacillus plantarum 423 to Caco-2 cells under conditions simulating the intestinal tract, and in the presence of antibiotics and anti-inflammatory medicaments. Arch. Microbiol. 2008, 190, 573–584. [Google Scholar] [CrossRef] [PubMed]
| Strains | Kinds of Fermented Food | L-Asparaginase Activity (U/mL) | L-Glutaminase Activity (U/mL) |
|---|---|---|---|
| MKHJ 1-1 | Gochujang | 1.20 ± 0.03 a* | 0 |
| 1-2 | Gochujang | 0.81 ± 0.02 c | 0 |
| 2-2 | Gochujang | 1.11 ± 0.06 a | 0 |
| 2-3 | Gochujang | 0.87 ± 0.05 bc | 0 |
| 4-2 | Soy sauce | 0.91 ± 0.05 bc | 0 |
| 4-3 | Soy sauce | 0.94 ± 0.05 bc | 0 |
| 8-1 | Doenjang | 0.96 ± 0.02 b | 0 |
| 8-4 | Doenjang | 0.91 ± 0.00 bc | 0 |
| 8-5 | Doenjang | 0.97 ± 0.06 b | 0 |
| 12-8 | Doenjang | 0.97 ± 0.03 b | 0 |
| 13-2 | Doenjang | 0.67 ± 0.02 d | 0 |
| 17-1 | Doenjang | 0.90 ± 0.10 bc | 0 |
| 19-2 | Doenjang | 0.89 ± 0.03 bc | 0 |
| 25-7 | Soy sauce | 0.96 ± 0.04 b | 0 |
| Strains | Hemolysis | β-Glucuronidase Activity | |
|---|---|---|---|
| β-Glucuronidase Activity (U/mL) 4 | Visible Color | ||
| MKHJ 1-1 | γ-hemolysis | 0.25 ± 0.02 | Colorless |
| 1-2 | γ-hemolysis | 0.25 ± 0.16 | Colorless |
| 2-2 | γ-hemolysis | 0.25 ± 0.05 | Colorless |
| 2-3 | β-hemolysis | 0.43 ± 0.13 | Colorless |
| 4-2 | β-hemolysis | 0.33 ± 0.05 | Colorless |
| 4-3 | β-hemolysis | 0.24 ± 0.05 | Colorless |
| 8-1 | β-hemolysis | 0.31 ± 0.20 | Colorless |
| 8-4 | β-hemolysis | 0.24 ± 0.04 | Colorless |
| 8-5 | β-hemolysis | 0.36 ± 0.16 | Colorless |
| 12-8 | β-hemolysis | 0.29 ± 0.14 | Colorless |
| 13-2 | γ-hemolysis | 0.31 ± 0.11 | Colorless |
| 17-1 | γ-hemolysis | 0.11 ± 0.11 | Colorless |
| 19-2 | γ-hemolysis | 0.36 ± 0.06 | Colorless |
| 25-7 | β-hemolysis | 0.21 ± 0.13 | Colorless |
| B. cereus1 | β-hemolysis | ND | ND |
| E. coli2 | ND 3 | 0.81 ± 0.16 5 | Yellow |
| Pathogens | Antimicrobial Activity (mm) | |||||
|---|---|---|---|---|---|---|
| MKHJ 1-1 | 1-2 | 2-2 | 13-2 | 17-1 | 19-2 | |
| E. coli (KCTC 1682) | 13.17 ± 0.76 | 0 | 0 | 0 | 0 | 14.37 ± 0.58 |
| P. aeruginosa (KCTC 2513) | 16.50 ± 1.41 | 0 | 0 | 16.00 ± 1.41 | 0 | 17.17 ± 1.04 |
| S. sonnei (KCTC 2518) | 20.50 ± 1.06 | 0 | 0 | 21.83 ± 0.76 | 0 | 20.83 ± 1.26 |
| S. flexneri (KCTC 22192) | 17.00 ± 1.00 | 0 | 0 | 15.80 ± 1.39 | 0 | 17.50 ± 0.50 |
| K. pneumoniae (KCTC 2242) | 16.47 ± 0.45 | 0 | 0 | 15.87 ± 0.32 | 0 | 17.33 ± 1.53 |
| S. aureus (KCTC 3881) | 13.00 ± 2.83 | 18.50 ± 2.12 | 0 | 16.00 ± 2.83 | 0 | 0 |
| B. cereus (KCTC 3624) | 11.67 ± 0.58 | 0 | 0 | 0 | 0 | 13.75 ± 0.35 |
| L. monocytogenes (KCTC 3710) | 0 | 0 | 0 | 0 | 0 | 0 |
| L. innocua (KCTC 3586) | 0 | 20.00 ± 0.00 | 0 | 17.50 ± 1.41 | 18.25 ± 1.06 | 0 |
| Treatment | RSR (%) | |
|---|---|---|
| MKHJ 1-1 | ||
| 0.1% pepsin | 1 h | 89.88 ± 3.41 b* |
| 2 h | 94.77 ± 1.28 b | |
| 3 h | 97.45 ± 0.46 a | |
| 0.3% bile salt | 1 h | 42.33 ± 1.72 a |
| 2 h | 41.63 ± 1.90 a | |
| 3 h | 42.39 ± 2.05 a | |
| Microorganisms | 4 h | 5 h | 24 h | |
|---|---|---|---|---|
| Autoaggregation (%) | ||||
| MKHJ 1-1 | 60.60 ± 0.16 b,1 | 63.03 ± 3.71 b | 91.32 ± 0.74 a | |
| Coaggregation (%) | ||||
| MKHJ 1-1 with | S. aureus KCTC 3881 | 22.28 ± 1.45 bc | 24.75 ± 1.45 b | 60.49 ± 2.99 a |
| E. coli KCTC 1682 | 16.24 ± 0.81 b | 18.74 ± 1.48 b | 61.72 ± 1.83 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.; Oh, S.; Yu, S.; Kim, M. Isolation and Characterization of Probiotic Bacillus subtilis MKHJ 1-1 Possessing L-Asparaginase Activity. Appl. Sci. 2021, 11, 4466. https://doi.org/10.3390/app11104466
Lim H, Oh S, Yu S, Kim M. Isolation and Characterization of Probiotic Bacillus subtilis MKHJ 1-1 Possessing L-Asparaginase Activity. Applied Sciences. 2021; 11(10):4466. https://doi.org/10.3390/app11104466
Chicago/Turabian StyleLim, Hyeji, Sujin Oh, Sungryul Yu, and Misook Kim. 2021. "Isolation and Characterization of Probiotic Bacillus subtilis MKHJ 1-1 Possessing L-Asparaginase Activity" Applied Sciences 11, no. 10: 4466. https://doi.org/10.3390/app11104466
APA StyleLim, H., Oh, S., Yu, S., & Kim, M. (2021). Isolation and Characterization of Probiotic Bacillus subtilis MKHJ 1-1 Possessing L-Asparaginase Activity. Applied Sciences, 11(10), 4466. https://doi.org/10.3390/app11104466






