Characterization of Magnetite–Silica Magnetic Fluids by Laser Scattering
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Magnetic Fluid
2.2. Assessment of Physical Characteristics of Magnetic Fluid
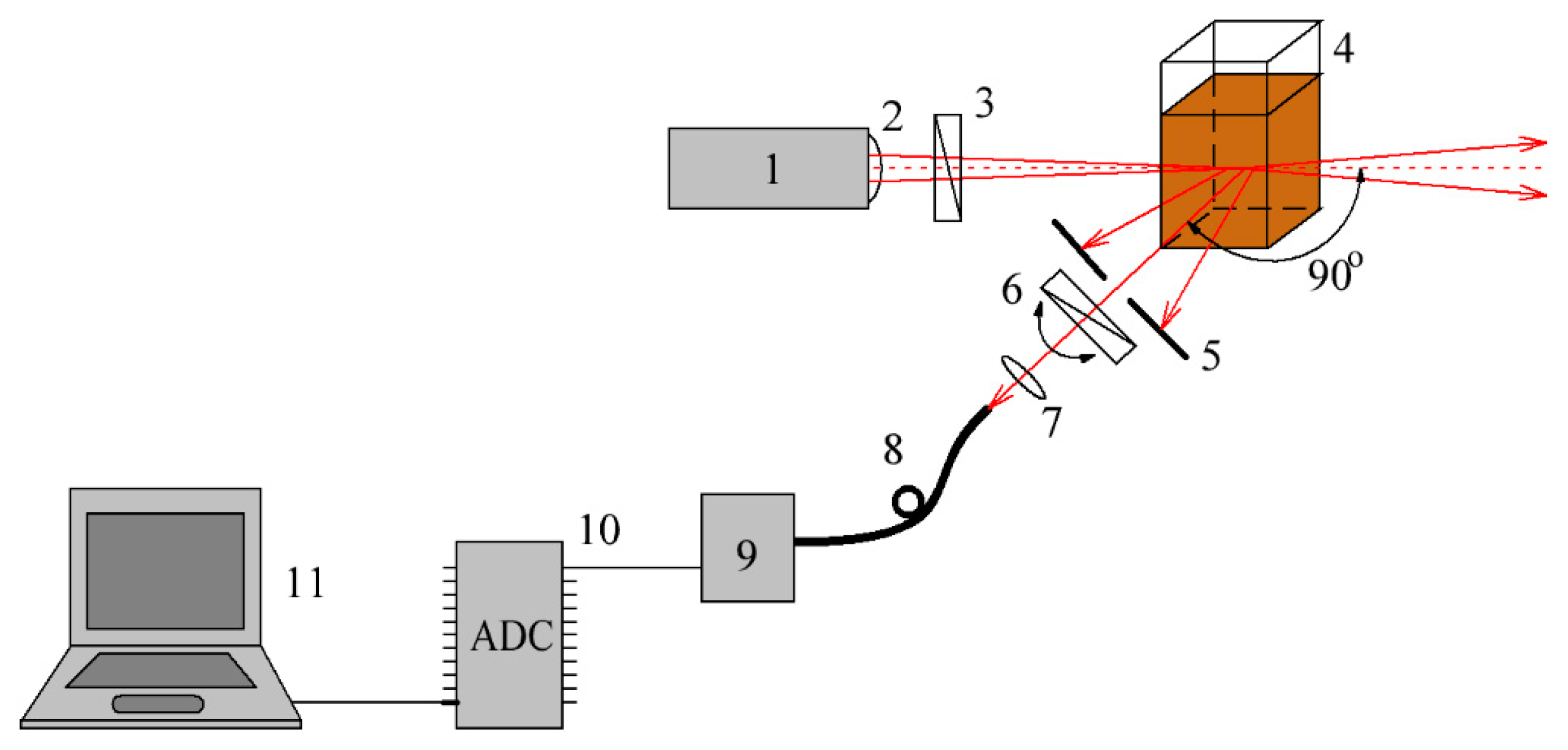
2.3. DLS-Based Original Experimental Technique
3. Results and Discussion
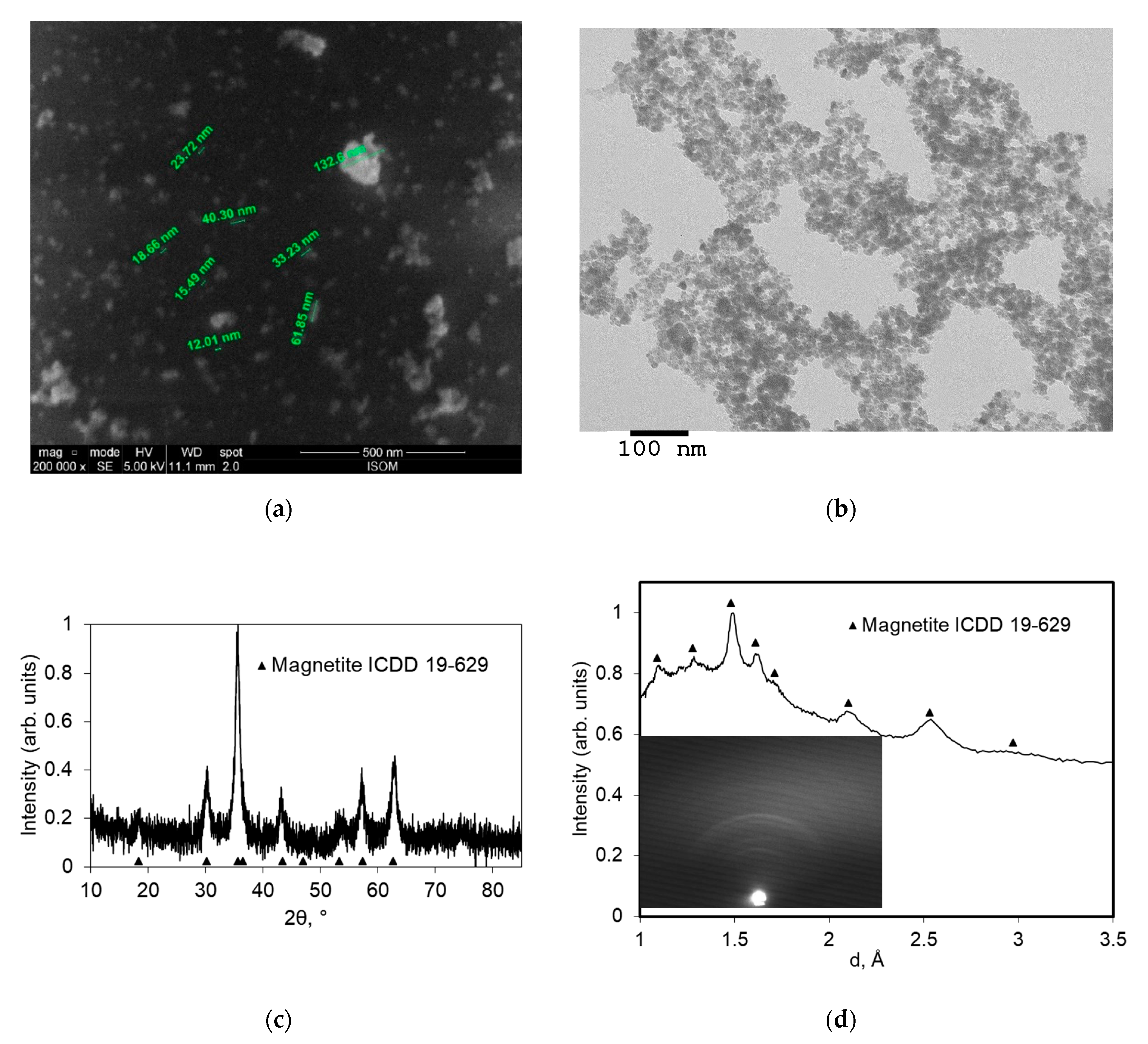
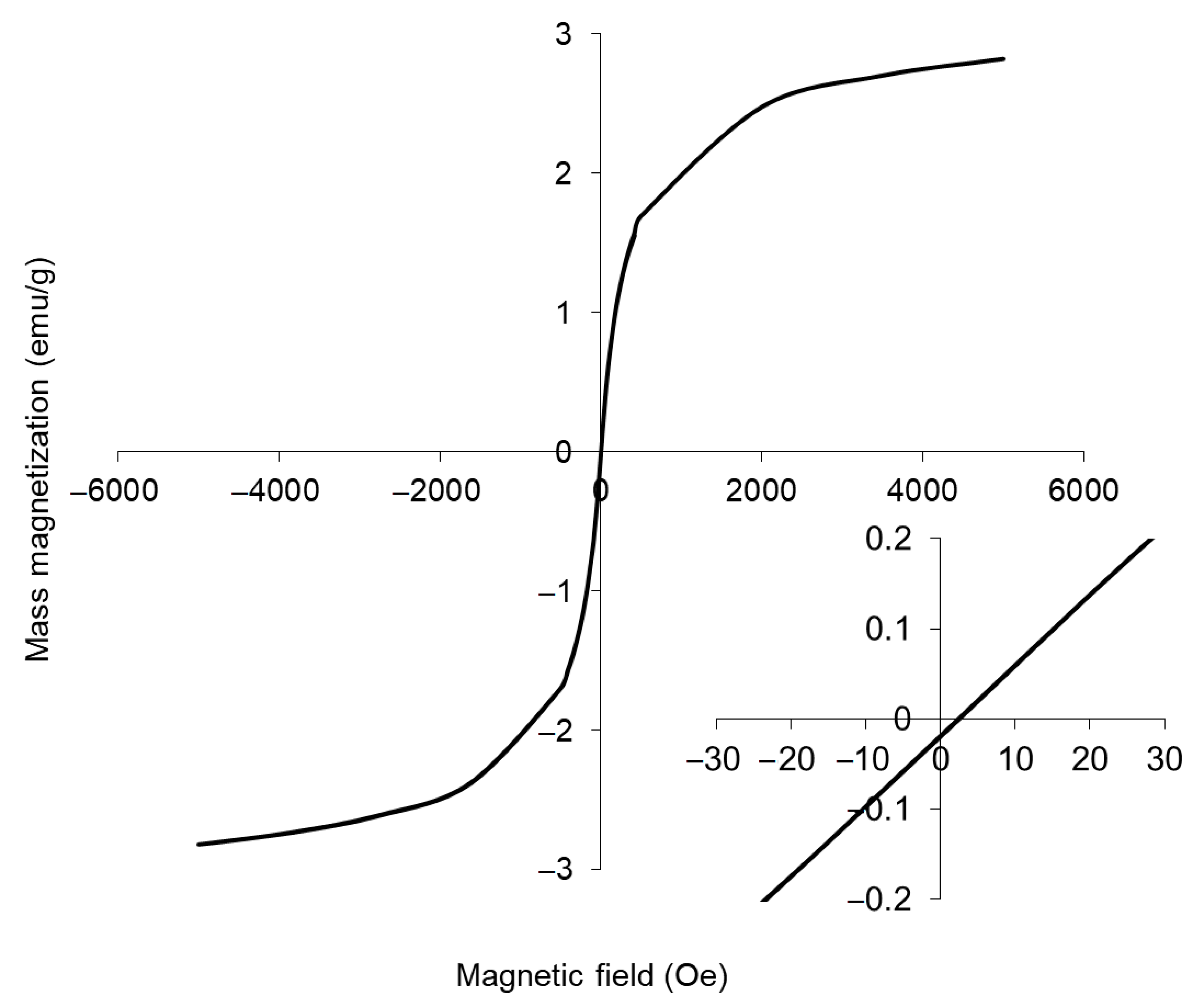
3.1. Physical Characteristics of Magnetic Fluid
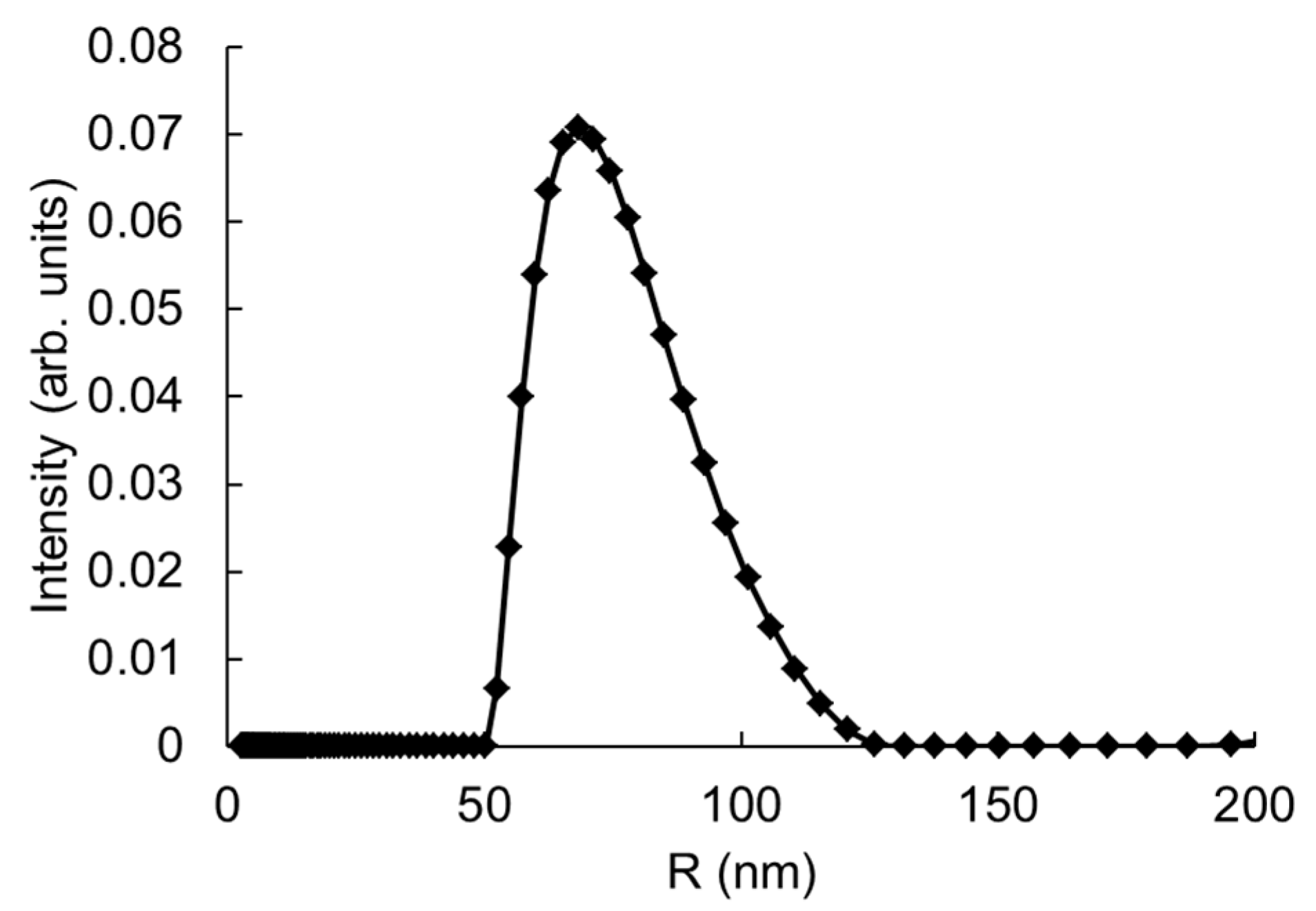
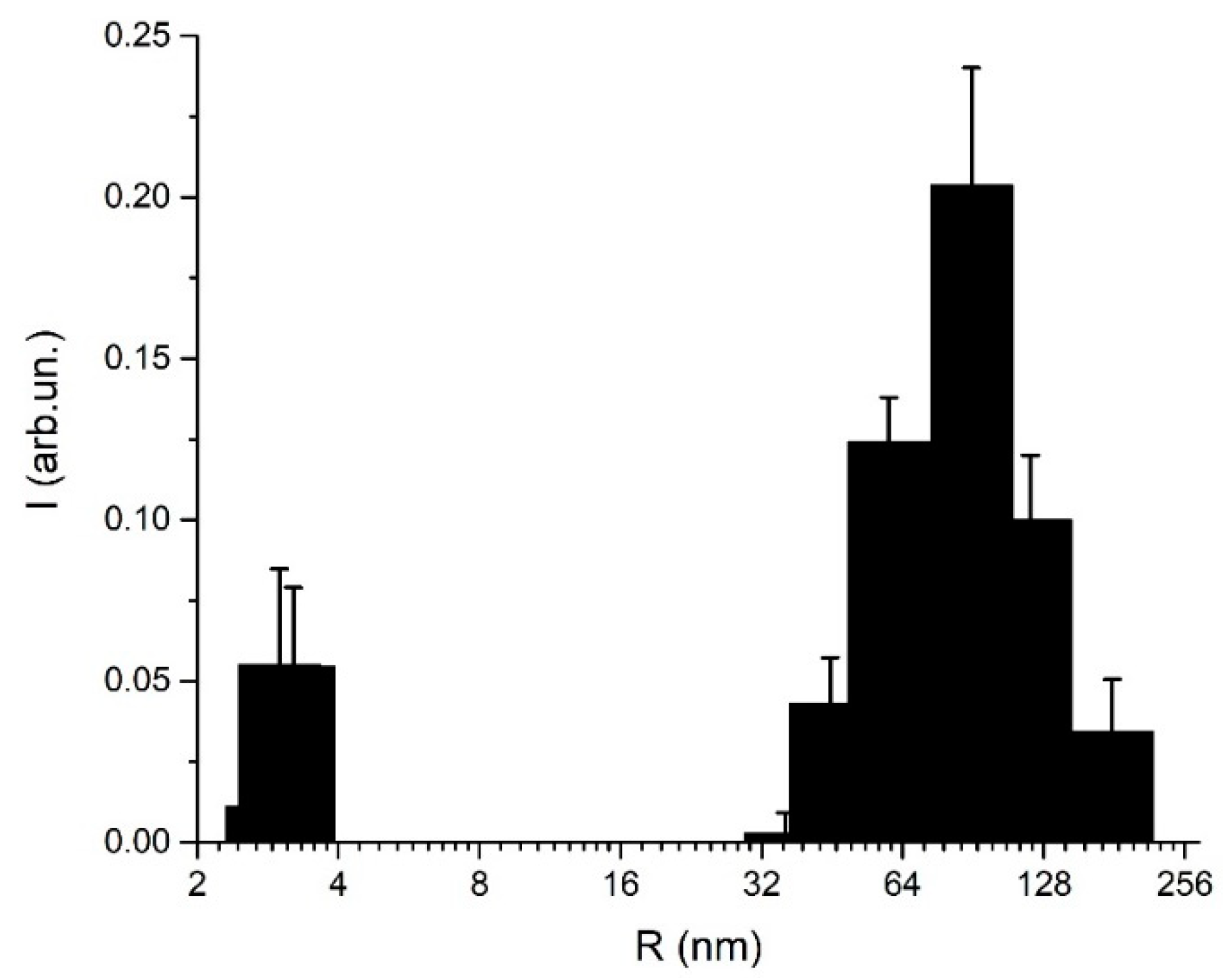
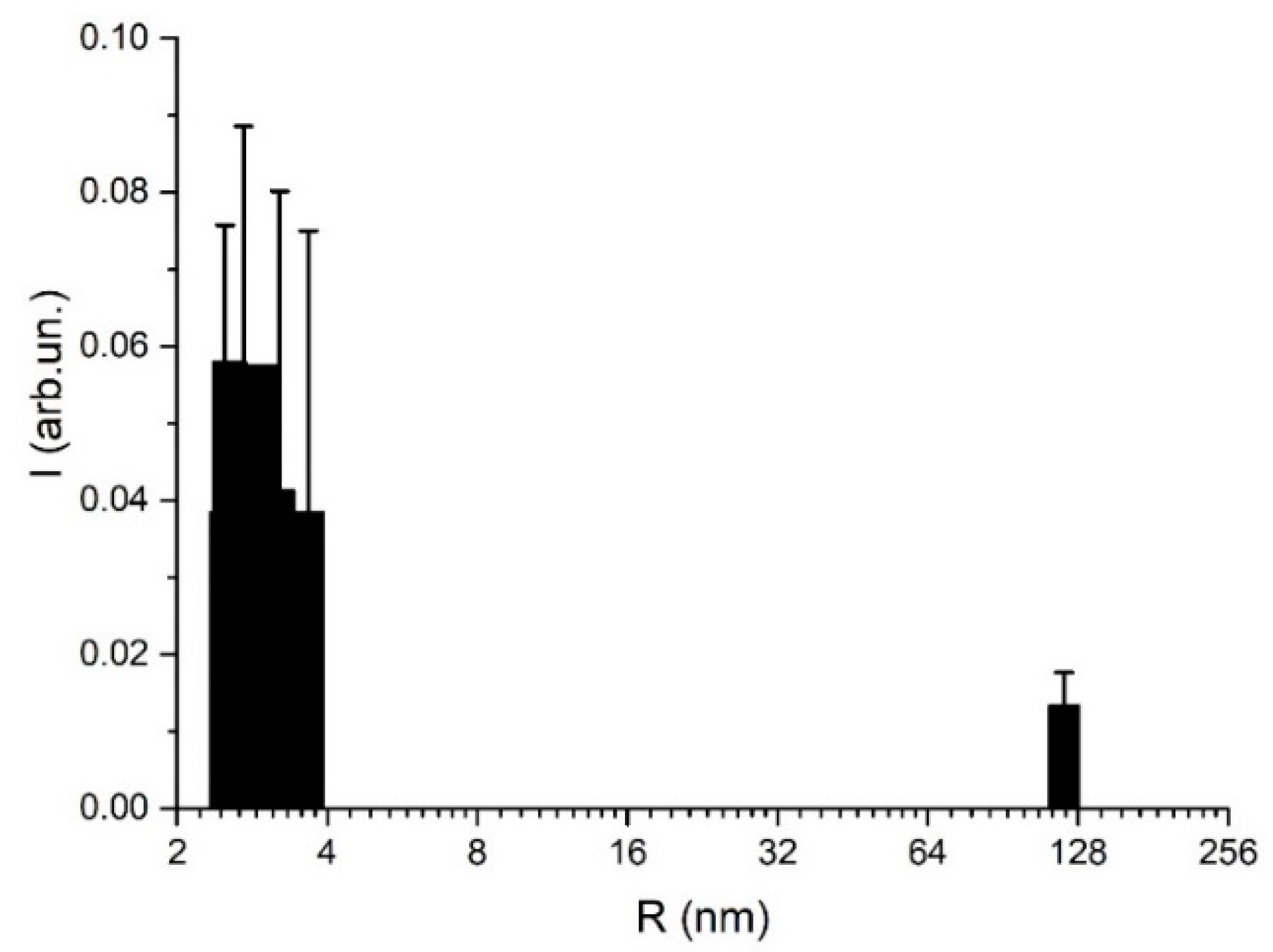
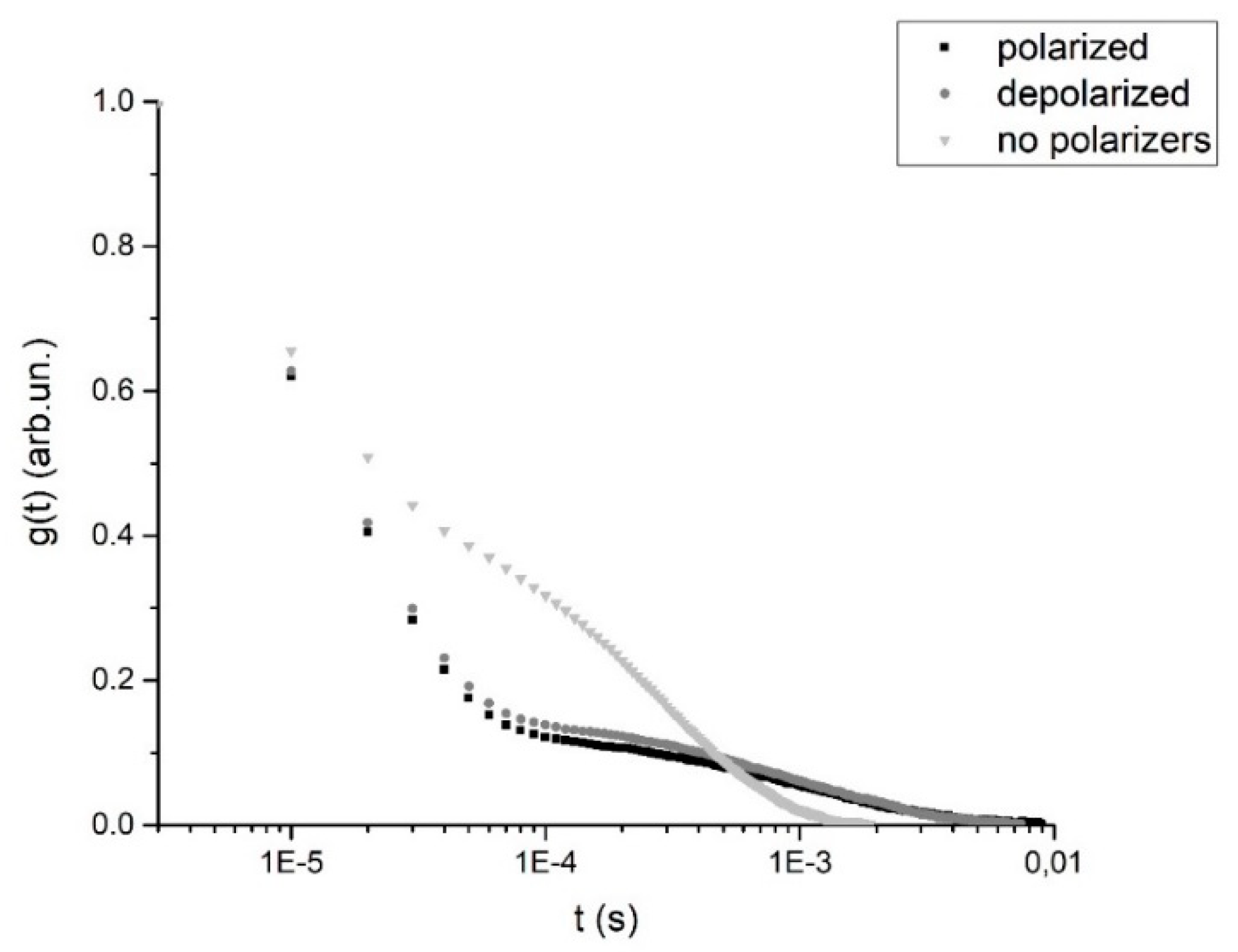
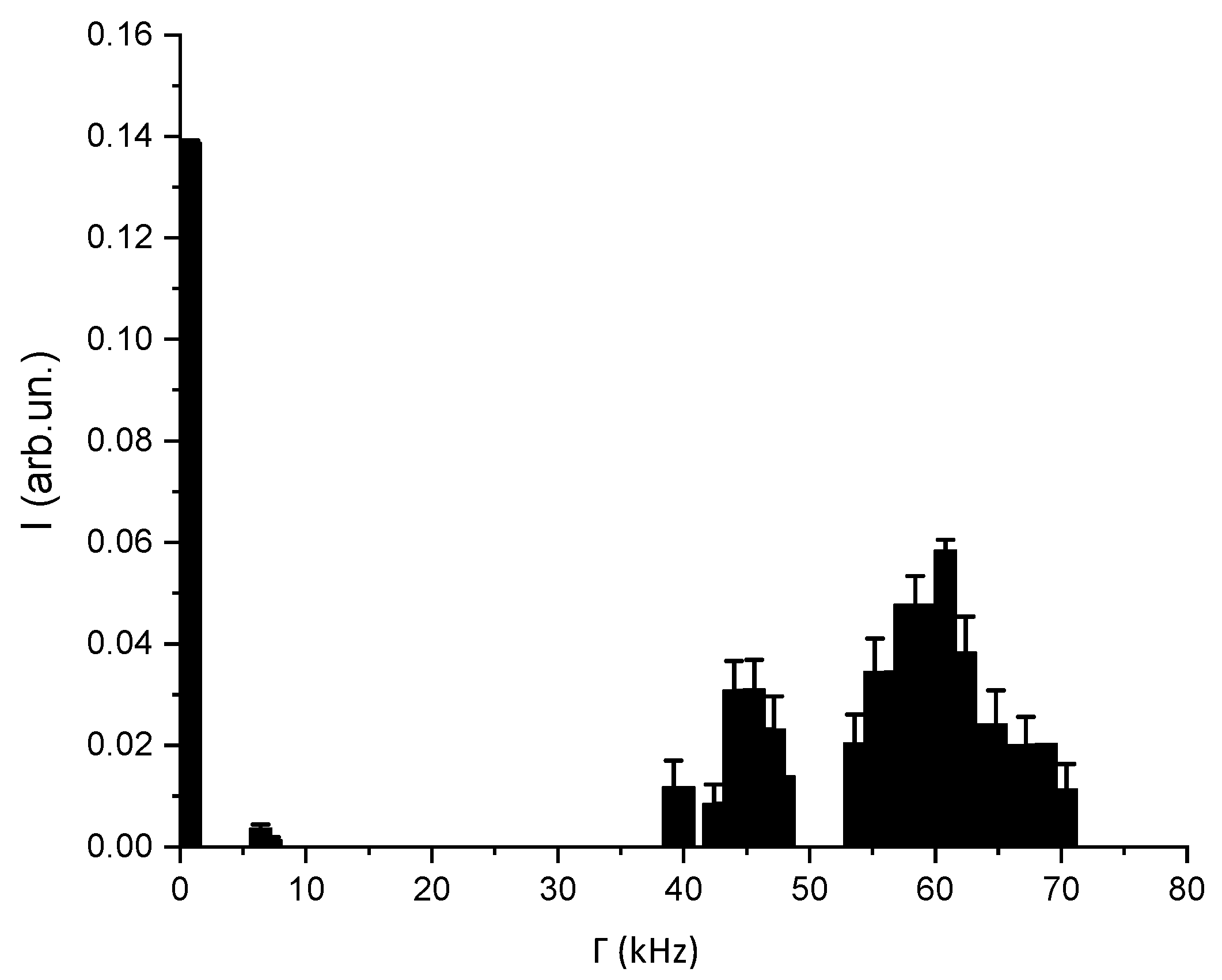
3.2. DLS-Based Original Experimental Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shliomis, M.I. Magnetic fluids. Soviet Phys. Uspekhi 1974, 17, 153–169. [Google Scholar] [CrossRef]
- Taketomi, S.; Shimbun, S. Magnetic Fluids—Principle and Application; Nikkan Kogyo Shinbun: Tokyo, Japan, 1988. [Google Scholar]
- Klimchitskaya, G.L.; Mostepanenko, V.M.; Nepomnyashchaya, E.K.; Velichko, E.N. Impact of magnetic nanoparticles on the Casimir pressure in three-layer systems. Phys. Rev. B 2019, 99, 45433. [Google Scholar] [CrossRef]
- Rinck, P.A. Magnetic Resonance in Medicine: The Basic Textbook of the European Magnetic Resonance Forum; Blackwell Scientific Publications: Oxford, UK, 1993. [Google Scholar]
- Li, Y.; Pu, S.; Zhao, Y.; Zhang, R.; Jia, Z.; Yao, J.; Li, X. All-fiber-optic vector magnetic field sensor based on side-polished fiber and magnetic fluid. Opt. Express 2019, 27, 35182–35188. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Liu, D.; Mallik, A.K.; Farrel, G.; Wu, Q.; Peng, G.D.; Semenova, Y. Magnetic Field Sensor Based on a Tri-Microfiber Coupler Ring in Magnetic Fluid and a Fiber Bragg Grating. Sensors 2019, 19, 5100. [Google Scholar] [CrossRef]
- Peng, J.; Jia, S.; Bian, J.; Zhang, S.; Liu, J.; Zhou, X. Recent Progress on Electromagnetic Field Measurement Based on Optical Sensors. Sensors 2019, 19, 2860. [Google Scholar] [CrossRef]
- Oda, S.; Kitamoto, Y. Relationship between ion concentration of ferrofluid and response signals of magnetic nanoparticles against ac magnetic fields. AIP Adv. 2017, 7. [Google Scholar] [CrossRef]
- Alam, J.; Riaz, U.; Ahmad, S. Effect of ferrofluid concentration on electrical and magnetic properties of the Fe3O4/PANI nanocomposites. J. Magn. Magn. Mater. 2007, 314, 93–99. [Google Scholar] [CrossRef]
- Skumiel, A.; Józefczak, A.; Hornowski, T.; Łabowski, M. The influence of the concentration of ferroparticles in a ferrofluid on its magnetic and acoustic properties. J. Phys. D Appl. Phys. 2003, 36, 3120–3124. [Google Scholar] [CrossRef]
- Hejazian, M.; Nguyen, N.T. Magnetofluidic concentration and separation of non-magnetic particles using two magnet arrays. Biomicrofluidics 2016, 10. [Google Scholar] [CrossRef]
- Sabet Sarvestany, N.; Farzad, A.; Ebrahimnia-Bajestan, E.; Mir, M. Effects of magnetic nanofluid fuel combustion on the performance and emission characteristics. J. Dispers. Sci. Technol. 2014, 35, 1745–1750. [Google Scholar] [CrossRef]
- Bogachev, Y.V.; Chernenco, J.S.; Gareev, K.G.; Kononova, I.E.; Matyushkin, L.B.; Moshnikov, V.A.; Nalimova, S.S. The Study of Aggregation Processes in Colloidal Solutions of Magnetite-Silica Nanoparticles by NMR Relaxometry, AFM, and UV-Vis-Spectroscopy. Appl. Magn. Reson. 2014, 45, 329–337. [Google Scholar] [CrossRef]
- Yerin, C.V.; Vivchar, V.I. Ellipsometry of magnetic fluid in a magnetic field. J. Magn. Magn. Mater. 2020, 498, 166144. [Google Scholar] [CrossRef]
- Brinker, C.; Scherer, G. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Toropova, Y.G.; Golovkin, A.S.; Malashicheva, A.B.; Korolev, D.V.; Gorshkov, A.N.; Gareev, K.G.; Afonin, M.V.; Galagudza, M.M. In vitro toxicity of FemOn, FemOn-SiO2 composite, and SiO2-FemOn core-shell magnetic nanoparticles. Int. J. Nanomed. 2017, 12, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Nikitin, M.P.; Alenichev, M.K.; Drozhzhennikova, E.B.; Grigorenko, V.G.; Ringaci, A.S.; Andreeva, I.P. Nano-biosensors based on dynamic light scattering. Opt. Methods Insp. Charact. Imaging Biomater. IV 2019, 11060, 110600Y. [Google Scholar]
- Ashikhmin, V.S. Photocor Mini User Manual; Photocor: Moscow, Russia, 2017. [Google Scholar]
- Martchenko, I.; Dietsch, H.; Moitzi, C.; Schurtenberger, P. Hydrodynamic Properties of Magnetic Nanoparticles with Tunable Shape Anisotropy: Prediction and Experimental Verification. J. Phys. Chem. B 2011, 115, 14838–14845. [Google Scholar] [CrossRef]
- Geers, C.; Rodriguez-Lorenzo, L.; Urban, D.; Kinnear, C.; Petri-Fink, A.; Balog, S. A new angle on dynamic depolarized light scattering: Number-averaged size distribution of nanoparticles in focus. Nanoscale 2016, 8, 15813–15821. [Google Scholar] [CrossRef]
- Moore, T.L.; Urban, D.A.; Rodriguez-Lorenzo, L.; Milosevic, A.; Crippa, F.; Spuch-Calvar, M.; Balog, S.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle administration method in cell culture alters particle-cell interaction. Sci. Rep. 2019, 9, 900. [Google Scholar] [CrossRef]
- Gan Jia Gui, N.; Stanley, C.; Nguyen, N.-T.; Rosengarten, G. Ferrofluids for heat transfer enhancement under an external magnetic field. Int. J. Heat Mass Transf. 2018, 123, 110–121. [Google Scholar] [CrossRef]
- Gareev, K.G.; Rejnyuk, A.V.; Testov, D.O.; Luchinin, V.V.; Moshnikov, V.A. Method of Producing Magnetic Fluid. Russian Federation Patent 2,639,709, 22 December 2017. [Google Scholar]
- Levin, A.D.; Shmytkova, E.A.; Khlebtsov, B.N. Multipolarization Dynamic Light Scattering of Nonspherical Nanoparticles in Solution. J. Phys. Chem. C 2017, 3070, 3077. [Google Scholar] [CrossRef]
- Nepomnyashchaya, E.; Aksenov, E.; Velichko, E. Molecular dynamics as studied by laser correlation spectroscopy. In Proceedings of the 2017 Progress in Electromagnetics Research Symposium, St. Petersburg, Russia, 22–25 May 2017; pp. 3556–3562. [Google Scholar]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Pecora, R. Doppler shifts in light scattering from pure liquids and polymer solutions. J. Chem. Phys. 1964, 40, 1604–1614. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering; John Wiley: New York, NY, USA, 1976. [Google Scholar]
- Lehner, D.; Lindner, H.; Glatter, O. Determination of the translational and rotational diffusion coefficients of rodlike particles using depolarized dynamic light scattering. Langmuir 2000, 16, 1689–1695. [Google Scholar] [CrossRef]
- Shetty, A.M.; Wilkins, G.M.H.; Nanda, J.; Solomon, M.J. Multiangle depolarized dynamic light scattering of short functionalized single-walled carbon nanotubes. J. Phys. Chem. C 2009, 113, 7129–7133. [Google Scholar] [CrossRef]
- Escobedo-Sánchez, M.A.; de La Cruz-Burelo, H.A.; Arauz-Lara, J.L.; Haro-Pérez, C.; Rojas-Ochoa, L.F. Study of translational and rotational dynamics of birefringent colloidal particles by depolarized light scattering in the far- and near-field regimes. J. Chem. Phys. 2015, 143, 044902. [Google Scholar] [CrossRef]
- Quirantes, A.; Ben-Taleb, A.; Delgado, A.V. Determination of size/shape parameters of colloidal ellipsoids by photon correlation spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 1996, 119, 73–80. [Google Scholar] [CrossRef]
- Bossert, D.; Natterodt, J.; Urban, D.A.; Weder, C.; Petri-Fink, A.; Balog, S. Speckle-Visibility Spectroscopy of Depolarized Dynamic Light Scattering. J. Phys. Chem. B 2017, 121, 7999–8007. [Google Scholar] [CrossRef]
- Nepomnyashchaya, E.; Velichko, E.; Kotov, O. Determination of Noise Components in Laser Correlation Spectroscopic Devices for Signal-to-Noise Ratio Estimation. In Proceedings of the 2019 IEEE International Conference on Electrical Engineering and Photonics (EExPolytech), St. Petersburg, Russia, 17–18 October 2019. [Google Scholar]
- Nepomnyashchaya, E.; Velichko, E.; Aksenov, E. Inverse problem of laser correlation spectroscopy for analysis of polydisperse solutions of nanoparticles. J. Phys. Conf. Ser. 2016, 769, 012025. [Google Scholar] [CrossRef]
- Kharitonskii, P.V.; Gareev, K.G.; Ionin, S.A.; Ryzhov, V.A.; Bogachev, Y.V.; Klimenkov, B.D.; Kononova, I.E.; Moshnikov, V.A. Microstructure and magnetic state of Fe3O4-SiO2 colloidal particles. J. Magn. 2015, 20, 221–228. [Google Scholar] [CrossRef][Green Version]
- Vezo, O.S.; Gareev, K.G.; Korolev, D.V.; Kuryshev, I.A.; Lebedev, S.V.; Moshnikov, V.A.; Sergienko, E.S.; Kharitonskii, P.V. Aggregate stability and magnetic characteristics of colloidal FemOn–SiO2 particles obtained by sol–gel method. Phys. Solid State 2017, 59, 1008–1013. [Google Scholar] [CrossRef]
- Tohver, V.; Smay, J.E.; Braem, A.; Braun, P.V.; Lewis, J.A. Nanoparticle halos: A new colloid stabilization mechanism. Proc. Natl. Acad. Sci. USA 2001, 98, 8950–8954. [Google Scholar] [CrossRef]
- Mo, S.; Shao, X.; Chen, Y.; Cheng, Z. Increasing entropy for colloidal stabilization. Sci. Rep. 2016, 6, 36836. [Google Scholar] [CrossRef] [PubMed]
- Watarai, H.; Memon Sakurai, S.S. Critical Detection of Agglomeration of Magnetic Nanoparticles by Magnetic Orientational Linear Dichroism. Langmuir 2020, 36, 12414–12422. [Google Scholar] [CrossRef] [PubMed]
- Gareev, K.G. Interaction of Nanocomposites Based on the FemOn–SiO2 System with an Electromagnetic Field in an Ultra-Wide Frequency Range. Magnetochemistry 2020, 6, 24. [Google Scholar] [CrossRef]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-81614-4. [Google Scholar]
- Kirschvink, J.; Jones, D.; MacFadden, B. Magnetite Biomineralization and Magnetoreception in Organisms: A New Biomagnetism; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Nepomnyashchaya, N.; Velichko, E. Modification of laser correlation spectroscopy method for analyzing polydisperse nanoparticle suspensions. St. Petersburg Polytech. Univ. J. Phys. Math. 2019, 44, 73–87. [Google Scholar] [CrossRef]
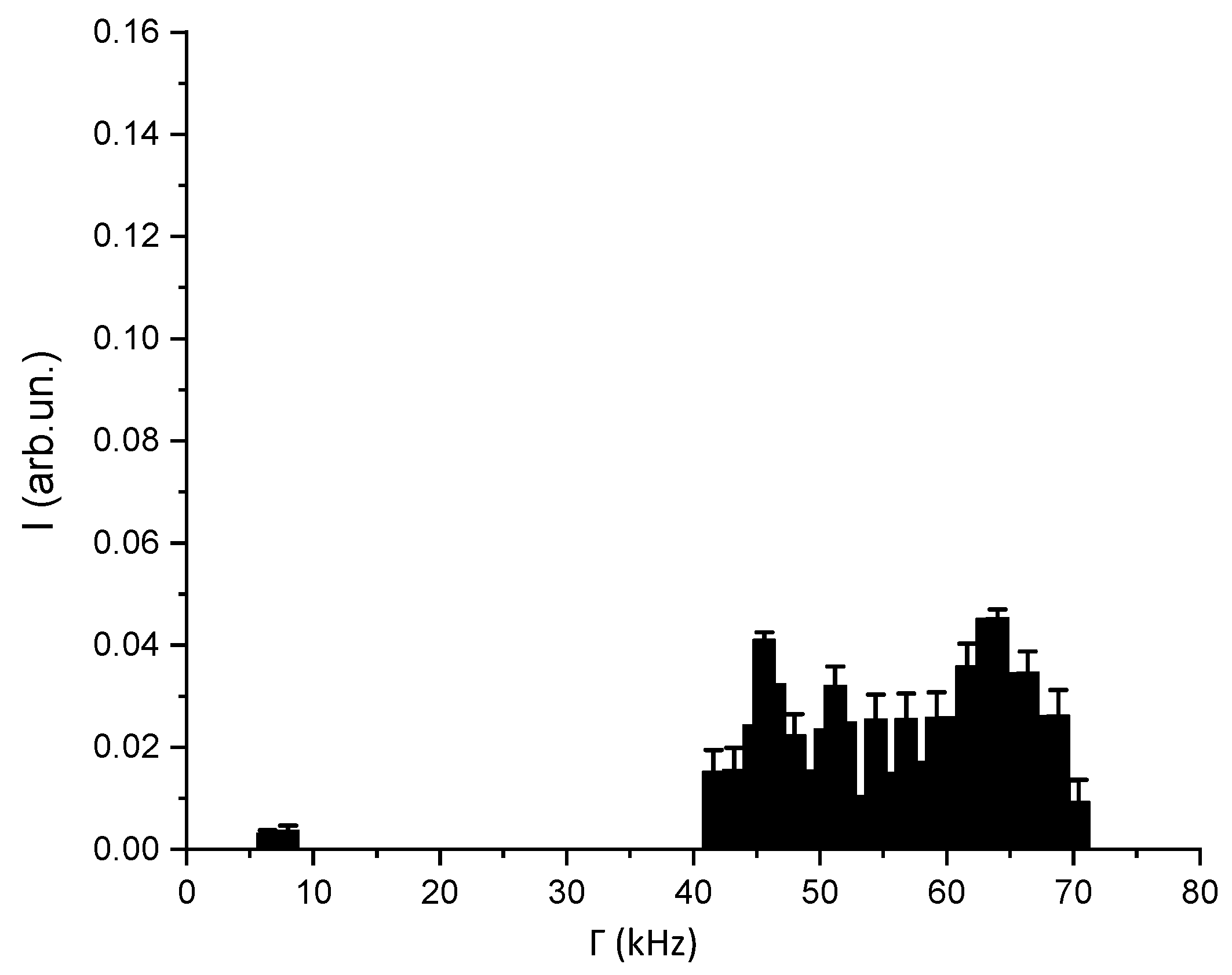
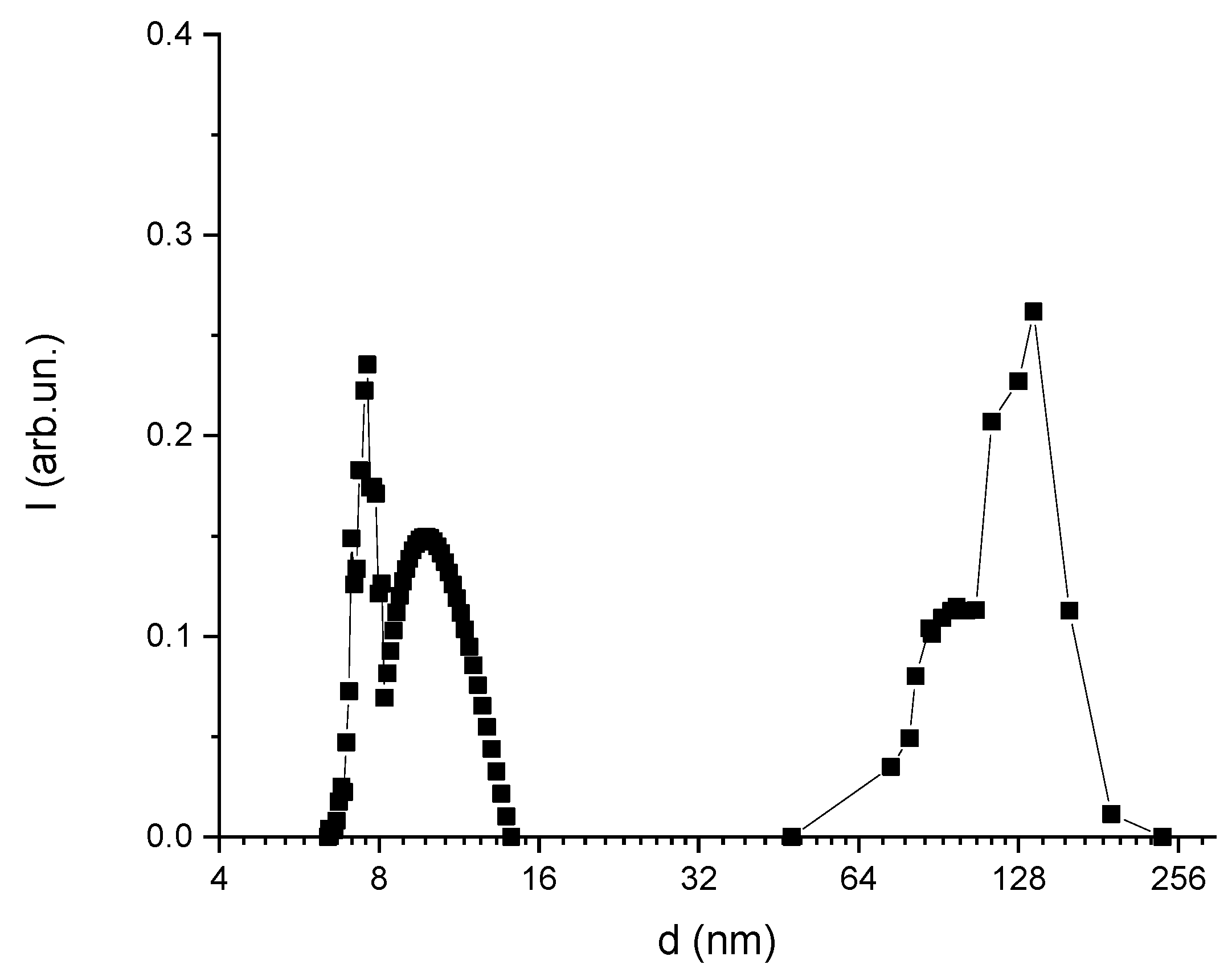
| DT (µm2/s) | DR (1/s) | da (nm) | db (nm) | ε |
|---|---|---|---|---|
| (2.8 ± 0.2)–(3.5 ± 0.3) | (700 ± 64)–(1060 ± 100) | (146 ± 14)–(188 ± 18) | (86 ± 8)–(140 ± 1.2) | (0.55 ± 0.04)–(0.96 ± 0.04) |
| (58.0 ± 5.6)–(80.0 ± 7.5) | (4.0 ± 0.3) × 105–(6.0 ± 0.5) × 105 | (8.2 ± 0.7)–(10.0 ± 0.9) | (7.0 ± 0.6)–(9.4 ± 0.8) | (0.85 ± 0.04)–(0.90 ± 0.09) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velichko, E.N.; Nepomnyashchaya, E.K.; Gareev, K.G.; Martínez, J.; Maicas, M.C. Characterization of Magnetite–Silica Magnetic Fluids by Laser Scattering. Appl. Sci. 2021, 11, 183. https://doi.org/10.3390/app11010183
Velichko EN, Nepomnyashchaya EK, Gareev KG, Martínez J, Maicas MC. Characterization of Magnetite–Silica Magnetic Fluids by Laser Scattering. Applied Sciences. 2021; 11(1):183. https://doi.org/10.3390/app11010183
Chicago/Turabian StyleVelichko, Elena N., Elina K. Nepomnyashchaya, Kamil G. Gareev, Javier Martínez, and Marco C. Maicas. 2021. "Characterization of Magnetite–Silica Magnetic Fluids by Laser Scattering" Applied Sciences 11, no. 1: 183. https://doi.org/10.3390/app11010183
APA StyleVelichko, E. N., Nepomnyashchaya, E. K., Gareev, K. G., Martínez, J., & Maicas, M. C. (2021). Characterization of Magnetite–Silica Magnetic Fluids by Laser Scattering. Applied Sciences, 11(1), 183. https://doi.org/10.3390/app11010183








