Optimization and Validation of the GC/FID Method for the Quantification of Fatty Acids in Bee Products
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solvents and Reagents
2.3. Extraction of Fats from Bee Products
2.4. Quantitative and Qualitative Analyses
2.5. GC/FID Method Validation
2.6. Statistical Analysis
3. Results and Discussion
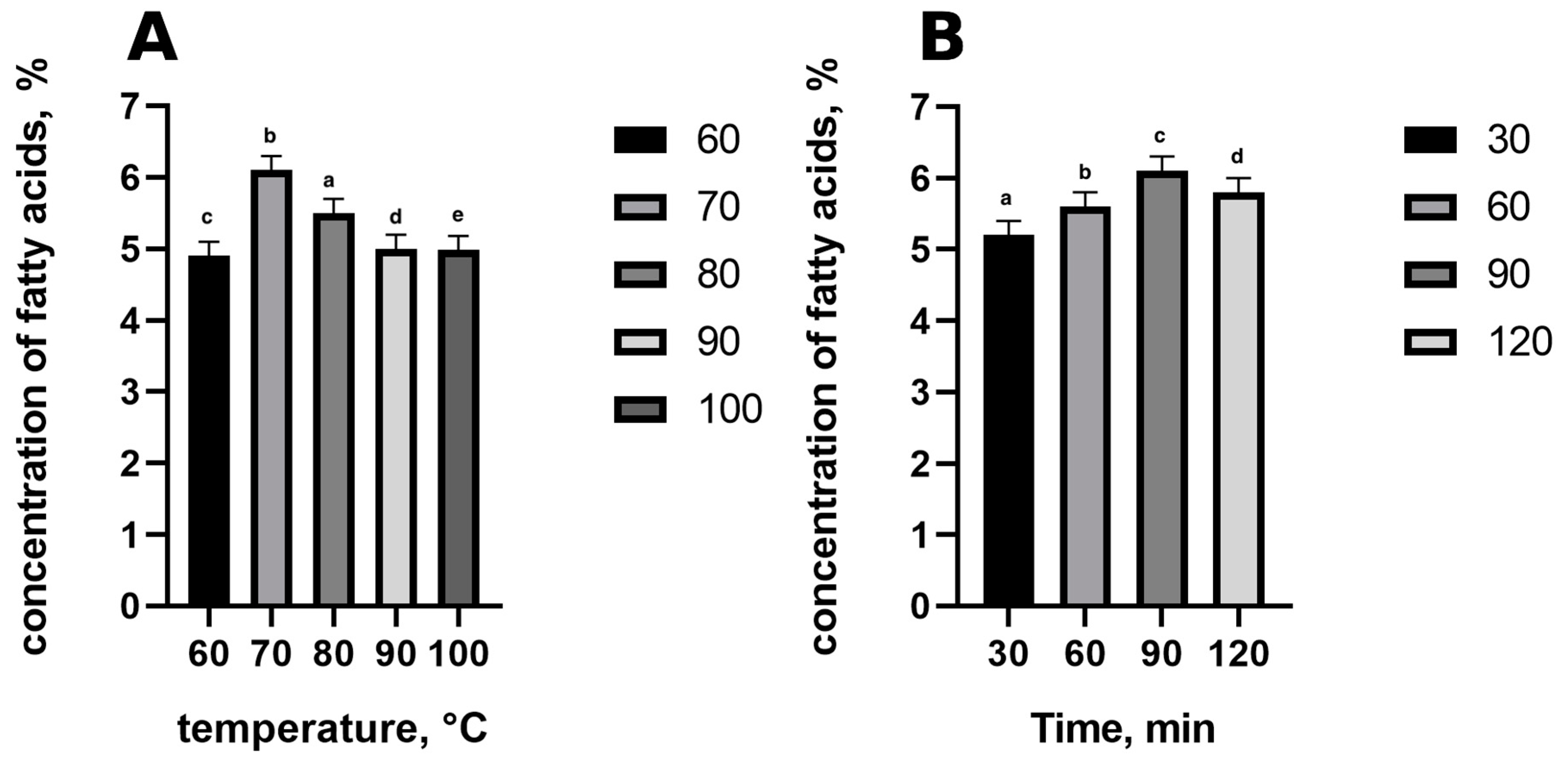
3.1. Methylation Parameters
3.2. Method Validation
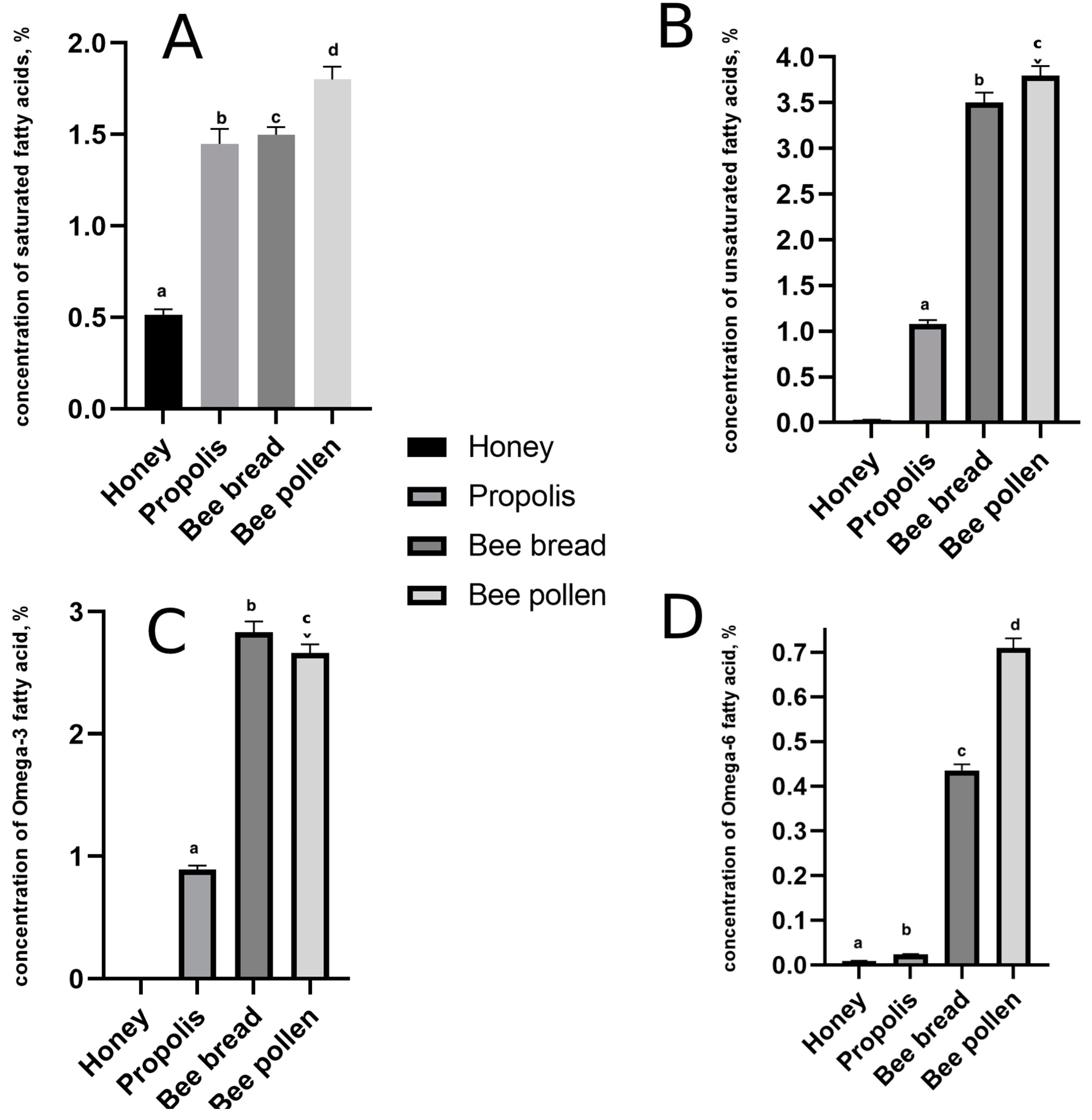
3.3. Determination of Total Fatty Acids in Bee Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Innis, S.M. Fatty acids and early human development. Earl. Hum. Dev. 2007, 83, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Tvrzicka, E.; Kremmyda, L.S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: their role in human metabolism, health and disease-a review. part 1: classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Palac. Uni. Olomouc. 2011, 155, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Čeksterytė, V.; Račys, J.; Kaškonienė, V.; Venskutonis, P.R. Fatty acid composition in beebread. Biology 2008, 4, 253–257. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Ad. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Arien, Y.; Dag, A.; Zarchin, S.; Masci, T.; Shafir, S. Omega-3 deficiency impairs honey bee learning. Proc. Natl. Acad. Sci. USA 2015, 112, 15761–15766. [Google Scholar] [CrossRef] [PubMed]
- Muth, F.; Breslow, P.R.; Masek, P.; Leonard, A.S. A pollen fatty acid enhances learning and survival in bumblebees. Behavior. Ecol. 2018, 29, 1371–1379. [Google Scholar] [CrossRef]
- Manning, R. Fatty acids in pollen: a review of their importance for honey bees. Bee W. 2001, 82, 60–75. [Google Scholar] [CrossRef]
- Ares, A.M.; Valverde, S.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Extraction and determination of bioactive compounds from bee pollen. J. Pharm. Biomed. Anal. 2018, 147, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Kezic, N.; Bolanča, V. Optimization of GC method for routine analysis of the fatty acid profile in several food samples. Food Chem. 2010, 1, 285–291. [Google Scholar] [CrossRef]
- Validation of Analytical Procedures: Text and methodology. ICH Harmonization Tripartite Guideline; European Medicines Agency: Geneva, Switzerland, 2005; pp. 1–13. [Google Scholar]
- Hang, M.; Yang, X.; Zhao, H.T.; Dong, A.J.; Wang, J.; Liu, G.Y.; Wang, P.; Cheng, C.L.; Zhang, H. A quick method for routine analysis of C18 trans fatty acids in non-hydrogenated edible vegetable oils by gas chromatography–mass spectrometry. Food Contr. 2015, 57, 293–301. [Google Scholar]
- Estevinho, L.M.; Rodrigues, S.; Pereira, A.P.; Feas, X. Portuguese bee pollen: palynological study, nutritional and micro- biological evaluation. Intern. J. Food Sci. Technol. 2012, 47, 429–435. [Google Scholar] [CrossRef]
- Subedi, U.; Jayawardhane, K.N.; Pan, X.; Ozga, J.; Chen, G.; Foroud, N.A.; Singer, S.D. The potential of genome editing for improving seed oil content and fatty acid composition in oilseed crops. Lipids 2020, 55, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Urcan, A.; Criste, A.; Dezmirean, D.; Mărgăoan, R.; Caeiro, A.; Graça Campos, M. Similarity of data from bee bread with the same taxa collected in India and Romania. Molecules 2018, 23, 2491. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.G.R.; Bogdanov, S.; Bicudo de Almeida-Muradian, L.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Agricult. Res. 2008, 47, 154–161. [Google Scholar]
- Čeksterytė, V.; Navakauskienė, R.; Treigytė, G.; Jansen, E.; Kurtinaitienė, B.; Dabkevičienė, G.; Balžekas, J. Fatty acid profiles of monofloral clover beebread and pollen and proteomics of red clover (Trifolium pratense) pollen. Biosci. Biotech. Biochem. 2016, 80, 2100–2108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urcan, A.; Marghitas, L.; Dezmirean, D.S.; Bobis, O.; Bonta, V. Chemical composition and biological activities of beebread–Review. Bull. Uni. Agri. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2017, 74, 6–14. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trend. Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Barajas, J.; Cortes-Rodriguez, M.; Rodríguez-Sandoval, E. Effect of temperature on the drying process of bee pollen from two zones of Colombia. J. Food Proc. Engineer. 2012, 35, 134–148. [Google Scholar] [CrossRef]

| Nr. | FAME | RT (Mean) | R2 | Range (µg/mL) | Detection (LOD), µg/mL | Quantitative (LOQ), µg/mL |
|---|---|---|---|---|---|---|
| 1. | Methyl butyrate | 10.794 | 0.999 | 6.24–399.6 | 0.51 | 1.54 |
| 2 | Meethyl hexanoate | 12.866 | 0.999 | 6.24–399.6 | 0.44 | 1.33 |
| 3 | Methyl octanoate | 16.514 | 0,999 | 6.24–399.6 | 0.42 | 1.28 |
| 4 | Methyl decanoate | 21.548 | 0.999 | 6.24–399.6 | 0.39 | 1.18 |
| 5 | Methyl laurate | 27.024 | 0.999 | 6.24–399.6 | 0.33 | 1.01 |
| 6 | Methyl tridecanoate | 29.679 | 0.999 | 3.08–197.5 | 0.32 | 0.98 |
| 7 | Methyl myristate | 32.263 | 0.999 | 6.24–399.6 | 0.29 | 0.89 |
| 8 | Myristoleic acid methyl ester | 34.256 | 0.999 | 3.12–199.8 | 0.31 | 0.93 |
| 9 | Methyl pentadecanoate | 34.706 | 0.999 | 3.12–199.8 | 0.28 | 0.84 |
| 10 | Cis-10-pentadecanoic acid methyl ester | 36.649 | 0.999 | 3.09–198.0 | 0.28 | 0.86 |
| 11 | Methyl palmitate | 37.09 | 0.999 | 9.36–599.4 | 0.25 | 0.76 |
| 12 | Methyl palmitoleate | 38.629 | 0.999 | 3.12–199.8 | 0.27 | 0.81 |
| 13 | Methyl heptadecanoate | 39.292 | 0.999 | 3.11–199.2 | 0.31 | 0.94 |
| 14 | Cis-10-heptadecanoic acid methyl ester | 40.815 | 0.998 | 3.12–199.8 | 0.24 | 0.72 |
| 15 | Methyl stearate | 41.474 | 0.999 | 6.24–399.6 | 0.22 | 0.68 |
| 16 | Trans-9-elaidic acid methyl ester | 42.343 | 0.998 | 3.12–199.8 | 0.21 | 0.64 |
| 17 | Cis-9-oleic acid methyl ester | 42.757 | 0.999 | 6.24–399.6 | 0.24 | 0.71 |
| 18 | Methyl linoleate | 44.667 | 0.999 | 3.09–197.9 | 0.25 | 0.76 |
| 20 | Methyl arachidate | 45.518 | 0.998 | 6.24–399.6 | 0.21 | 0.63 |
| 21 | Gamma-linolenic acid methyl ester | 46.089 | 0.999 | 3.12–199.8 | 0.25 | 0.77 |
| 22 | Methyl eicosanoate | 46.672 | 0.999 | 3.11–199.2 | 0.21 | 0.65 |
| 23 | Methyl linolenate | 46.854 | 0.999 | 3.12–199.8 | 0.27 | 0.81 |
| 24 | Cis-11,14-eicosadienoic acid methyl ester | 48.475 | 0.999 | 3.12–199.8 | 0.23 | 0.69 |
| 26 | Methyl behenate | 49.268 | 0.998 | 6.23–398.8 | 0.26 | 0.79 |
| 30 | Cis-11,14,17-eicotrienoic acid methyl ester | 50.507 | 0.999 | 3.03–193.6 | 0.27 | 0.82 |
| 33 | Cis-13,16-docosadienoic acid methyl ester | 52.014 | 0.999 | 3.12–199.8 | 0.28 | 0.84 |
| 34 | Methyl lignocerate | 52.786 | 0.999 | 6.24–399.6 | 0.26 | 0.79 |
| 35 | Methyl cis-5,8,11,14,17-eicosapentaenoate | 52.949 | 0.999 | 3.12–199.8 | 0.33 | 1.01 |
| 36 | Methyl nervonate | 53.843 | 0.999 | 3.12–199.8 | 0.29 | 0.87 |
| 37 | Cis-4,7,10,13,16,19-docosahexaenoic acid methyl ester | 57.949 | 0.999 | 3.12–199.8 | 0.54 | 1.63 |
| Bee Product | Total Number of Saturated Fatty Acids | Total Number of Unsaturated Fatty Acids | Omega-6/Omega-3 | TUS/TS |
|---|---|---|---|---|
| Propolis | 10 | 12 | 36.6:1 | 0.74:1 |
| Bee bread | 10 | 11 | 6.52:1 | 2.29:1 |
| Bee pollen | 12 | 9 | 3.79:1 | 2.07:1 |
| Honey | 6 | 2 | 0 | 0.06:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarukas, L.; Kuraite, G.; Baranauskaite, J.; Marksa, M.; Bezruk, I.; Ivanauskas, L. Optimization and Validation of the GC/FID Method for the Quantification of Fatty Acids in Bee Products. Appl. Sci. 2021, 11, 83. https://doi.org/10.3390/app11010083
Jarukas L, Kuraite G, Baranauskaite J, Marksa M, Bezruk I, Ivanauskas L. Optimization and Validation of the GC/FID Method for the Quantification of Fatty Acids in Bee Products. Applied Sciences. 2021; 11(1):83. https://doi.org/10.3390/app11010083
Chicago/Turabian StyleJarukas, Laurynas, Greta Kuraite, Juste Baranauskaite, Mindaugas Marksa, Ivan Bezruk, and Liudas Ivanauskas. 2021. "Optimization and Validation of the GC/FID Method for the Quantification of Fatty Acids in Bee Products" Applied Sciences 11, no. 1: 83. https://doi.org/10.3390/app11010083
APA StyleJarukas, L., Kuraite, G., Baranauskaite, J., Marksa, M., Bezruk, I., & Ivanauskas, L. (2021). Optimization and Validation of the GC/FID Method for the Quantification of Fatty Acids in Bee Products. Applied Sciences, 11(1), 83. https://doi.org/10.3390/app11010083





