Arsenic (V) Removal by an Adsorbent Material Derived from Acid Mine Drainage Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Arsenic Adsorption Batch Test
2.3.1. Adsorption Kinetic
2.3.2. Adsorption Isotherm
2.3.3. Effect of pH
2.3.4. Adsorption from Environment Samples of Acid Mine Drainage
2.4. Continuous Column Test
2.4.1. Lab-Scale Test
2.4.2. Pilot-Scale Test
3. Results and Discussion
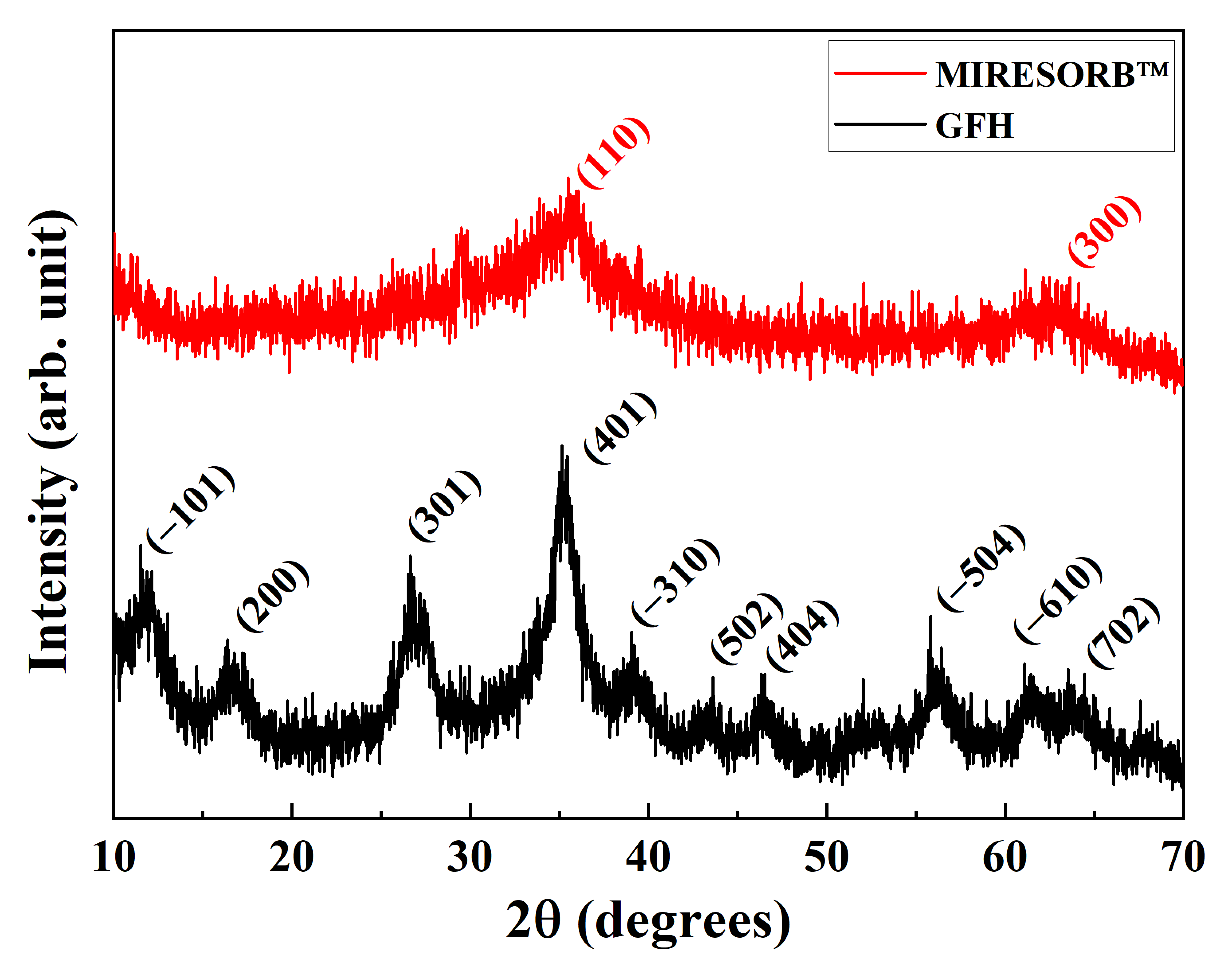
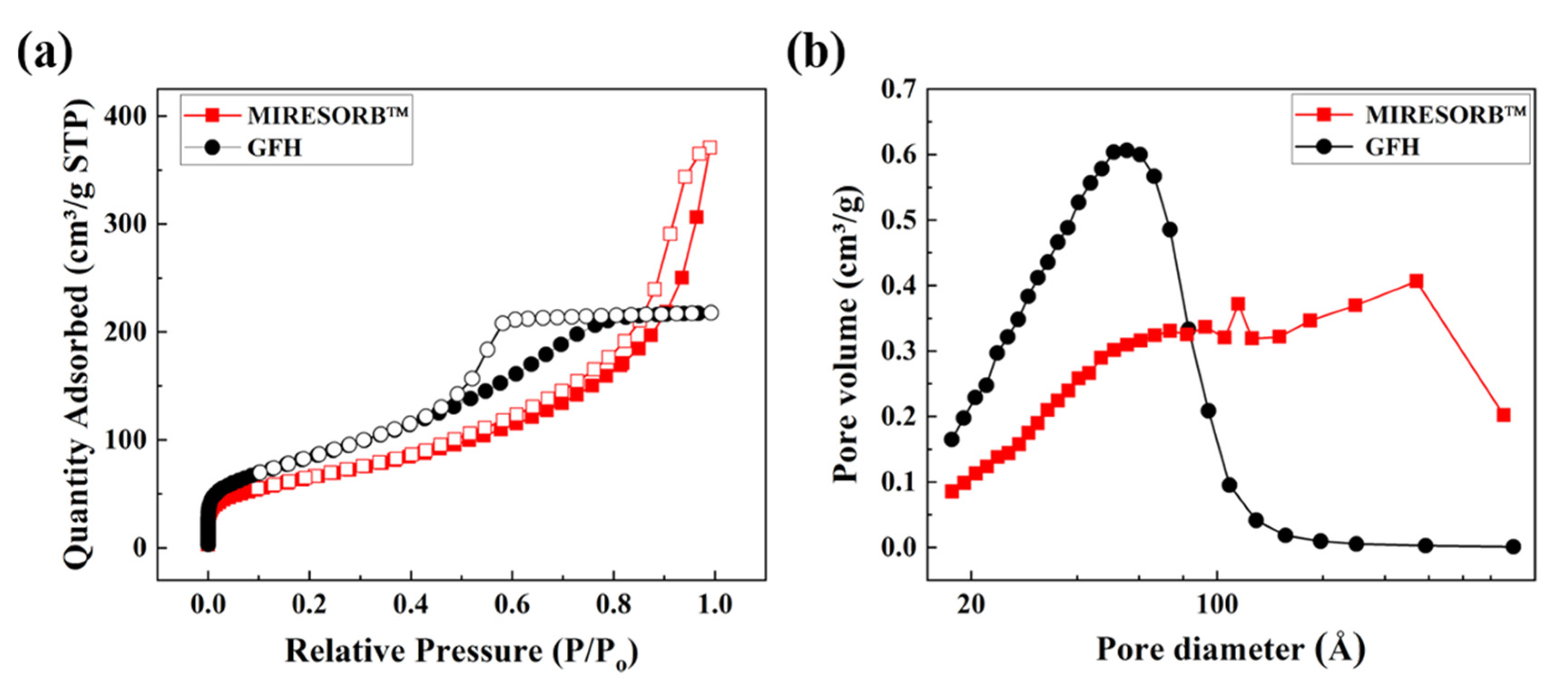
3.1. Adsorbent Characterization
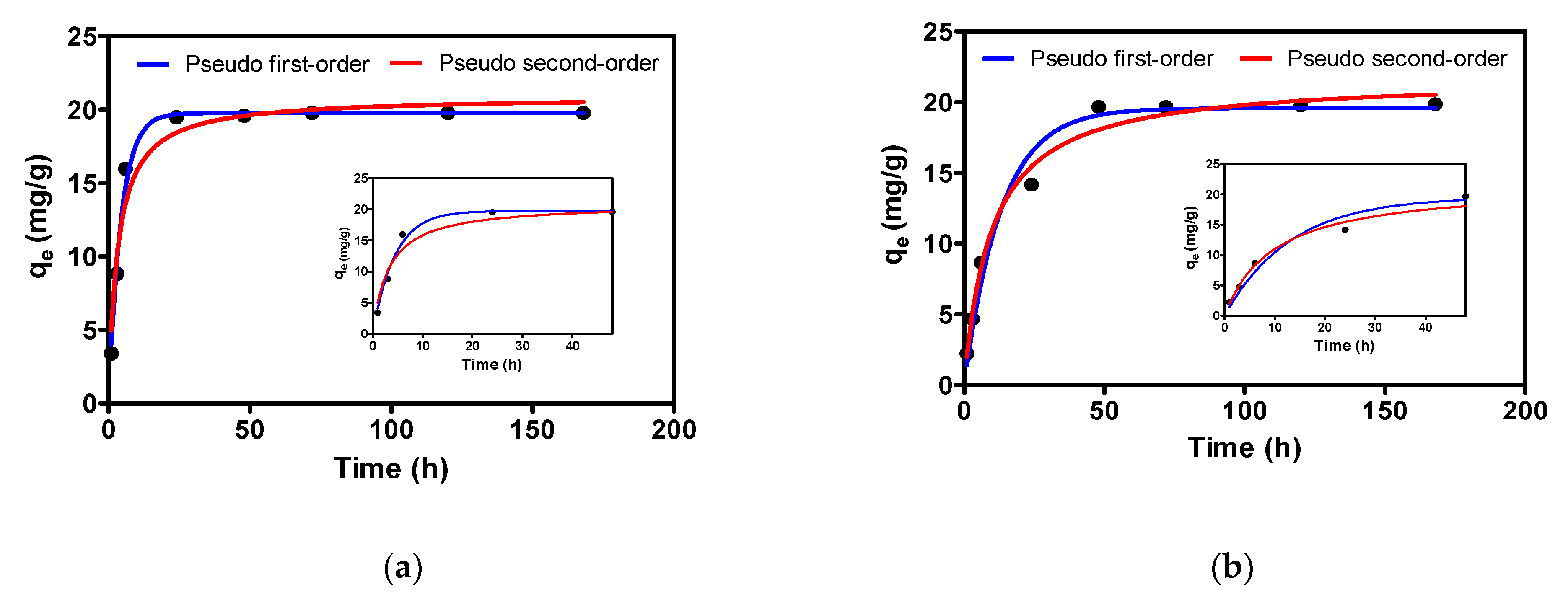
3.2. Adsorption Kinetics
3.3. Adsorption Isotherm
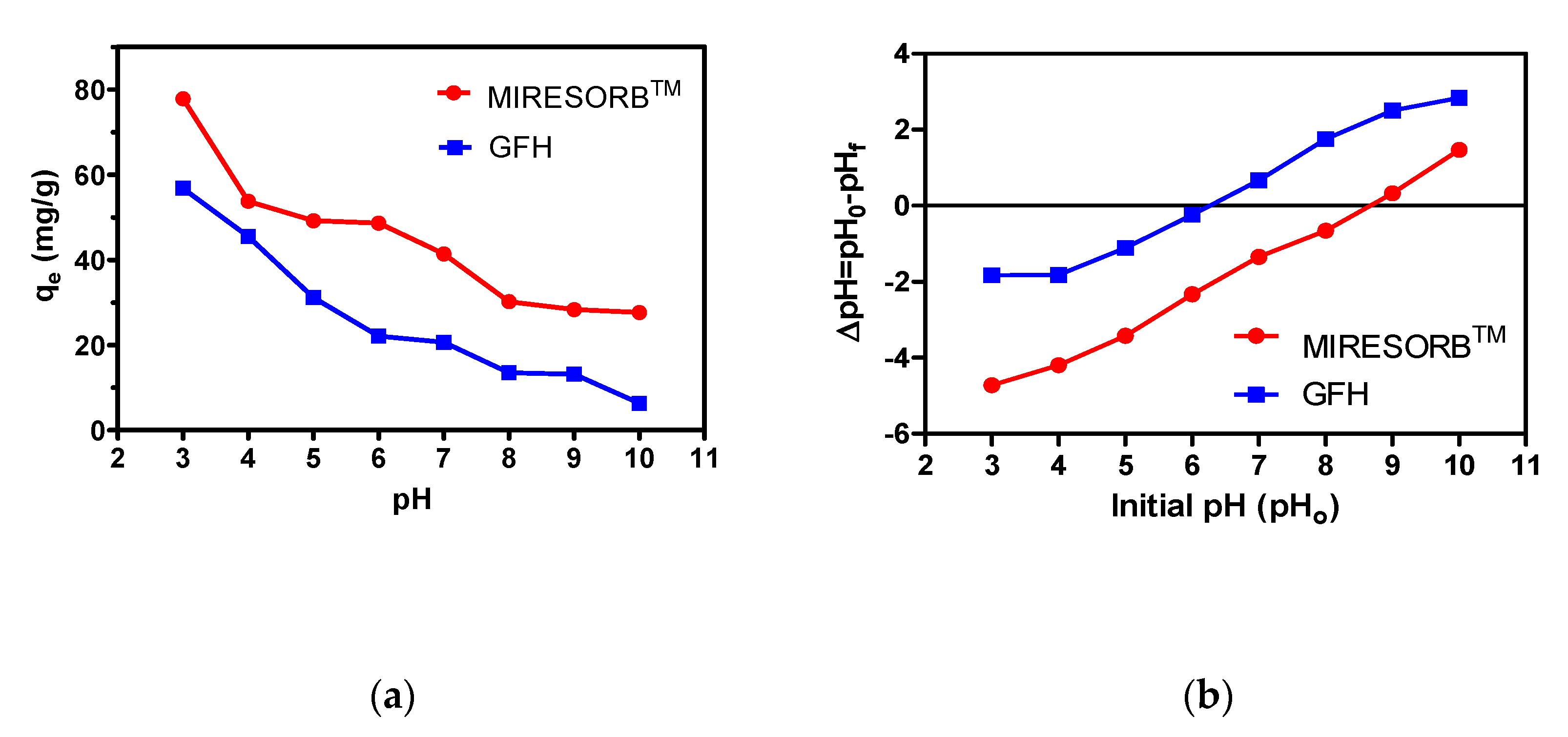
3.4. Effect of pH
3.5. Practical Applicability of Adsorbent
3.5.1. Treatment of an Environmental Acid Mine Drainage Sample
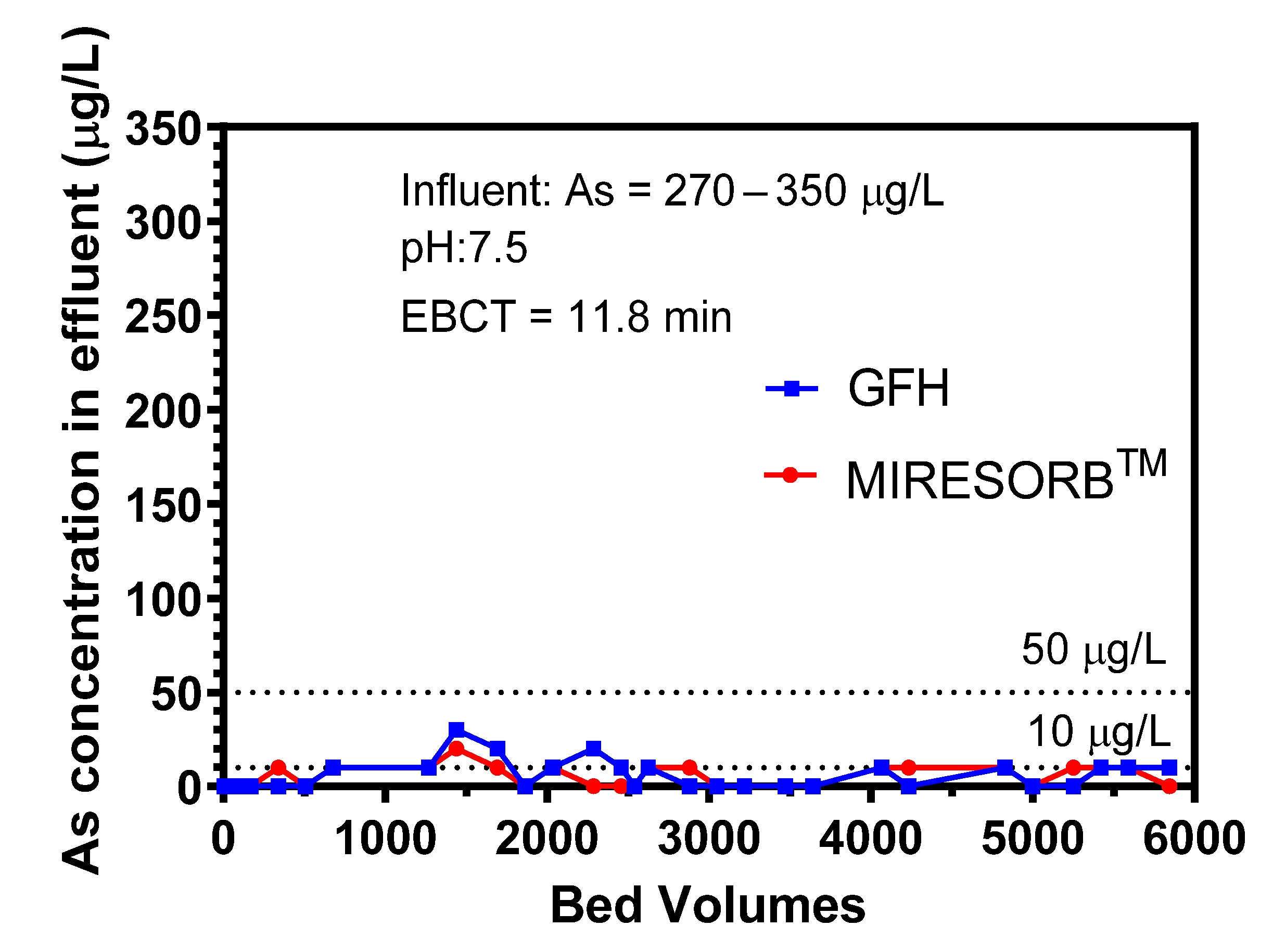
3.5.2. Lab-Scale Column Study
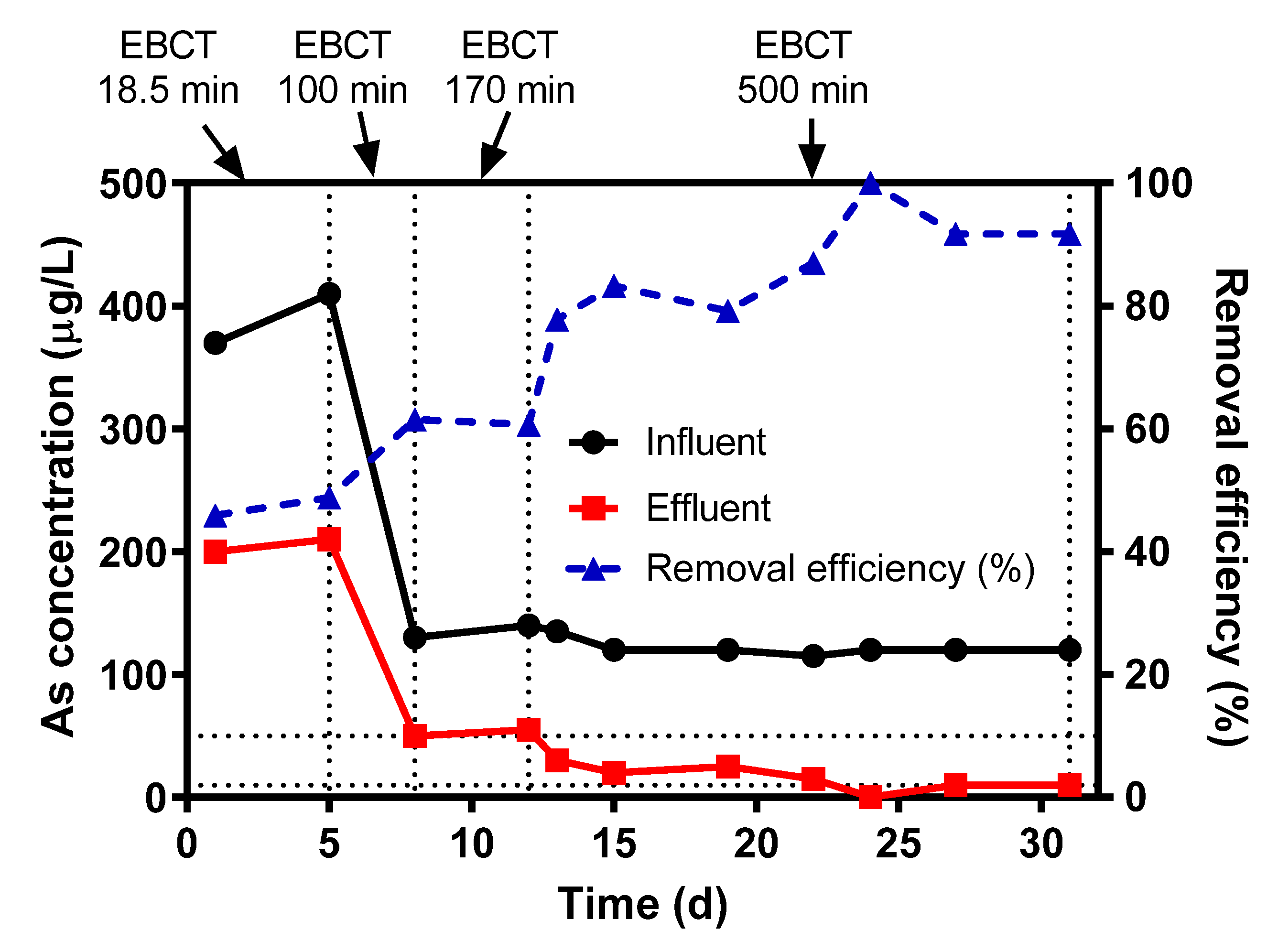
3.5.3. Pilot-Scale Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siddiqui, S.I.; Chaudhry, S.A. Arsenic Removal from Water Using Nanocomposites: A Review. Curr. Environ. Eng. Contin. Curr. Environ. Manag. 2017, 4, 81–102. [Google Scholar] [CrossRef]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Kwak, J.I.; Nam, S.H.; An, Y.J. Water quality standards for the protection of human health and aquatic ecosystems in Korea: Current state and future perspective. Environ. Sci. Pollut. Res. Int. 2018, 25, 3108–3119. [Google Scholar] [CrossRef]
- Brinkel, J.; Khan, M.H.; Kraemer, A. A Systematic Review of Arsenic Exposure and Its Social and Mental Health Effects with Special Reference to Bangladesh. Int. J. Environ. Res. Public Health 2009, 6, 1609–1619. [Google Scholar] [CrossRef]
- Zheng, Y. Global solutions to a silent poison. Science 2020, 368, 818–819. [Google Scholar] [CrossRef]
- Doyle, P. Environmental Geology. In Encyclopedia of Geology; Selley, R.C., Cocks, L.R.M., Plimer, I.R., Eds.; Elsevier: Oxford, UK, 2005; pp. 25–33. [Google Scholar]
- Pfeiffer, M.; Batbayar, G.; Hofmann, J.; Siegfried, K.; Karthe, D.; Hahn-Tomer, S. Investigating arsenic (As) occurrence and sources in ground, surface, waste and drinking water in northern Mongolia. Environ. Earth Sci. 2015, 73, 649–662. [Google Scholar] [CrossRef]
- Batsaikhan, B.; Kwon, J.; Kim, K.; Lee, Y.; Lee, J.; Badarch, M.; Yun, S. Hydrochemical evaluation of the influences of mining activities on river water chemistry in central northern Mongolia. Environ. Sci. Pollut. Res. 2017, 24, 2019–2034. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, H.J.; Cheong, H.K.; Choi, K.; Jang, J.Y.; Jeong, W.C.; Kim, D.S.; Yu, S.; Kim, Y.W.; Lee, K.Y.; et al. Investigation on health effects of an abandoned metal mine. J. Korean Med. Sci. 2008, 23, 452–458. [Google Scholar] [CrossRef]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Guan, X.; Wang, J.; Chusuei, C.C. Removal of arsenic from water using granular ferric hydroxide: Macroscopic and microscopic studies. J. Hazard. Mater. 2008, 156, 178–185. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, Z.; Dong, L.; Qiu, Y.; Zhao, J. Removal of Arsenate from Aqueous Solution by Manganese and Iron (hydr)oxides Coated Resin. Sep. Sci. Technol. 2010, 46, 130–136. [Google Scholar] [CrossRef]
- Chowdhury, R. Using adsorption and sulphide precipitation as the principal removal mechanisms of arsenic from a constructed wetland—A critical review. Chem. Ecol. 2017, 33, 560–571. [Google Scholar] [CrossRef]
- Kumar, R.; Kang, C.; Mohan, D.; Khan, M.A.; Lee, J.; Lee, S.S.; Jeon, B. Waste sludge derived adsorbents for arsenate removal from water. Chemosphere 2020, 239, 124832. [Google Scholar] [CrossRef]
- Egashira, R.; Tanabe, S.; Habaki, H. Adsorption of heavy metals in mine wastewater by Mongolian natural zeolite. Proc. Eng. 2012, 42, 49–57. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, Y.; Wang, F.; Zhang, J.; Li, D. Arsenic(V) removal by granular adsorbents made from water treatment residuals materials and chitosan. Colloids Surf. Physicochem. Eng. Asp. 2020, 585, 124036. [Google Scholar] [CrossRef]
- Lee, H.; Kim, D.; Kim, J.; Ji, M.; Han, Y.; Park, Y.; Yun, H.; Choi, J. As(III) and As(V) removal from the aqueous phase via adsorption onto acid mine drainage sludge (AMDS) alginate beads and goethite alginate beads. J. Hazard. Mater. 2015, 292, 146–154. [Google Scholar] [CrossRef]
- Demers, I.; Mbonimpa, M.; Benzaazoua, M.; Bouda, M.; Awoh, S.; Lortie, S.; Gagnon, M. Use of acid mine drainage treatment sludge by combination with a natural soil as an oxygen barrier cover for mine waste reclamation: Laboratory column tests and intermediate scale field tests. Miner. Eng. 2017, 107, 43–52. [Google Scholar] [CrossRef]
- Macías, F.; Pérez-López, R.; Caraballo, M.A.; Cánovas, C.R.; Nieto, J.M. Management strategies and valorization for waste sludge from active treatment of extremely metal-polluted acid mine drainage: A contribution for sustainable mining. J. Clean. Prod. 2017, 141, 1057–1066. [Google Scholar] [CrossRef]
- Ko, D.; Lee, J.S.; Patel, H.A.; Jakobsen, M.H.; Hwang, Y.; Yavuz, C.T.; Hansen, H.C.B.; Andersen, H.R. Selective removal of heavy metal ions by disulfide linked polymer networks. J. Hazard. Mater. 2017, 332, 140–148. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Chwastowski, J.; Bradło, D.; Żukowski, W. Adsorption of Cadmium, Manganese and Lead Ions from Aqueous Solutions Using Spent Coffee Grounds and Biochar Produced by Its Pyrolysis in the Fluidized Bed Reactor. Materials 2020, 13, 2782. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Bac, B.H.; Lee, Y.; Kim, M.H.; Kang, I.M. Highly ordered Ge-incorporated akaganeite (β-FeOOH): A tunnel-type nanorod. Cryst. Eng. Commun. 2011, 13, 287–292. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Cao, R.; Tao, J. Preparation and Characterization of Nanosized Hematite Colloids Using Green Vitriol as Ferrum Source. J. Nanomater. 2014, 2014, 749562. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef]
- Baskan, M.B.; Pala, A. Removal of arsenic from drinking water using modified natural zeolite. Desalination 2011, 281, 396–403. [Google Scholar] [CrossRef]
- Mohammad, S.G.; Ahmed, S.M.; Amr, A.; Kamel, A.H. Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetampirid Pesticide from Aqueous Solutions. Molecules 2020, 2339. [Google Scholar]
- Yang, J.; Kim, Y.; Park, S.; Baek, K. Removal of As(III) and As(V) using iron-rich sludge produced from coal mine drainage treatment plant. Environ. Sci. Pollut. Res. 2014, 21, 10878–10889. [Google Scholar] [CrossRef]
- Ko, M.; Kim, J.; Lee, J.; Ko, J.; Kim, K. Arsenic immobilization in water and soil using acid mine drainage sludge. Appl. Geochem. 2013, 35, 1–6. [Google Scholar] [CrossRef]
- Rait, R.; Trumm, D.; Pope, J.; Craw, D.; Newman, N.; MacKenzie, H. Adsorption of arsenic by iron rich precipitates from two coal mine drainage sites on the West Coast of New Zealand. N. Z. J. Geol. Geophys. 2010, 53, 177–193. [Google Scholar] [CrossRef]
- Saha, B.; Bains, R.; Greenwood, F. Physicochemical Characterization of Granular Ferric Hydroxide (GFH) for Arsenic(V) Sorption from Water. Sep. Sci. Technol. 2005, 40, 2909–2932. [Google Scholar] [CrossRef]
- Aredes, S.; Klein, B.; Pawlik, M. The removal of arsenic from water using natural iron oxide minerals. J. Clean. Prod. 2012, 29, 208–213. [Google Scholar] [CrossRef]
- Escudero, C.; Fiol, N.; Villaescusa, I.; Bollinger, J. Arsenic removal by a waste metal (hydr)oxide entrapped into calcium alginate beads. J. Hazard. Mater. 2009, 164, 533–541. [Google Scholar] [CrossRef] [PubMed]

| Adsorbent | Mg | Al | Si | Ca | Fe | Ni | Zn |
|---|---|---|---|---|---|---|---|
| MIRESORBTM | 4.00 | 9.20 | 1.92 | 5.73 | 78.1 | 0.12 | 0.12 |
| GFH | 4.40 | 2.19 | 0.15 | 0.15 | 93.0 | ND | ND |
| Characteristic | MIRESORBTM | GFH |
|---|---|---|
| Surface area (m2/g) | 233 | 306 |
| Pore volume (cm/g3) | 0.57 | 0.33 |
| Pore size (nm) | 7.30 | 4.39 |
| Adsorbent | Pseudo First-Order | Pseudo Second-Order | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (1/h) | R2 | qe (mg/g) | k2 (g/(mg·h)) | R2 | |
| MIRESORBTM | 19.76 | 0.227 | 0.990 | 20.88 | 0.015 | 0.958 |
| GFH | 19.58 | 0.076 | 0.977 | 21.74 | 0.005 | 0.986 |
| Adsorbent | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF ([mg/g]·[mg/L]1/n) | n (1/n) | R2 | |
| MIRESORBTM | 50.38 | 0.38 | 0.91 | 19.04 | 3.68 (0.27) | 0.98 |
| GFH | 29.07 | 0.33 | 0.94 | 12.00 | 4.35 (0.23) | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byambaa, E.; Seon, J.; Kim, T.-H.; Kim, S.D.; Ji, W.H.; Hwang, Y. Arsenic (V) Removal by an Adsorbent Material Derived from Acid Mine Drainage Sludge. Appl. Sci. 2021, 11, 47. https://doi.org/10.3390/app11010047
Byambaa E, Seon J, Kim T-H, Kim SD, Ji WH, Hwang Y. Arsenic (V) Removal by an Adsorbent Material Derived from Acid Mine Drainage Sludge. Applied Sciences. 2021; 11(1):47. https://doi.org/10.3390/app11010047
Chicago/Turabian StyleByambaa, Erdenechimeg, Jaeyoung Seon, Tae-Hyun Kim, Shin Dong Kim, Won Hyun Ji, and Yuhoon Hwang. 2021. "Arsenic (V) Removal by an Adsorbent Material Derived from Acid Mine Drainage Sludge" Applied Sciences 11, no. 1: 47. https://doi.org/10.3390/app11010047
APA StyleByambaa, E., Seon, J., Kim, T.-H., Kim, S. D., Ji, W. H., & Hwang, Y. (2021). Arsenic (V) Removal by an Adsorbent Material Derived from Acid Mine Drainage Sludge. Applied Sciences, 11(1), 47. https://doi.org/10.3390/app11010047






