Facile Preparation of Biocompatible and Transparent Silica Aerogels as Ionogels Using Choline Dihydrogen Phosphate Ionic Liquid
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silica Ionogels
2.3. Characterization of Ionogels
3. Results
3.1. FT-IR Spectra
3.2. TGA Analyses
3.3. N2 Adsorption Isotherm
3.4. Solid-State NMR Spectra
3.5. Rheological Studies
3.6. Particle Size Analyses
3.7. XRD Analyses
3.8. SAXS Analyses
3.9. SEM Studies
3.10. TEM Studies
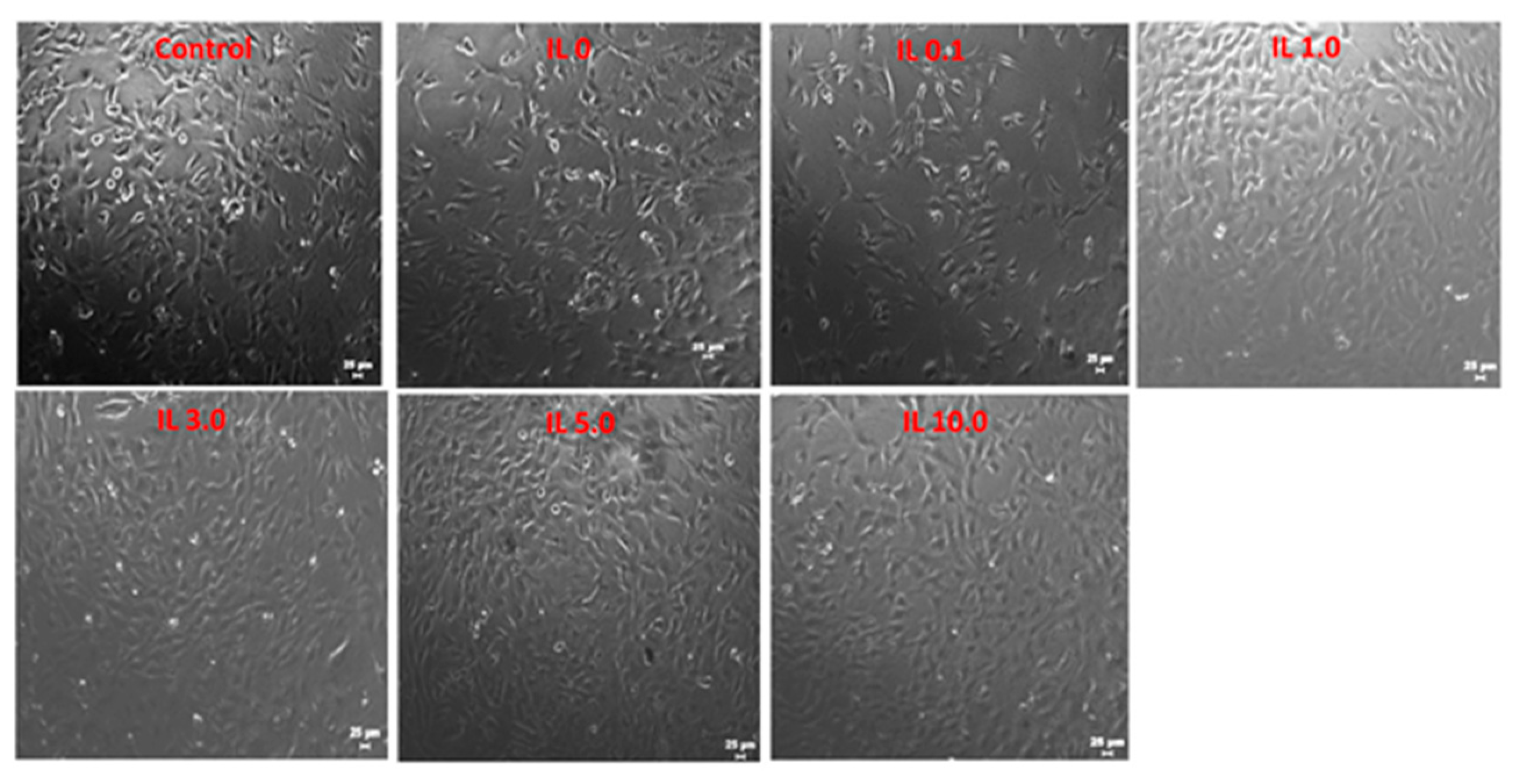
3.11. Biocompatibility Studies of the Ionogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwan, M.; Ratke, L. Flexibilisation of resorcinol-formaldehyde aerogels. J. Mater. Chem. A 2013, 1, 13462–13468. [Google Scholar] [CrossRef]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.-K.; Gaponik, N.; Eychmüller, A. Modern Inorganic Aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive manufacturing of silica aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Zhang, R.; Qiao, W.; Zhang, L.; Liang, X.; Ling, L. Biomolecular adsorption behavior on spherical carbon aerogels with various mesopore sizes. J. Colloid Interface Sci. 2009, 331, 40–46. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shahat, A.; Ismael, M. Mesoporous aluminosilica monoliths for the adsorptive removal of small organic pollutants. J. Hazard. Mater. 2012, 201–202, 23–32. [Google Scholar] [CrossRef]
- Lee, S.; Cha, Y.C.; Hwang, H.J.; Moon, J.-W.; Han, I.S. The effect of pH on the physicochemical properties of silica aerogels prepared by an ambient pressure drying method. Mater. Lett. 2007, 61, 3130–3133. [Google Scholar] [CrossRef]
- Bhatia, R.B.; Brinker, C.J.; Gupta, A.K.; Singh, A.K. Aqueous Sol-Gel Process for Protein Encapsulation. Chem. Mater. 2000, 12, 2434–2441. [Google Scholar] [CrossRef]
- Jin, W.; Brennan, J.D. Properties and applications of proteins encapsulated within sol-gel derived materials. Analytica Chimica Acta 2002, 461, 1–36. [Google Scholar] [CrossRef]
- Li, Y.K.; Chou, M.J.; Wu, T.-Y.; Jinn, T.-R.; Chen-Yang, Y.W. A novel method for preparing a protein-encapsulated bioaerogel: Using a red fluorescent protein as a model. Acta Biomaterialia 2008, 4, 725–732. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Liu, X.; Wang, J.; Wang, J.; Dong, K.; Sun, J.; Xu, B. Ionic liquid clusters: Structure, formation mechanism, and effect on the behavior of ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 5893–5906. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chen, N.; Chen, R.; Wang, L.; Li, L. Organically modified silica-supported ionogels electrolyte for high temperature lithium-ion batteries. Nano Energy 2017, 31, 9–18. [Google Scholar] [CrossRef]
- Tröger-Müller, S.; Brandt, J.; Antonietti, M.; Liedel, C. Green Imidazolium Ionics—From Truly Sustainable Reagents to Highly Functional Ionic Liquids. Chem. Eur. J. 2017, 23, 11810–11817. [Google Scholar] [CrossRef]
- Donato, R.K.; Matějka, L.; Schrekker, H.S.; Pleštil, J.; Jigounov, A.; Brus, J.; Šlouf, M. The multifunctional role of ionic liquids in the formation of epoxy-silica nanocomposites. J. Mater. Chem. 2011, 21, 13801–13810. [Google Scholar] [CrossRef]
- Sun, J.-K.; Antonietti, M.; Yuan, J. Nanoporous ionic organic networks: From synthesis to materials applications. Chem. Soc. Rev. 2016, 45, 6627–6656. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A.; Nordstierna, L. An investigation of the sol-gel process in ionic liquid-silica gels by time resolved Raman and 1H NMR spectroscopy. Phys. Chem. Chem. Phys. 2012, 14, 13216–13223. [Google Scholar] [CrossRef] [PubMed]
- Viau, L.; Néouze, M.-A.; Biolley, C.; Volland, S.; Brevet, D.; Gaveau, P.; Dieudonné, P.; Galarneau, A.; Vioux, A. Ionic Liquid Mediated Sol-Gel Synthesis in the Presence of Water or Formic Acid: Which Synthesis for Which Material? Chem. Mater. 2012, 24, 3128–3134. [Google Scholar] [CrossRef]
- Dai, S.; Ju, Y.H.; Gao, H.J.; Lin, J.S.; Pennycook, S.J.; Barnes, C.E. Preparation of silica aerogel using ionic liquids as solvents. Chem. Commun. 2000, 243–244. [Google Scholar] [CrossRef]
- Migliorini, M.V.; Donato, R.K.; Benvegnú, M.A.; Gonçalves, R.S.; Schrekker, H.S. Imidazolium ionic liquids as bifunctional materials (morphology controller and pre-catalyst) for the preparation of xerogel silica’s. J. Sol Gel Sci. Technol. 2008, 48, 272–276. [Google Scholar] [CrossRef]
- Vioux, A.; Viau, L.; Volland, S.; Le Bideau, J. Use of ionic liquids in sol-gel; ionogels and applications. Comptes Rendus Chimie 2010, 13, 242–255. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Lunstroot, K.; Driesen, K.; Nockemann, P.; Van Hecke, K.; Van Meervelt, L.; Görller-Walrand, C.; Binnemans, K.; Bellayer, S.; Viau, L.; Le Bideau, J.; et al. Lanthanide-Doped luminescent ionogels. Dalton Trans. 2009, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Néouze, M.-A.; Le Bideau, J.; Gaveau, P.; Bellayer, S.; Vioux, A. Ionogels, New Materials Arising from the Confinement of Ionic Liquids within Silica-Derived Networks. Chem. Mater. 2006, 18, 3931–3936. [Google Scholar] [CrossRef]
- Gupta, A.K.; Singh, R.K.; Chandra, S. Studies on mesoporous silica ionogels prepared by sol-gel method at different gelation temperatures. RSC Adv. 2013, 3, 13869–13877. [Google Scholar] [CrossRef]
- Vekariya, R.L.; Dhar, A.; Lunagariya, J. Synthesis and characterization of double-SO3H functionalized Brönsted acidic hydrogensulfate ionic liquid confined with silica through sol-gel method. Compos. Interfaces 2017, 24, 801–816. [Google Scholar] [CrossRef]
- Xue, C.; Zhu, H.; Du, X.; An, X.; Wang, E.; Duan, D.; Shi, L.; Hao, X.; Xiao, B.; Peng, C. Unique allosteric effect-driven rapid adsorption of carbon dioxide in a newly designed ionogel [P4444][2-Op]@MCM-41 with excellent cyclic stability and loading-dependent capacity. J. Mater. Chem. A 2017, 5, 6504–6514. [Google Scholar] [CrossRef]
- Göbel, R.; Friedrich, A.; Taubert, A. Tuning the phase behavior of ionic liquids in organically functionalized silica ionogels. Dalton Trans. 2010, 39, 603–611. [Google Scholar] [CrossRef]
- Göbel, R.; White, R.J.; Titirici, M.-M.; Taubert, A. Carbon-Based ionogels: Tuning the properties of the ionic liquid via carbon-ionic liquid interaction. Phys. Chem. Chem. Phys. 2012, 14, 5992–5997. [Google Scholar] [CrossRef]
- Sharma, M.; Mondal, D.; Mukesh, C.; Prasad, K. Preparation of tamarind gum based soft ion gels having thixotropic properties. Carbohydr. Polym. 2014, 102, 467–471. [Google Scholar] [CrossRef]
- Viau, L.; Tourné-Péteilh, C.; Devoisselle, J.-M.; Vioux, A. Ionogels as drug delivery system: One-step sol-gel synthesis using imidazolium ibuprofenate ionic liquid. Chem. Commun. 2010, 46, 228–230. [Google Scholar] [CrossRef]
- Sen, K.; Mendes, E.; Wolterbeek, H.T. Combined effort of Fe-dextran and an RTIL towards formation of ionogel. J. Sol Gel Sci. Technol. 2012, 63, 135–139. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Rao, K.S.; Kumar, A. Facile preparation of agarose-chitosan hybrid materials and nanocomposite ionogels using an ionic liquid via dissolution, regeneration and sol-gel transition. Green Chem. 2014, 16, 320–330. [Google Scholar] [CrossRef]
- Subianto, S.; Mistry, M.K.; Choudhury, N.R.; Dutta, N.K.; Knott, R. Composite Polymer Electrolyte Containing Ionic Liquid and Functionalized Polyhedral Oligomeric Silsesquioxanes for Anhydrous PEM Applications. ACS Appl. Mater. Interfaces 2009, 1, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Lin, B.; Yan, F.; Qiu, L.; Lu, J. Macromolecular protic ionic liquid-based proton-conducting membranes for anhydrous proton exchange membrane application. J. Power Sources 2011, 196, 7979–7984. [Google Scholar] [CrossRef]
- Maity, S.; Singha, S.; Jana, T. Low acid leaching PEM for fuel cell based on polybenzimidazole nanocomposites with protic ionic liquid modified silica. Polymer 2015, 66, 76–85. [Google Scholar] [CrossRef]
- Aquino, S.A.; Vieira, M.O.; Ferreira, A.S.D.; Cabrita, E.J.; Einloft, S.; de Souza, M.O. Hybrid Ionic Liquid-Silica Xerogels Applied in CO2 Capture. Appl. Sci. 2019, 9, 2614. [Google Scholar] [CrossRef]
- Lee, S.H.; Doan, T.T.N.; Ha, S.H.; Koo, Y.-M. Using ionic liquids to stabilize lipase within sol–gel derived silica. J. Mol. Catal. B Enzym. 2007, 45, 57–61. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Li, J.; Li, Z.; He, P.; Liu, H.; Li, J. Highly active horseradish peroxidase immobilized in 1-butyl-3-methylimidazolium tetrafluoroborate room-temperature ionic liquid based sol-gel host materials. Chem. Commun. 2005, 1778–1780. [Google Scholar] [CrossRef]
- Wood, N.; Stephens, G. Accelerating the discovery of biocompatible ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 1670–1674. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Pei, Y.; Wang, J.; He, M. Design of environmentally friendly ionic liquid aqueous two-phase systems for the efficient and high activity extraction of proteins. Green Chem. 2012, 14, 2941–2950. [Google Scholar] [CrossRef]
- Winther-Jensen, O.; Vijayaraghavan, R.; Sun, J.; Winther-Jensen, B.; MacFarlane, D.R. Self polymerising ionic liquid gel. Chem. Commun. 2009, 3041–3043. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C. Proteins in Ionic Liquids: Current Status of Experiments and Simulations. Top. Curr. Chem. 2017, 375, 127–152. [Google Scholar] [CrossRef]
- Elhi, F.; Priks, H.; Rinne, P.; Kaldalu, N.; Žusinaite, E.; Johanson, U.; Aabloo, A.; Tamm, T.; Põhako-Esko, K. Electromechanically active polymer actuators based on biofriendly choline ionic liquids. Smart Mater. Struct. 2020, 29, 055021. [Google Scholar] [CrossRef]
- Silva, L.P.; Moya, C.; Sousa, M.; Santiago, R.; Sintra, T.E.; Carreira, A.R.F.; Palomar, J.; Coutinho, J.A.P.; Carvalho, P.J. Encapsulated Amino-Acid-Based Ionic Liquids for CO2 Capture. Eur. J. Inorg. Chem. 2020, 2020, 3158–3166. [Google Scholar] [CrossRef]
- Foureau, D.M.; Vrikkis, R.M.; Jones, C.P.; Weaver, K.D.; MacFarlane, D.R.; Salo, J.C.; McKillop, I.H.; Elliott, G.D. In Vitro Assessment of Choline Dihydrogen Phosphate (CDHP) as a Vehicle for Recombinant Human Interleukin-2 (rhIL-2). Cell. Mol. Bioeng. 2012, 5, 390–401. [Google Scholar] [CrossRef]
- Fujita, K.; MacFarlane, D.R.; Forsyth, M. Protein solubilising and stabilising ionic liquids. Chem. Commun. 2005, 4804–4806. [Google Scholar] [CrossRef]
- Weaver, K.D.; Vrikkis, R.M.; Van Vorst, M.P.; Trullinger, J.; Vijayaraghavan, R.; Foureau, D.M.; McKillop, I.H.; MacFarlane, D.R.; Krueger, J.K.; Elliott, G.D. Structure and function of proteins in hydrated choline dihydrogen phosphate ionic liquid. Phys. Chem. Chem. Phys. 2012, 14, 790–801. [Google Scholar] [CrossRef]
- Fujita, K.; MacFarlane, D.R.; Forsyth, M.; Yoshizawa-Fujita, M.; Murata, K.; Nakamura, N.; Ohno, H. Solubility and Stability of Cytochrome c in Hydrated Ionic Liquids: Effect of Oxo Acid Residues and Kosmotropicity. Biomacromolecules 2007, 8, 2080–2086. [Google Scholar] [CrossRef]
- Mukesh, C.; Mondal, D.; Sharma, M.; Prasad, K. Rapid dissolution of DNA in a novel bio-based ionic liquid with long-term structural and chemical stability: Successful recycling of the ionic liquid for reuse in the process. Chem. Commun. 2013, 49, 6849–6851. [Google Scholar] [CrossRef]
- Hoshino, T.; Fujita, K.; Higashi, A.; Sakiyama, K.; Ohno, H.; Morishima, K. Contracting cardiomyocytes in hydrophobic room-temperature ionic liquid. Biochem. Biophys. Res. Commun. 2012, 427, 379–384. [Google Scholar] [CrossRef]
- Tateishi-Karimata, H.; Sugimoto, N. A-T Base Pairs are More Stable than G-C Base Pairs in a Hydrated Ionic Liquid. Angewandte Chemie 2012, 51, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Meera, K.M.S.; Sankar, R.M.; Jaisankar, S.N.; Mandal, A.B. Mesoporous and biocompatible surface active silica aerogel synthesis using choline formate ionic liquid. Colloids Surf. B Biointerfaces 2011, 86, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Seeni Meera, K.; Murali Sankar, R.; Jaisankar, S.N.; Baran Mandal, A. Studies on Biocompatible Surface-Active Silica Aerogel and Polyurethane-Siloxane Cross-Linked Structures for Various Surfaces. In Encyclopedia of Biocolloid and Biointerface Science 2V Set; Ohshima, H., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 1–16. [Google Scholar]
- Seeni Meera, K.M.; Murali Sankar, R.; Murali, A.; Jaisankar, S.N.; Mandal, A.B. Sol-Gel network silica/modified montmorillonite clay hybrid nanocomposites for hydrophobic surface coatings. Colloids Surf. B Biointerfaces 2012, 90, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Delahaye, E.; Göbel, R.; Löbbicke, R.; Guillot, R.; Sieber, C.; Taubert, A. Silica ionogels for proton transport. J. Mater. Chem. 2012, 22, 17140–17146. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds; John Wiley and Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Karout, A.; Pierre, A.C. Porous texture of silica aerogels made with ionic liquids as gelation catalysts. J. Sol Gel Sci. Technol. 2009, 49, 364–372. [Google Scholar] [CrossRef]
- Ashby, D.S.; DeBlock, R.H.; Lai, C.-H.; Choi, C.S.; Dunn, B.S. Patternable, Solution-Processed Ionogels for Thin-Film Lithium-Ion Electrolytes. Joule 2017, 1, 344–358. [Google Scholar] [CrossRef]
- Verma, Y.L.; Gupta, A.K.; Singh, R.K.; Chandra, S. Preparation and characterisation of ionic liquid confined hybrid porous silica derived from ultrasonic assisted non-hydrolytic sol-gel process. Microporous Mesoporous Mater. 2014, 195, 143–153. [Google Scholar] [CrossRef]
- Yoshizawa-Fujita, M.; Fujita, K.; Forsyth, M.; MacFarlane, D.R. A new class of proton-conducting ionic plastic crystals based on organic cations and dihydrogen phosphate. Electrochem. Commun. 2007, 9, 1202–1205. [Google Scholar] [CrossRef]
- Rana, U.A.; Bayley, P.M.; Vijayaraghavan, R.; Howlett, P.; MacFarlane, D.R.; Forsyth, M. Proton transport in choline dihydrogen phosphate/H3PO4 mixtures. Phys. Chem. Chem. Phys. 2010, 12, 11291–11298. [Google Scholar] [CrossRef]
- Wang, X.; Jana, S.C. Synergistic Hybrid Organic-Inorganic Aerogels. ACS Appl. Mater. Interfaces 2013, 5, 6423–6429. [Google Scholar] [CrossRef]
- Bothwell, K.M.; Marr, P.C. Taming the Base Catalyzed Sol-Gel Reaction: Basic Ionic Liquid Gels of SiO2 and TiO2. ACS Sustain. Chem. Eng. 2017, 5, 1260–1263. [Google Scholar] [CrossRef]
- Singh, M.P.; Singh, R.K.; Chandra, S. Ionic liquids confined in porous matrices: Physicochemical properties and applications. Progress Mater. Sci. 2014, 64, 73–120. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). J. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sert Çok, S.; Koç, F.; Balkan, F.; Gizli, N. Revealing the pore characteristics and physicochemical properties of silica ionogels based on different sol-gel drying strategies. J. Solid State Chem. 2019, 278, 120877. [Google Scholar] [CrossRef]
- Gupta, A.K.; Singh, M.P.; Singh, R.K.; Chandra, S. Low density ionogels obtained by rapid gellification of tetraethyl orthosilane assisted by ionic liquids. Dalton Trans. 2012, 41, 6263–6271. [Google Scholar] [CrossRef]
- Seeni Meera, K.M.; Murali Sankar, R.; Jaisankar, S.N.; Mandal, A.B. Physicochemical Studies on Polyurethane/Siloxane Cross-Linked Films for Hydrophobic Surfaces by the Sol–Gel Process. J. Phys. Chem. B 2013, 117, 2682–2694. [Google Scholar] [CrossRef]
- Seeni Meera, K.M.; Murali Sankar, R.; Paul, J.; Jaisankar, S.N.; Mandal, A.B. The influence of applied silica nanoparticles on a bio-renewable castor oil based polyurethane nanocomposite and its physicochemical properties. Phys. Chem. Chem. Phys. 2014, 16, 9276–9288. [Google Scholar] [CrossRef]
- Wang, G.-H.; Zhang, L.-M. A Biofriendly Silica Gel for in Situ Protein Entrapment: Biopolymer-Assisted Formation and Its Kinetic Mechanism. J. Phys. Chem. B 2009, 113, 2688–2694. [Google Scholar] [CrossRef]
- Poolman, J.M.; Boekhoven, J.; Besselink, A.; Olive, A.G.L.; van Esch, J.H.; Eelkema, R. Variable gelation time and stiffness of low-molecular-weight hydrogels through catalytic control over self-assembly. Nat. Protoc. 2014, 9, 977–988. [Google Scholar] [CrossRef]
- Carvalho, A.P.A.; Soares, B.G.; Livi, S. Organically modified silica (ORMOSIL) bearing imidazolium—Based ionic liquid prepared by hydrolysis/co-condensation of silane precursors: Synthesis, characterization and use in epoxy networks. Eur. Polym. J. 2016, 83, 311–322. [Google Scholar] [CrossRef]
- Pandey, P.K.; Rawat, K.; Aswal, V.K.; Kohlbrecher, J.; Bohidar, H.B. DNA ionogel: Structure and self-assembly. Phys. Chem. Chem. Phys. 2017, 19, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, A.; Ghosh, S.; Maity, S.; Jana, T. Polybenzimidazole gel membrane for the use in fuel cell. Polymer 2011, 52, 4319–4330. [Google Scholar] [CrossRef]
- Dawedeit, C.; Kim, S.H.; Braun, T.; Worsley, M.A.; Letts, S.A.; Wu, K.J.; Walton, C.C.; Chernov, A.A.; Satcher, J.H.; Hamza, A.V.; et al. Tuning the rheological properties of sols for low-density aerogel coating applications. Soft Matter 2012, 8, 3518–3521. [Google Scholar] [CrossRef]
- Song, H.; Luo, Z.; Zhao, H.; Luo, S.; Wu, X.; Gao, J.; Wang, Z. High tensile strength and high ionic conductivity bionanocomposite ionogels prepared by gelation of cellulose/ionic liquid solutions with nano-silica. RSC Adv. 2013, 3, 11665–11675. [Google Scholar] [CrossRef]
- Rennie, A.J.R.; Martins, V.L.; Smith, R.M.; Hall, P.J. Influence of Particle Size Distribution on the Performance of Ionic Liquid-based Electrochemical Double Layer Capacitors. Sci. Rep. 2016, 6, 22062. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-P.; Yan, B. Luminescent nanoparticles prepared by encapsulating lanthanide chelates to silica sphere. Colloid Polym. Sci. 2014, 292, 1385–1393. [Google Scholar] [CrossRef]
- Ward, A.J.; Pujari, A.A.; Costanzo, L.; Masters, A.F.; Maschmeyer, T. Ionic liquid-templated preparation of mesoporous silica embedded with nanocrystalline sulfated zirconia. Nanoscale Res. Lett. 2011, 6, 192. [Google Scholar] [CrossRef]
- Ak, F.; Oztoprak, Z.; Karakutuk, I.; Okay, O. Macroporous Silk Fibroin Cryogels. Biomacromolecules 2013, 14, 719–727. [Google Scholar] [CrossRef]
- Polarz, S.; Smarsly, B. Nanoporous Materials. J. Nanosci. Nanotechnol. 2002, 2, 581–612. [Google Scholar] [CrossRef]
- Taubert, A.; Löbbicke, R.; Kirchner, B.; Leroux, F. First examples of organosilica-based ionogels: Synthesis and electrochemical behavior. Beilstein J. Nanotechnol. 2017, 8, 736–751. [Google Scholar] [CrossRef]
- Smarsly, B.; Göltner, C.; Antonietti, M.; Ruland, W.; Hoinkis, E. SANS Investigation of Nitrogen Sorption in Porous Silica. J. Phys. Chem. B 2001, 105, 831–840. [Google Scholar] [CrossRef]
- Wu, C.-M.; Lin, S.-Y. Close Packing Existence of Short-Chain Ionic Liquid Confined in the Nanopore of Silica Ionogel. J. Phys. Chem. C 2015, 119, 12335–12344. [Google Scholar] [CrossRef]
- Nayeri, M.; Nygård, K.; Karlsson, M.; Maréchal, M.; Burghammer, M.; Reynolds, M.; Martinelli, A. The role of the ionic liquid C6C1ImTFSI in the sol-gel synthesis of silica studied using in situ SAXS and Raman spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 9841–9848. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.D.; Kim, Y.-H.; Suh, K.-H.; Ahn, Y.-S.; Yeo, J.-G.; Han, J.-H. Superhydrophobic silica aerogel powders with simultaneous surface modification, solvent exchange and sodium ion removal from hydrogels. Microporous Mesoporous Mater. 2008, 112, 504–509. [Google Scholar] [CrossRef]
- Aravind, P.R.; Shajesh, P.; Mukundan, P.; Warrier, K.G.K. Silica-Titania aerogel monoliths with large pore volume and surface area by ambient pressure drying. J. Sol Gel Sci. Technol. 2009, 52, 328–334. [Google Scholar] [CrossRef]
- Göbel, R.; Hesemann, P.; Weber, J.; Möller, E.; Friedrich, A.; Beuermann, S.; Taubert, A. Surprisingly high, bulk liquid-like mobility of silica-confined ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 3653–3662. [Google Scholar] [CrossRef]
- Wu, C.-M.; Lin, S.-Y.; Chen, H.-L. Structure of a monolithic silica aerogel prepared from a short-chain ionic liquid. Microporous Mesoporous Mater. 2012, 156, 189–195. [Google Scholar] [CrossRef]








| Codes | TEOS:H2O:H3PO4 | IL (wt. %) | Gelation Time (min) a |
|---|---|---|---|
| IL 0 | 1:2:0.5 | 0 | 195 |
| IL 0.1 | 0.1 | 185 | |
| IL 0.5 | 0.5 | 170 | |
| IL 1.0 | 1.0 | 165 | |
| IL 3.0 | 3.0 | 155 | |
| IL 5.0 | 5.0 | 140 | |
| IL 10.0 | 10.0 | 120 |
| Codes | TGA |
|---|---|
| Weight Loss at 100 °C (%) | |
| IL 0 | 13.1 |
| IL 0.1 | 11.6 |
| IL 0.5 | 10.9 |
| IL 1.0 | 10.2 |
| IL 3.0 | 9.4 |
| IL 5.0 | 9.2 |
| IL 10.0 | 8.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal Mohamed, S.M.; Murali Sankar, R.; Kiran, M.S.; Jaisankar, S.N.; Milow, B.; Mandal, A.B. Facile Preparation of Biocompatible and Transparent Silica Aerogels as Ionogels Using Choline Dihydrogen Phosphate Ionic Liquid. Appl. Sci. 2021, 11, 206. https://doi.org/10.3390/app11010206
Kamal Mohamed SM, Murali Sankar R, Kiran MS, Jaisankar SN, Milow B, Mandal AB. Facile Preparation of Biocompatible and Transparent Silica Aerogels as Ionogels Using Choline Dihydrogen Phosphate Ionic Liquid. Applied Sciences. 2021; 11(1):206. https://doi.org/10.3390/app11010206
Chicago/Turabian StyleKamal Mohamed, Seeni Meera, Rajavelu Murali Sankar, Manikantan Syamala Kiran, Sellamuthu N. Jaisankar, Barbara Milow, and Asit Baran Mandal. 2021. "Facile Preparation of Biocompatible and Transparent Silica Aerogels as Ionogels Using Choline Dihydrogen Phosphate Ionic Liquid" Applied Sciences 11, no. 1: 206. https://doi.org/10.3390/app11010206
APA StyleKamal Mohamed, S. M., Murali Sankar, R., Kiran, M. S., Jaisankar, S. N., Milow, B., & Mandal, A. B. (2021). Facile Preparation of Biocompatible and Transparent Silica Aerogels as Ionogels Using Choline Dihydrogen Phosphate Ionic Liquid. Applied Sciences, 11(1), 206. https://doi.org/10.3390/app11010206







