Abstract
Lemon balm and dandelion are commonly used medicinal herbs exhibiting numerous pharmacological activities that are beneficial for human health. In this study, we explored the protective effects of a 2:1 (w/w) mixture of lemon balm and dandelion extracts (MLD) on carbon tetrachloride (CCl4)-induced acute liver injury in mice. CCl4 (0.5 mL/kg; i.p.) injection inhibited body weight gain and increased relative liver weight. Pre-administration of MLD (50–200 mg/kg) for 7 days prevented these CCl4-mediated changes. In addition, histopathological analysis revealed that MLD synergistically alleviated CCl4-mediated hepatocyte degeneration and infiltration of inflammatory cells. MLD decreased serum aspartate aminotransferase and alanine transferase activities and reduced the number of liver cells that stained positive for cleaved caspase-3 and cleaved poly(ADP-ribose) polymerase, suggesting that MLD protects against CCl4-induced hepatic damage via the inhibition of apoptosis. Moreover, MLD attenuated CCl4-mediated lipid peroxidation and protein nitrosylation by restoring impaired hepatic nuclear factor erythroid 2-related factor 2 mRNA levels and its dependent antioxidant activities. Furthermore, MLD synergistically decreased mRNA and protein levels of tumor necrosis factor-α, interleukin-1β, and interleukin-6 in the liver. Together, these results suggest that MLD has potential for preventing acute liver injury by inhibiting apoptosis, oxidative stress, and inflammation.
1. Introduction
The production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is indispensable for aerobic organisms and tightly regulated by a sophisticated system. ROS under normal physiological conditions act as secondary messengers responsible for the regulation of diverse cellular processes including cell proliferation, gene expression, and antimicrobial defenses [1]. However, oxidative and nitrosative stress, caused by an imbalance of redox homeostasis resulting in an excessive and chronic accumulation of ROS/RNS, inhibits the function of various biomolecules and changes the permeability of plasma and subcellular membranes, which in turn triggers cell death [2]. In addition, oxidative stress initiates a vicious cycle of tissue injury by causing the release of diverse damage-associated molecular patterns from injured cells, activating neighboring immune cells, producing proinflammatory cytokines, and accelerating inflammation-mediated tissue injury [3]. Due to the high consumption of oxygen when metabolizing xenobiotic or endogenous substances, the liver is one of the primary organs susceptible to oxidative damage [4]. Hence, oxidative stress remains central to the onset and progression of liver disease regardless of disease etiology, with effects seen in drug- and alcohol-induced liver injury, nonalcoholic fatty liver disease, viral hepatitis, fibrosis, cirrhosis, and cancer [2,4].
Due to the critical role of oxidative stress in liver disease, herbs possessing antioxidant and anti-inflammatory activities have garnered significant attention due to their potential to manage liver disease with fewer side effects [4]. Lemon balm (Melisa officinalis L.; Lamiaceae family) is an fragrant herb that has been traditionally used for the treatment of anxiety, depression, and heart disease, as well as an enhancer of cognitive function [5,6]. Numerous studies of lemon balm have revealed a wide array of pharmacological activities, including antioxidant, anti-inflammation, anti-nociceptive, neuroprotective, hypoglycemic, and hypolipidemic activities [5]. Furthermore, lemon balm extract has been reported to restore impaired lipids metabolism in the liver [7]. In addition, throughout East Asia, dandelion (Taraxacum officinale [L.] Weber ex F.H.Wigg; Asteraceae family) is known to reduce inflammation in the liver and lung [8]. Modern scientific studies have sought to validate many of these potential benefits, revealing a range of benefits that include diuretic, antioxidative, anti-inflammatory, and anti-cancer activities [8]. Moreover, dandelion extract was shown to prevent a wide range of liver diseases induced by hepatotoxins (e.g., carbon tetrachloride (CCl4), acetaminophen, and alcohol) or diet in experimental animal models [9,10,11,12,13] and sensitize the tumor necrosis factor (TNF)-related apoptosis-inducing ligand-mediated cell death of hepatocellular carcinoma cells [14]. However, more studies are needed on the hepatoprotective effect of the combination of two herbs.
As an effort to discover valuable hepatoprotective medicinal herbs, we previously found that leaf extracts from lemon balm and dandelion significantly alleviated CCl4-mediated acute liver injury. Of the diverse combination of ratios tested, a 2:1 (w/w) mixture of lemon balm and dandelion extracts (MLD) exhibited the most potent hepatoprotective effect against CCl4 [15]. Therefore, the present study investigated the dose-dependent effects of MLD on CCl4-induced acute liver injury, in comparison with silymarin, a representative natural product used as protection against diverse stages of liver disease, and each herbal extract alone. Furthermore, the effects of MLD on hepatocyte apoptosis, oxidative stress, and inflammation were assessed to elucidate MLD’s response mechanism in CCl4-induced acute liver injury.
2. Materials and Methods
2.1. Preparation of MLD and Reagents
Lemon balm leaf extract (LB) and dandelion leaf extract (DL) were supplied by Evear Extraction (Coutures, France). LB and DL were dissolved in distilled water and mixed at a ratio of 2:1 (w/w) for preparing MLD. Anti-cleaved poly(ADP-ribose) polymerase (PARP) and anti-cleaved caspase-3 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Cell Signaling Technology (Beverly, MA, USA), respectively. The antibody used to direct nitrotyrosine was obtained from Millipore (Temecula, CA, USA), and the anti-4-hydroxynonenal antibody from Abcam (Cambridge, UK). CCl4, olive oil, silymarin, hematoxylin and eosin staining solution, and other reagents were supplied by Sigma-Aldrich (St. Louis, MO, USA).
2.2. Animal Husbandry and Treatment
Eighty male SPF/VAF Outbred CrlOri:CD1 (ICR) mice (age 6 weeks; body weight, 29–32 g) were supplied by OrientBio (Seungnam, Korea) and maintained in standard conditions (temperature, 20–25 °C; relative humidity 30–35%; light:dark cycle, 12:12 h; food and water, ad libitum). After acclimatization for 7 days, the mice were divided into the following eight groups (n = 10 mice per group): vehicle, CCl4, CCl4 + silymarin, CCl4 + LB, CCl4 + DL, CCl4 + MLD 200, CCl4 + MLD 100, and CCl4 + MLD 50. After herbal extract or silymarin was dissolved in distilled water, 200 mg/kg silymarin, 100 mg/kg LB, 100 mg/kg DL, or three doses of MLD (50, 100, or 200 mg/kg) were orally administered to ICR mice once daily for 7 days (i.e., the first administration of herbal extract or silymarin = day 0). To induce acute liver injury, CCl4 (0.5 mL/kg in olive oil; i.p.) was injected at 1 h after treatment with herbal extracts or silymarin on day 6. Vehicle group were treated with equal volumes of distilled water and olive oil. Concentrations of silymarin, herbal extracts, and CCl4 were determined based on previous reports [15,16]. At 24 h after CCl4 treatment, animals were anesthetized under 2–3% isoflurane and euthanized by cervical dislocation.
2.3. Body Weight Gain and Relative Liver Weight
Before measuring body weight using a balance (XB320M; Precisa Instrument, Zürich, Switzerland), all mice were fasted for 12 h to minimize differences from feeding. Body weight gain was calculated from the weight differences between days 7 and 0, and relative liver weight was calculated as the proportion of the liver weight to body weight on day 7.
2.4. Histology and Immunohistochemistry
Paraffin-embedded sectioning of the liver followed by hematoxylin and eosin staining was conducted as described previously [15,16]. A certified pathologist observed the stained tissues under a light microscope (Eclipse 80i; Nikon, Tokyo, Japan), counted the number of degenerated hepatocytes and infiltrated inflammatory cells, and assessed the modified histological activity index (HAI) score as described previously [16,17]. In addition, hepatic tissues were immunostained using specific primary antibodies with an appropriate avidin-biotin-peroxidase complex and a peroxidase substrate kit (Vector Labs, Burlingame, CA, USA) as described previously [16]. Hepatic parenchymal cells around the central vein showing over 20% of immunoreactivity were counted as positive cells using an automated image analyzer (iSolution FL ver 9.1; IMT i-solution Inc., Burnaby, BC, Canada).
2.5. Measurement of Serum Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) Activities
Blood collected from the vena cava were centrifuged to obtain the serum. Serum AST and ALT activities were measured using an automated blood analyzer (Dri-Chem NX500i; Fuji Medical System, Tokyo, Japan).
2.6. Measurement of Lipid Peroxidation
Liver homogenates were prepared by homogenization in Tris buffer (0.01 M; pH 7.4) followed by centrifugation at 12,000× g for 15 min. The resulting supernatants were reacted with thiobarbituric acid at 100 °C for 90 min. Absorbance at 525 nm was monitored using a spectrophotometer (OPTZEN POP; Mecasys, Daejeon, Korea). The concentration of malondialdehyde was calculated by interpolating a standard curve and normalized to protein concentration.
2.7. Real-Time Polymerase Chain Reaction (PCR)
Total RNA was isolated from hepatic tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and reverse-transcribed using an oligo (dT)16. Real-time PCR was conducted, as described previously [18]. Primer sequences are listed in Table 1. The relative quantification of specific genes was conducted using β-actin as an endogenous control, as described previously [19].

Table 1.
Primer sequences for amplifying specific genes.
2.8. Determination of Glutathione Level, and Superoxide Dismutase (SOD) and Catalase Activities
Glutathione levels and SOD and catalase activities in the liver homogenates were measured [18] and normalized to protein concentration.
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
Liver tissues were homogenized in radioimmunoprecipitation assay buffer using a bead beater (TacoTM Prep; GeneReach Biotechnology, Taichung, Taiwan) and an ultrasonic cell disruptor (KS-750; Madell Technology, Ontario, CA, USA), incubated on ice for 30 min, and centrifuged at 20,000× g for 15 min. Protein level of TNF-α, interleukin (IL)-1β, and IL-6 was read on an automated microplate reader (Sunrise; Tecan, Männedorf, Switzerland) in accordance with the manufacturer’s instructions (MyBioSource, San Diego, CA, USA).
2.10. Statistical Analyses
All numerical values were presented as the mean ± standard deviation (SD) of 10 mice. One-way analysis of variance or Welch test was conducted to compare means among experimental groups. Tukey honestly significant difference (for equal variances) or Dunnett’s T3 test (for unequal variances) was used as post-hoc analysis, with p values < 0.05 considered as significance.
3. Results
3.1. MLD Synergistically Protects the Liver in CCl4-Treated Mice
To investigate the hepatoprotective effects of MLD, CCl4 (0.5 mL/kg in olive oil; i.p.) was treated once into mice that had been administered with silymarin (200 mg/kg), LB (100 mg/kg), DL (100 mg/kg), or MLD (200, 100, or 50 mg/kg) for 7 days. On day 0, body weight of vehicle-treated group was 30.15 ± 0.84 g, and there were no differences among experimental groups. Body weight on day 7 after treatment with CCl4, CCl4 + silymarin, CCl4 + LB, CCl4 + DL, CCl4 + MLD 200, CCl4 + MLD 100, and CCl4 + MLD 50 was 88.65 ± 4.49, 95.66 ± 4.80, 95.83 ± 6.02, 93.91 ± 4.75, 101.39 ± 5.17, 100.44 ± 3.26, and 101.15 ± 5.75% of vehicle-treated group, respectively. Compared to the vehicle, CCl4 injection significantly decreased body weight gain (p < 0.01; Figure 1a), and this was parallel with previous observation that CCl4 causes anorexia [15,20]. However, MLD (50–200 mg/kg) significantly inhibited the CCl4-mediated reduction in body weight gain (p < 0.01). In addition, three doses of MLD tended to further increase the body weight gain compared to silymarin, LB, or DL. But body weight gains due to silymarin versus 100–200 mg/kg MLD, LB versus 200 mg/kg MLD, and DL versus 100 mg/kg MLD were only statistically significant (p < 0.05; Figure 1a). Furthermore, CCl4-mediated increases in relative liver weight were significantly attenuated by the three doses of MLD, silymarin, LB, and DL (p < 0.01; Figure 1b). The magnitude of the MLD-mediated reduction in relative liver weight was greater than that associated with silymarin or either of the herbal extracts alone (p < 0.01; Figure 1b).
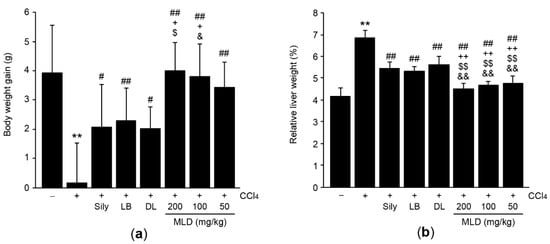
Figure 1.
Effects of mixture of lemon balm and dandelion extracts (MLD) on body weight gain and relative liver weight in CCl4-treated mice. Mice were treated with silymarin (Sily), lemon balm leaf extract (LB), dandelion leaf extract (DL) or one of three doses of MLD once daily for 7 days. On day 6, CCl4 was injected at 1 h after the final administration of herbal drugs. Body weight gain (a) and relative liver weight (b) were measured. All values are expressed as mean ± SD of ten mice. ** p < 0.01 between the vehicle and CCl4 groups; ## p < 0.01, # p < 0.05 versus CCl4-injected mice; ++ p < 0.01, + p < 0.05 between Sily- and MLD-treated mice; $$ p < 0.01, $ p < 0.05 between LB- and MLD-treated mice; && p < 0.01, & p < 0.05 between DL- and MLD-treated mice.
Histopathological analyses using hematoxylin and eosin-stained tissue sections indicated that CCl4 increased modified HAI scores as a result of hepatocyte degeneration and infiltration of inflammatory cells into the hepatic parenchyma (p < 0.01). These CCl4-induced hepatic histopathological changes were significantly prevented by the administration of 50–200 mg/kg MLD (p < 0.01; Figure 2a,b). These three different doses of MLD yielded a greater reduction in CCl4-induced acute hepatic damage relative to silymarin or either of the herbal extracts alone, though statistically significant differences in the number of degenerated hepatocytes was only seen in the 200 mg/kg MLD-administered group (p < 0.01; Figure 2b, upper). Although the magnitude of the decrease in the number of degenerated hepatocytes seen in the 100 mg/kg MLD group was greater than that seen in either the LB (p < 0.05) or DL (p < 0.05) groups, no differences were evident between the 100 mg/kg MLD and silymarin groups. Furthermore, the decrease in number of degenerated hepatocytes seen in the 50 mg/kg MLD group was not statistically different than that seen in the silymarin, LB, or DL group (Figure 2b, upper). Finally, the magnitude of the decreases in number of infiltrated inflammatory cells and modified HAI scores induced by 50–200 mg/kg MLD was greater than that associated with silymarin or either of the herbal extracts alone, with the exception of the comparison between LB and 50 mg/kg MLD (p < 0.05, number of infiltrated inflammatory cells in the DL versus the 50 mg/kg MLD group; p < 0.05, modified HAI score in the LB versus the 100 mg/kg MLD group, in the silymarin versus the 50 mg/kg MLD group, and in the DL versus the 50 mg/kg MLD group; p < 0.01, other comparisons; Figure 2b, middle and lower).
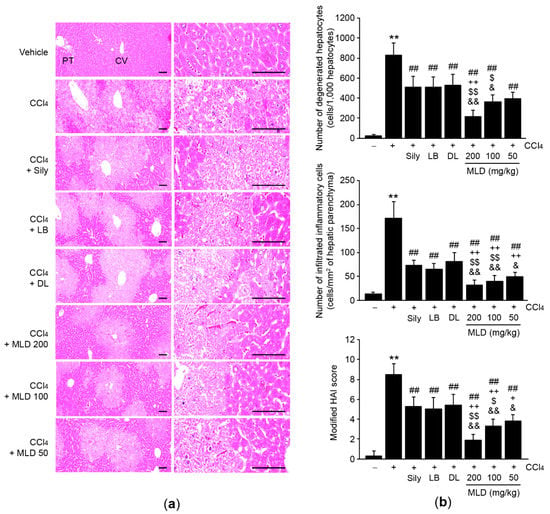
Figure 2.
MLD protects the liver from CCl4-induced damage. (a) Representative hepatic tissues stained with hematoxylin and eosin. Scale bars, 200 μm. (b) The numbers of degenerated hepatocytes (upper) and infiltrated inflammatory cells (middle), and modified HAI scores (lower) were quantified using an image analyzer. All values are expressed as mean ± SD of ten mice. ** p < 0.01 between the vehicle and CCl4 groups; ## p < 0.01 versus CCl4-injected mice; ++ p < 0.01, + p < 0.05 between Sily- and MLD-treated mice; $$ p < 0.01, $ p < 0.05 between LB- and MLD-treated mice; && p < 0.01, & p < 0.05 between DL- and MLD-treated mice. CV, central vein; PT, portal triad.
3.2. MLD Synergistically Attenuates Hepatocyte Apoptosis
To explore whether MLD protects the liver by reducing hepatocyte damage, we measured the activities of serum biomarkers related to hepatotoxicity. Administration of MLD at three different doses, silymarin, LB, or DL significantly attenuated CCl4-mediated increases in serum AST and ALT activities (p < 0.01). The effects seen in response to the three doses of MLD were more potent than those seen in response to silymarin, LB, or DL (p < 0.05, AST activity in the LB versus the 50 mg/kg MLD group; p < 0.01, other comparisons; Figure 3a). Next, we quantified the number of apoptotic cells via immunohistochemical staining of hepatic tissues using antibodies against cleaved caspase-3 and cleaved PARP. Compared to the vehicle, CCl4 injection significantly increased the numbers of cleaved caspase-3- and cleaved PARP-positive cells in hepatic tissue (p < 0.01; Figure 3b,c), indicating that CCl4 provokes apoptosis in hepatocytes. By contrast, the three doses of MLD, silymarin, LB, and DL all significantly inhibited the increases in number of cleaved caspase-3- and cleaved PARP-positive cells (p < 0.05, number of cleaved PARP-positive cells in the CCl4 versus the DL group; p < 0.01, other comparisons; Figure 3b,c). Moreover, the magnitude of the inhibition by MLD was greater than that by silymarin or either of the herbal extracts alone (p < 0.05, number of cleaved caspase-3-positive cells in the silymarin versus the 100 mg/kg MLD group and in the LB versus the 50 or 100 mg/kg MLD group; p < 0.05, number of cleaved PARP-positive cells in the LB or DL versus the 50 mg/kg MLD group; p < 0.01, other comparisons; Figure 3c).
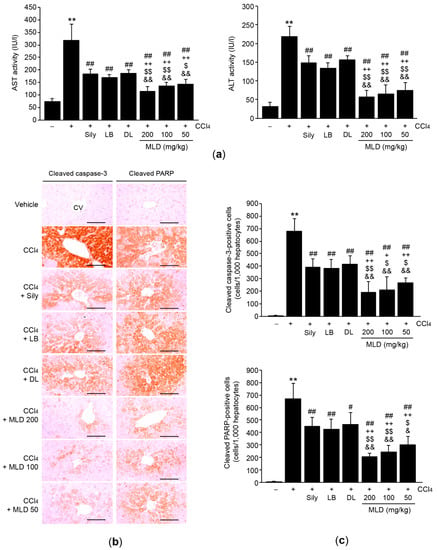
Figure 3.
MLD attenuates apoptosis of hepatocytes. (a) Effects of MLD on serum AST and ALT activities. (b) Hepatic tissues were immunostained using cleaved caspase-3 or cleaved polymerase (PARP) antibodies. Scale bars, 200 μm. (c) Positive cells in the hepatic parenchyma around the central vein were counted. All values are expressed as mean ± SD of ten mice. ** p < 0.01 between the vehicle and CCl4 groups; ## p < 0.01, # p < 0.05 versus CCl4-injected mice; ++ p < 0.01, + p < 0.05 between Sily- and MLD-treated mice; $$ p < 0.01, $ p < 0.05 between LB- and MLD-treated mice; && p < 0.01, & p < 0.05 between DL- and MLD-treated mice. CV, central vein.
3.3. MLD Synergistically Alleviates CCl4-Mediated Oxidative Stress by Restoring Antioxidant Activity
To investigate whether MLD prevents CCl4-induced hepatic apoptosis by reducing oxidative stress, hepatic tissues were immunohistochemically stained using a nitrotyrosine (a marker of nitrosative stress) antibody. Compared to the vehicle, CCl4 significantly increased the number of nitrotyrosine-positive cells in the liver (p < 0.01; Figure 4a, left and Figure 4b, upper). Although administration of silymarin or DL attenuated the CCl4-mediated increases in number of nitrotyrosine-positive cells, these differences were not statistically significant. However, all three doses of MLD and LB significantly decreased the number of nitrotyrosine-positive cells, relative to CCl4 treatment (p < 0.01, CCl4 versus MLD; p < 0.05, CCl4 versus LB), and the magnitude of the reduction in number of nitrotyrosine-positive cells by MLD was greater than that by silymarin or either of the single herbal extracts alone (p < 0.01; Figure 4b, upper). Immunohistochemical staining of 4-hydroxynonenal and quantification of malondialdehyde were conducted to assess the effects of MLD on hepatic lipid peroxidation. CCl4-mediated increases in lipid peroxidation were significantly inhibited by the three different doses of MLD (p < 0.01; Figure 4a, right; Figure 4b, lower; and Figure 4c). The inhibition of 4-hydroxynonenal-positive cells and malondialdehyde by 100 or 200 mg/kg MLD was more potent than that seen in response to silymarin or either of the herbal extracts alone (p < 0.01; Figure 4b, lower and Figure 4c). Although the magnitude of the reduction in number of 4-hydroxynonenal-positive cells induced by 50 mg/kg MLD was greater than that induced by DL (p < 0.01), the number of 4-hydroxynonenal-positive cells observed in the 50 mg/kg MLD group did not differ from that in either the silymarin or LB group (Figure 4b, lower). In addition, 50 mg/kg MLD synergistically decreased malondialdehyde levels in comparison to silymarin or either of the herbal extracts alone (p < 0.05, LB versus 50 mg/kg MLD; p < 0.01, other comparisons; Figure 4c).
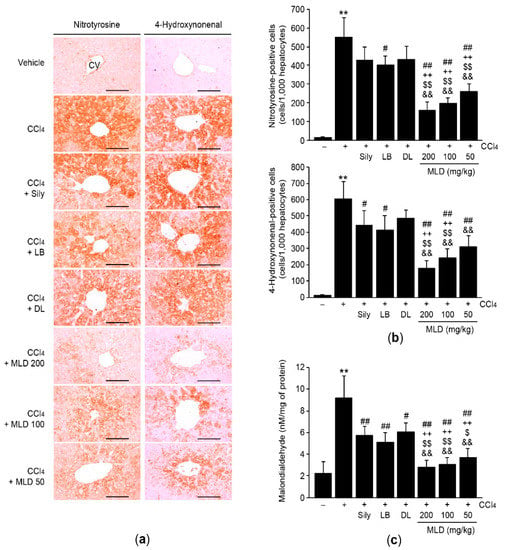
Figure 4.
MLD alleviates CCl4-mediated oxidative stress. Hepatic tissues were immunostained using anti-nitrotyrosine or anti-4-hydroxynonenal antibodies (a), and positive cells were counted (b). Scale bars, 200 μm. (c) Lipid peroxidation. Malondialdehyde concentration was determined using liver homogenates. All values are expressed as mean ± SD of ten mice. ** p < 0.01 between the vehicle and CCl4 groups; ## p < 0.01, # p < 0.05 versus CCl4-injected mice; ++ p < 0.01 between Sily- and MLD-treated mice; $$ p < 0.01, $ p < 0.05 between LB- and MLD-treated mice; && p < 0.01 between DL- and MLD-treated mice. CV, central vein.
Next, we explored the effects of MLD on antioxidant activity in CCl4-treated mice. Compared to the vehicle, CCl4 injection significantly reduced the mRNA levels of nuclear factor erythroid 2-related factor 2 (Nrf2) (p < 0.01; Figure 5a), an important transcription factor for antioxidant gene expression [21]. Administration of MLD, silymarin, LB, and DL significantly reversed the reduction in levels of Nrf2 mRNA (p < 0.05, CCl4 versus DL; p < 0.01, other comparisons). This restoration of Nrf2 mRNA by three different doses of MLD was more potent than that by silymarin or either the herbal extracts alone (p < 0.01; Figure 5a). Consistent with the reduction in Nrf2 mRNA levels, CCl4 also significantly depleted glutathione (an endogenous antioxidant) levels and inhibited SOD and catalase activities (p < 0.01; Figure 5b–d). All three doses of MLD significantly alleviated the depletion of glutathione and the reduction of SOD and catalase activities (p < 0.01), with the magnitude of these restorative effects greater than those seen in response to silymarin or either of the herbal extracts alone (p < 0.05, glutathione levels in the silymarin versus the 50 mg/kg MLD group; p < 0.05, SOD activity in the silymarin versus the 100 mg/kg MLD group and in the LB versus the 50 or 100 mg/kg MLD group; p < 0.05, catalase activity in the DL versus 50 or 100 mg/kg MLD group; p < 0.01, other comparisons; Figure 5b–d), except for glutathione levels, which did not differ significantly between the 50 mg/kg MLD group and the LB group (Figure 5b).
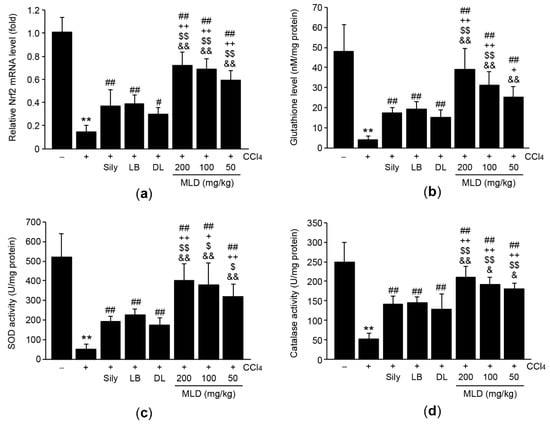
Figure 5.
MLD increases antioxidant activities in CCl4-treated mice. Effects of MLD on Nrf2 mRNA levels (a), glutathione levels (b), SOD activity (c), and catalase activity (d) in hepatic tissues. All values are expressed as mean ± SD of ten mice. ** p < 0.01 between the vehicle and CCl4 groups; ## p < 0.01, # p < 0.05 versus CCl4-injected mice; ++ p < 0.01, + p < 0.05 between Sily- and MLD-treated mice; $$ p < 0.01, $ p < 0.05 between LB- and MLD-treated mice; && p < 0.01, & p < 0.05 between DL- and MLD-treated mice.
3.4. MLD Synergistically Decreases the Levels of Proinflammatory Cytokines in CCl4-Treated Mice
To explore whether MLD protects the liver by reducing CCl4-induced inflammation, mRNA and protein levels of proinflammatory cytokines were measured using real-time PCR and ELISA, respectively. CCl4 significantly increased the mRNA and protein levels of TNF-α (p < 0.01; Figure 6a), IL-1β (p < 0.01; Figure 6b), and IL-6 (p < 0.01; Figure 6c) compared to the vehicle. Administration of three different doses of MLD significantly inhibited the increases in mRNA and protein levels of proinflammatory cytokines induced by CCl4 (p < 0.01; Figure 6a–c). The magnitude of the inhibition in mRNA and protein levels by MLD was greater than that achieved in response to silymarin or either of the herbal extracts alone (p < 0.05, IL-1β protein levels in the silymarin or DL versus the 50 mg/kg MLD group; p < 0.01, other comparisons; Figure 6a–c), with the exception of TNF-α protein levels in the 50 mg/kg MLD and IL-1β protein levels in the 50–100 mg/kg MLD group, which did not differ significantly from those in the LB group.
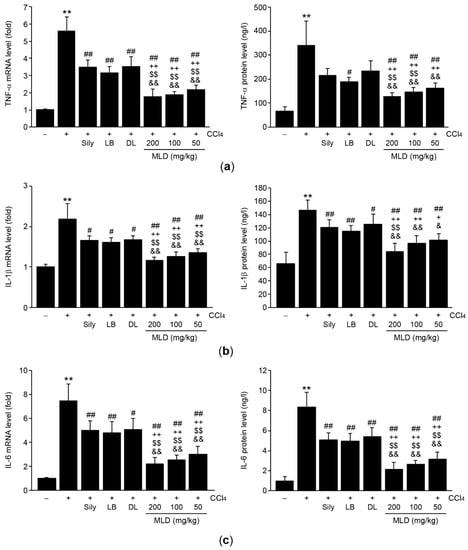
Figure 6.
MLD inhibits the production of proinflammatory cytokines in CCl4-treated mice. Levels of mRNA (left) and proteins (right) of TNF-α (a), IL-1β (b), and IL-6 (c) in the liver were determined using real-time PCR and ELISA, respectively. All values are expressed as mean ± SD of ten mice. ** p < 0.01 between the vehicle and CCl4 groups; ## p < 0.01, # p < 0.05 versus CCl4-injected mice; ++ p < 0.01, + p < 0.05 between Sily- and MLD-treated mice; $$ p < 0.01 between LB- and MLD-treated mice; && p < 0.01, & p < 0.05 between DL- and MLD-treated mice.
4. Discussion
Recent advances in pharmacognostics have revealed that a wide range of bioactive compounds are present in medicinal herbs. Of these, phytochemical studies have identified numerous volatile compounds (e.g., geranial and neral), polyphenolic compounds (e.g., rosmarinic acid, luteolin, and naringin), and terpenoids (e.g., ursolic acid) in lemon balm [5]. Similarly, numerous polyphenolic compounds (e.g., chicoric acid, chlorogenic acid, luteolin, and isorhamnetin), terpenoids (e.g., taraxacinic acids), and alkaloids (e.g., taraxacine and taraxafolin) have been isolated from the aerial parts of dandelion [8]. The vast majority of antioxidant and anti-inflammatory activities of herbs are attributed to the presence of polyphenols. Two such compounds, rosmarinic acid and chicoric acid are both enriched in lemon balm and dandelion [6,22] and are effectively solubilized in MLD (e.g., 34.07 ± 0.55 mg/g rosmarinic acid and 2.26 ± 0.01 mg/g chicoric acid) [23]. Because it has been reported that the aforementioned polyphenolic compounds can attenuate oxidative stress-mediated liver injury [24,25,26,27,28,29], not only the presence of these polyphenols but also other unidentified compounds in MLD may collaboratively contribute to protect the liver against CCl4-induced acute liver damage. Further studies are warranted to elucidate the major bioactive compounds in MLD.
The results presented here indicated that MLD synergistically prevented CCl4-mediated increases in hepatocyte degeneration characterized by ballooning, fatty vacuole formation, and eosinophilic cell death. In addition, MLD significantly reduced the serum activities of ALT and AST that were released from injured hepatocytes into the bloodstream, suggesting that MLD protects hepatocytes from CCl4-mediated acute injury effectively. Although centrilobular necrosis of hepatocytes has been identified as the predominant form of pathological death induced by CCl4 [30], other types of programmed cell death (e.g., pyroptosis, necroptosis, and apoptosis) are also associated with CCl4-induced liver injury [16,25,31,32]. Mitochondria, core subcellular organelles for the activation of the intrinsic apoptotic pathway, are the primary organelles targeted by CCl4. Exposure to CCl4 has been shown to downregulate mitochondrial membrane potential and promote the opening of transition pores, leading to the release of Ca2+ and membrane proteins (e.g., cytochrome c) into the cytoplasm, thereby facilitating the cleavage of initiator caspases through the activation of the apoptosome complex [30,32]. In addition, it has been reported that CCl4 activates the death receptor-dependent extrinsic apoptotic pathway by increasing the expression of Fas, Fas ligand, and the Fas-associated death domain in the liver [33,34]. Cleavage of executor caspases (e.g., caspase-3) caused by the activation of both apoptotic pathways facilitates the disruption of the nuclear envelope, inactivation of DNA repair enzymes (e.g., PARP), and fragmentation of chromosomes [35]. Although the effects of MLD on other types of cell death will require further study, the results presented here showing that MLD decreased the number of cleaved caspase-3- and cleaved PARP-positive cells in the hepatic tissues provide clear evidence that MLD-dependent hepatic protection from CCl4 can be attributed to the inhibition of apoptosis.
CCl4 absorbed by hepatocytes is primarily metabolized by cytochrome P450 2E1, producing trichloromethyl and trichloromethylperoxyl radicals in the process, which in turn accelerates lipid peroxidation by extracting hydrogen from polyunsaturated fatty acids [30]. Among the major oxidation products, 4-hyroxynonenal preferentially reacts with thiol-containing redox signaling proteins, resulting in mitochondrial dysfunction via the inactivation of mitochondrial ATPase [36]. In addition, CCl4 directly produces ROS and RNS via the uncoupling of oxidative phosphorylation in the mitochondrial membrane and induction of inducible nitric oxide synthase [30,37]. In parallel with our previous report [16], we showed that hepatic lipid peroxidation (e.g., 4-hydroxynonenal and malondialdehyde) and nitrotyrosine levels were increased in the CCl4-treated mice, suggesting that CCl4 induces hepatic oxidative stress, which was synergistically attenuated by MLD administration.
Nrf2 interacted with Keap1 is continuously degraded by the ubiquitin-proteasome system. Oxidative stress allows Nrf2 to dissociate from Keap1 and translocate into the nucleus, where Nrf2 induces the transcription of diverse antioxidant genes, such as glutamate cysteine ligase (an enzyme involved in the glutathione biogenesis), SOD, catalase, and NAD(P)H: quinone oxidoreductase 1, which act as a defense against oxidative stress-mediated cell injury [21]. Because genetic ablation of Nrf2 potentiates the severity of liver injury in experimental animals [38,39,40], Nrf2 is regarded as a key transcription factor coping with oxidative stress and inflammation in the liver. Consistent with previous reports [41,42], the results presented here showed that CCl4 downregulated hepatic mRNA levels of Nrf2, resulting in the depletion of hepatic glutathione, and reduced SOD and catalase activities, which may facilitate liver injury via the disruption of the antioxidant response. Moreover, our preliminary results using HepG2 cells (hepatocyte-surrogate cells) indicate that MLD significantly transactivated antioxidant response element-harboring reporter genes and induced the mRNA level of NAD(P)H: quinone oxidoreductase 1 in a concentration-dependent manner [23]. Together, these results indicate that MLD synergistically restored impaired Nrf2 mRNA expression as well as antioxidant defense responses, suggesting that MLD-mediated Nrf2 activation protects against CCl4-mediated liver injury by alleviating oxidative stress.
Present results revealed that MLD significantly decreased the CCl4-mediated infiltration of inflammatory cells and the production of proinflammatory cytokines. Among the proinflammatory cytokines produced, TNF-α is unique in terms of its rapid induction and ability to induce cell death after CCl4 injection [30,43]. The targeted disruption of TNF-α or TNF receptor I has been shown to attenuate hepatic injury and decrease ALT activity induced by CCl4 [44], implying that TNF-α is the most important cytokine for the progression of CCl4-mediated acute liver injury. In addition, TNF-α accelerates the infiltration of inflammatory cells into the damaged lesions by upregulating the expression of adhesion molecules [44]. The subsequent binding of TNF-α to receptors expressed by these inflammatory cells and damaged hepatocytes activates diverse signaling cascades including that of nuclear factor-κB (NF-κB) which further stimulate the production of proinflammatory cytokines (e.g., IL-1β and IL-6) [30,45]. Moreover, the CCl4-mediated release of damage-associated molecular patterns from injured hepatocytes activates pyroptosis [25] and may promote the maturation and secretion of IL-1β. In parallel with a previous report [46], our preliminary results also showed that MLD synergistically blocked the increases in mRNA levels of Rel A, a major subunit of NF-κB, induced by CCl4 (data not shown). Furthermore, it has been reported that the induction of Nrf2-dependent antioxidant genes regulates the production of proinflammatory cytokines by inhibiting NF-κB [47]. More interestingly, Nrf2 inhibits the transcription of proinflammatory cytokines via direct binding to the promoter [48]. Therefore, the downregulation of NF-κB as well as the activation of Nrf2 by MLD may help to inhibit hepatic inflammation, thereby attenuating inflammation-mediated hepatocyte injury in CCl4-treated mice.
As a process to develop novel functional food for preventing liver disease, we previously found that MLD (200 mg/kg) was the most potent hepatoprotective herbal mixture against CCl4 when mice were given fixed doses of lemon balm and dandelion herbal extracts at various combination ratios (e.g., 1:1, 1:2, 1:4, 1:6, 1:8, 2:1, 4:1, 6:1, and 8:1) [15]. In the present study, the doses-dependent hepatoprotective effects of MLD were compared to silymarin as well as to each single herbal extract alone. The hepatoprotective effects of either 100 or 200 mg/kg of MLD were more potent than those of either herbal extract (100 mg/kg each). Although the majority of biochemical and histopathological parameters assessed in the 50 mg/kg MLD group improved significantly, the body weight gain, number of degenerated hepatocytes, inflammatory cell counts, modified HAI score, number of 4-hydroxynonenal-positive cells, glutathione levels, hepatic TNF-α protein levels, and hepatic IL-1β protein levels did not differ from those observed in response to single herbal extracts alone. Moreover, the magnitude of MLD-mediated hepatic protection was greater than that mediated by silymarin, with the exception of numbers of degenerated hepatocytes and 4-hydroxynonenal-positive cells.
5. Conclusions
In conclusion, the present results showed that MLD synergistically alleviated CCl4-mediated acute liver injury by inhibiting apoptosis, oxidative stress, and proinflammatory cytokine production. In addition, oxidative stress and inflammation are considered the major etiology in the progression of chronic liver disease. If additional experiments for verifying the beneficial effect of MLD on the chronic liver disease are conducted appropriately, MLD may be a promising functional food for the treatment or prevention of oxidative stress-mediated acute and chronic liver injury.
Author Contributions
Conceptualization, B.-R.C., K.-M.P. and S.-K.K.; methodology, S.-J.J. and S.-K.K.; formal analysis, I.-J.C., D.-G.L. and S.-K.K.; investigation, B.-R.C., D.-G.L. and S.-K.K.; resources, B.-R.C. and S.-J.J.; data curation, J.-K.K., I.-J.C. and S.-K.K.; writing—original draft preparation, B.-R.C., I.-J.C. and S.-K.K.; writing—review and editing, S.-K.K. and K.-M.P.; visualization, J.-K.K., I.-J.C. and S.-K.K.; supervision, S.-K.K. and K.-M.P.; B.-R.C. and I.-J.C. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Animal experiment was conducted according to the national regulations regarding the use and welfare of laboratory animals and was approved by Institutional Animal Care and Use of Daegu Haany University (Approval No. DHU2019-016).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signaling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Brenner, C.; Galluzzi, L.; Keep, O.; Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013, 59, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver disease. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar]
- World Health Organization. Folium Melissae. In WHO Monographs on Selected Medicinal Plants, 1st ed.; World Health Organization, Ed.; World Health Organization: Geneva, Switzerland, 2004; Volume 2, pp. 180–187. [Google Scholar]
- Jun, H.J.; Lee, J.H.; Jia, Y.; Hoang, M.H.; Byun, H.; Kim, K.H.; Lee, S.J. Melissa officinalis essential oil reduces plasma triglycerides in human apolipoprotein E2 transgenic mice by inhibiting sterol regulatory element-binding protein-1c-dependent fatty acid synthesis. J. Nutr. 2012, 142, 432–440. [Google Scholar] [CrossRef][Green Version]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.T.; Park, J.H.; Kim, H.J.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem. Toxicol. 2013, 58, 30–36. [Google Scholar] [CrossRef]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.T.; Park, J.H.; Kim, H.J.; Kim, M.S.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Dandelion leaf extract protects against liver injury induced by methionine- and choline-deficient diet in mice. J. Med. Food. 2013, 16, 26–33. [Google Scholar] [CrossRef]
- Colle, D.; Arantes, L.P.; Gubert, P.; da Luz, S.C.; Athayde, M.L.; Teixeira Rocha, J.B.; Soares, F.A. Antioxidant properties of Taraxacum officinale leaf extract are involved in the protective effect against hepatoxicity induced by acetaminophen in mice. J. Med. Food. 2012, 15, 549–556. [Google Scholar] [CrossRef]
- Park, C.M.; Cha, Y.S.; Youn, H.J.; Cho, C.W.; Song, Y.S. Amelioration of oxidative stress by dandelion extract through CYP2E1 suppression against acute liver injury induced by carbon tetrachloride in Sprague-Dawley rats. Phytother. Res. 2010, 24, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Yoo, S.; Yoon, H.G.; Park, J.; Lee, Y.H.; Kim, S.; Oh, K.T.; Lee, J.; Cho, H.Y.; Jun, W. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem. Toxicol. 2010, 48, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Cho, H.S.; Lee, J.J.; Lee, H.J.; Jun, S.Y.; Lee, J.H.; Song, H.H.; Choi, S.; Saloura, V.; Park, C.G.; et al. Novel TRAIL sensitizer Taraxacum officinale F.H. Wigg enhances TRAIL-induced apoptosis in Huh7 cells. Mol. Carcinog. 2016, 55, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Cho, I.J.; Jung, S.J.; Kim, J.K.; Lee, D.G.; Ku, S.K.; Park, K.M. Study on the hepatoprotective effects of lemon balm and dandelion leaf extract combination in carbon tetrachloride-mediated liver injured mice. Herbal Formula Sci. 2019, 27, 199–211. [Google Scholar]
- Jung, J.Y.; Park, S.M.; Ko, H.L.; Lee, J.R.; Park, C.A.; Byun, S.H.; Ku, S.K.; Cho, I.J.; Kim, S.C. Epimedium koreanum ameliorates oxidative stress-mediated liver injury by activating nuclear factor erythroid 2-related factor 2. Am. J. Chin. Med. 2018, 46, 469–488. [Google Scholar] [CrossRef] [PubMed]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.M.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Hu, J.R.; Chun, Y.S.; Kim, J.K.; Cho, I.J.; Ku, S.K. Ginseng berry aqueous extract prevents scopolamine-induced memory impairment in mice. Exp. Ther. Med. 2019, 18, 4388–4396. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Okamoto, T.; Okabe, S. Carbon tetrachloride treatment induces anorexia independently of hepatitis in rats. Int. J. Mol. Med. 2000, 6, 181–183. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Cho, I.J.; Jung, S.J.; Kim, J.K.; Park, S.M.; Lee, D.G.; Ku, S.K.; Park, K.M. Lemon balm and dandelion leaf extract synergistically alleviate ethanol-induced hepatotoxicity by enhancing antioxidant and anti-inflammatory activity. J. Food Biochem. 2020, 44, e13232. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lai, Y.; Huang, P.; Xie, L.; Lin, H.; Zhou, Z.; Mo, C.; Deng, G.; Yan, W.; Gao, Z.; et al. Naringin attenuates alcoholic liver injury by reducing lipid accumulation and oxidative stress. Life Sci. 2019, 216, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Shi, H.; Wang, Y.; Liu, X.; Cheng, Y.; Li, H.; Zhao, H.; Wang, S.; Dong, L. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int. Immunopharmacol. 2018, 54, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Kim, S.C.; Kim, K.M.; Jang, C.H.; Cho, S.S.; Kim, S.J.; Ku, S.K.; Cho, I.J.; Ki, S.H. Isorhamnetin attenuates liver fibrosis by inhibiting TGF-β/Smad signaling and relieving oxidative stress. Eur. J. Pharmacol. 2016, 783, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Landmann, M.; Kanuri, G.; Spruss, A.; Stahl, C.; Bergheim, I. Oral intake of chicoric acid reduces acute alcohol-induced hepatic steatosis in mice. Nutrition 2014, 30, 882–889. [Google Scholar] [CrossRef]
- Domitrović, R.; Skoda, M.; Marchesi, V.V.; Cvijanović, O.; Pugel, E.P.; Stefan, M.B. Rosmarinic acid ameliorates acute liver damage and fibrogenesis in carbon tetrachloride-intoxicated mice. Food Chem. Toxicol. 2013, 51, 370–378. [Google Scholar]
- Domitrović, R.; Jakovac, H.; Milin, C.; Radosević-Stasić, B. Dose- and time-dependent effects of luteolin on carbon tetrachloride-induced hepatotoxicity in mice. Exp. Toxicol. Pathol. 2009, 61, 581–589. [Google Scholar] [CrossRef]
- Weber, L.W.; Boll, M.; Stampfl, A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef]
- Choi, H.S.; Kang, J.W.; Lee, S.M. Melatonin attenuates carbon tetrachloride-induced liver fibrosis via inhibition of necroptosis. Transl. Res. 2015, 166, 292–303. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Nowicki, M.J. Caspase-12 mediates carbon tetrachloride-induced hepatocyte apoptosis in mice. World J. Gastroenterol. 2014, 20, 18189–18198. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Ting, W.J.; Shen, C.Y.; Hsu, H.H.; Lin, Y.M.; Kuo, C.H.; Tsai, F.J.; Tsai, C.H.; Tsai, Y.; Huang, C.Y. Anti-apoptotic effect of San Huang Shel Shin Tang cyclodextrin complex (SHSSTc) on CCl4-induced hepatotoxicity in rats. Environ. Toxicol. 2016, 31, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Xu, Y.; Xu, L.; Cong, X.; Yin, L.; Li, H.; Peng, J. Mechanism investigation of dioscin against CCl4-induced acute liver damage in mice. Environ. Toxicol. Pharmacol. 2012, 34, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-Dos-Santos, Â. A cell’s fate: An overview of the molecular biology and genetics of apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef] [PubMed]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 2016, 311, C537–C543. [Google Scholar] [CrossRef]
- Tipoe, G.L.; Leung, T.M.; Liong, E.; So, H.; Leung, K.M.; Lau, T.Y.; Tom, W.M.; Fung, M.L.; Fan, S.T.; Nanji, A.A. Inhibitors of inducible nitric oxide (NO) synthase are more effective than an NO donor in reducing carbon-tetrachloride induced acute liver injury. Histol. Histopathol. 2006, 21, 1157–1165. [Google Scholar]
- Xu, W.; Hellerbrand, C.; Köhler, U.A.; Bugnon, P.; Kan, Y.W.; Werner, S.; Beyer, T.A. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab. Investig. 2008, 88, 1068–1078. [Google Scholar] [CrossRef]
- Lamlé, J.; Marhenke, S.; Borlak, J.; von Wasielewski, R.; Eriksson, C.J.; Geffers, R.; Manns, M.P.; Yamamoto, M.; Vogel, A. Nuclear factor-erythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology 2008, 134, 1159–1168. [Google Scholar] [CrossRef]
- Enomoto, A.; Itoh, K.; Nagayoshi, E.; Haruta, J.; Kimura, T.; O’Connor, T.; Harada, T.; Yamamoto, M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001, 59, 169–177. [Google Scholar] [CrossRef]
- Peng, C.; Zhou, Z.M.; Li, J.; Luo, Y.; Zhou, Y.S.; Ke, X.H.; Huang, K.E. CCl4-induced liver injury was ameliorated by Qi-Ge decoction through the antioxidant pathway. Evid. Based Complement. Alternat. Med. 2019, 2019, 5941263. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Alilou, M. Naringenin attenuates CCl4-induced hepatic inflammation by the activation of an Nrf2-mediated pathway in rats. Clin. Exp. Pharmacol. Physiol. 2014, 41, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Morio, L.A.; Chiu, H.; Sprowles, K.A.; Zhou, P.; Heck, D.E.; Gordon, M.K.; Laskin, D.L. Distinct role of tumor necrosis factor α and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicol. Appl. Pharmacol. 2001, 172, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, S.J.; Myers, C.L.; Wallace, R.W.; Parks, T.P. Intercellular adhesion molecule-1 gene expression in human endothelial cells: Differential regulation by tumor necrosis factor-a and phorbol myristate acetate. J. Biol. Chem. 1992, 267, 12030–12035. [Google Scholar] [PubMed]
- Turner, N.A.; Mughal, R.S.; Warburton, P.; O’Regan, D.J.; Ball, S.G.; Porter, K.E. Mechanism of TNFalpha-induced IL-1alpha, IL-1beta and IL-6 expression in human cardiac fibroblasts: Effects of statins and thiazolidinediones. Cardiovasc. Res. 2007, 76, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, D.; Li, D.; Chen, X.; Wang, B.; Wang, F.; Liu, X.; Shang, J.; Zheng, Q. Licochalcone E protects against carbon tetrachloride-induced liver toxicity by activating peroxisome proliferator-activated receptor gamma. Mol. Med. Rep. 2017, 16, 5269–5276. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking cytokine transcription. Nature Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).