Featured Application
This review article deals with the synthesis and applications of CuFe bimetallic nanoparti-cles. Although much research has been published on nanomaterials, overall review articles on CuFe nanoparticles are lacking and this article presents the latest data on the synthesis, prop-erties and possible applications of CuFe nanoparticles.
Abstract
Bimetal CuFe (copper-iron) nanoparticles, which are based on the earth-abundant and inexpensive metals, have generated a great deal of interest in recent years. The possible modification of the chemical and physical properties of these nanoparticles by changing their size, structure, and composition has contributed to the development of material science. At the same time, the strong tendency of these elements to oxidize under atmospheric conditions makes the synthesis of pure bimetallic CuFe nanoparticles still a great challenge. This review reports on different synthetic approaches to bimetallic CuFe nanoparticles and bimetallic CuFe nanoparticles supported on various materials (active carbide, carbide nanotubes, silica, graphite, cellulose, mesoporous carbide), their structure, physical, and chemical properties, as well as their utility as catalysts, including electrocatalysis and photocatalysis.
1. Introduction
Bimetallic composite nanoparticles have generated great interest because the combination, at the nanoscale, of different metals can result in new or enhanced physicochemical properties and vast potential applications in the areas of electronics, photonics, catalysis, and biomedicine [1,2,3,4,5]. Properties of obtained particles depend on and can be tailored according to their architectures (e.g., core@shell or multishell structures, hollow structures, heterostructures, alloys), composition, size, and shape [1,6,7,8,9,10].
Copper (Cu) is a 3rd period transition metal and has some interesting physical and chemical properties, such as catalytic activity, high electrical and thermal conductivity, good ductility, malleability, and tensile strength. Due to the catalytic activity of copper nanoparticles, they find a number of applications, including gas-phase reactions, Ullmann reactions, cross-coupling reactions, A3 coupling reactions, azide-alkyne cycloaddition, photocatalysis, and electrocatalysis [11].
An attribute of nanoparticles exhibiting magnetic properties is the ability to selectively attach functional particles and manipulate them using an external magnetic field produced by an electromagnet or permanent magnet [12]. Among them, iron (Fe) is a class of ferromagnetic materials with high magnetic moment density (218 emu/g) and is magnetically soft. Iron nanoparticles in the size range below 20 nm are in the superparamagnetic regime, and their stable dispersions with high magnetic moment are predicted to have wide range applications including data storage, environmental remediation, catalysis, and disease diagnosis and therapy [13]. Nanoscale zero-valence iron (nZVI) is a strong reducing agent. A large specific surface area and reactivity of these nanoparticles increase the efficiency of removing inorganic and organic pollutants as well as heavy metals. However, due to their small size and magnetic properties, they easily aggregate [14,15,16,17]. Between the variety of transition metal macrocycle complex catalysts with different central metal atoms [14,15], iron-based materials have been identified to exhibit the best activity for ORR under various metal loadings [16,17,18,19,20,21,22,23].
On account of their properties, nanomaterials based on copper and iron can effectively replace rare and expensive noble-metal catalysts commonly employed in commercial chemical processes. However, the synthesis and use of both copper and iron nanoparticles is still a challenge due to the high tendency of these materials to oxidize under atmospheric conditions. Therefore, complex nanoparticles (e.g., core@shell, alloys) have been recently adapted to overcome the instability of copper and iron nanoparticles in the presence of oxygen, water, and several chemicals.
Since the fusion of the unique properties of copper and iron in one single entity promises multifunctionality and potential applications, a lot of effort is put into preparing such nanoparticles containing Cu and Fe. This coupling may result in the formation of nanocomposites with an extraordinary catalytic activity and ferromagnetic properties, which would allow for convenient separation of the nanocomposite catalyst from the reaction system in the applied magnetic field (Figure 1).
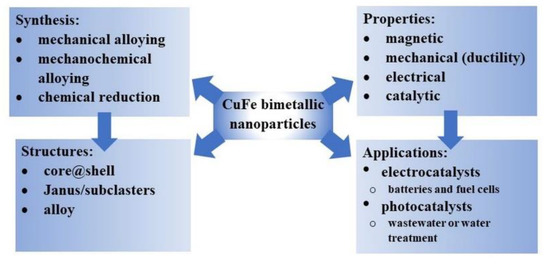
Figure 1.
CuFe bimetallic nanoparticles, their synthesis, architecture, properties, and applications.
2. Structure, Synthesis, and Properties of Bimetallic CuFe Nanoparticles
As already mentioned, bimetallic particles composed of two components are expected to display either a combination of the properties associated with each material or new or enhanced properties and capabilities due to coupling between two different materials. The properties of bimetallic nanoparticles can be tuned by varying the concentration of their constituent elements, their architecture, shape, and size. Although bimetallic nanoparticles consist of only two different metals, there are still many types of possible structures: random and ordered alloy, Janus, and core@shell (Figure 2) [5,24].
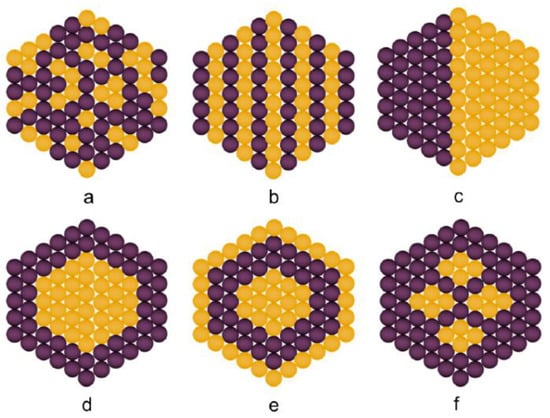
Figure 2.
Types of bimetallic nanoparticles: (a) random alloyed, (b) ordered alloy, (c) sub-clusters/Janus (d) core-shell, (e) multi-shell core-shell, and (f) multiple core materials coated by a single shell material. Yellow and purple spheres represent two different kinds of metal atoms. Reprinted from [5].
The CuFe bimetallic nanoparticles can be oriented in alloy, core@shell, Janus, and other architectures, depending mainly on their synthesis [25,26]. They are known to have face-centered cubic (fcc), body centered cubic (bcc), and hexagonal close-packed (hcp) crystal structures [25,26].
There are a lot of methods for designing various kinds of bimetallic nanoparticles; however, in the case of the CuFe system, both elements are unstable in the presence of air; therefore, using conventional methods is challenging.
2.1. Modeling
As can be found in the literature, molecular dynamics simulations allow for the creation of a model and determine the properties before starting experiments or full-scale industrial production of metallic nanoparticles [27,28,29,30,31,32]. Molecular dynamics calculations were also used to investigate the CuFe system, including the influence of particle size, cooling rate, and Cu concentration on the morphology of the CuFe nanoparticles. Rojas-Nunez et al. studied the dependence of the morphologies of FeCu on different Fe and Cu concentrations and the appearance of possible defects in the stabilized NPs, as well as the energy contributions to the bimetallic systems [25]. The researchers studied the Fe1−xCux systems with Cu concentration at 10%, 40%, and 70% (Figure 3). They examined three Fe cores (DFe) with diameters 5, 7, and 9 nm with their respective Cu concentrations. At the low concentration of Cu (10%), the Fe core@shell is created. With increasing the Cu concentration to 40%, core@shell structures are observed for small (5 nm) Fe particles, while Janus structures are preferred for medium (7 nm) and big (9 nm) Fe particles. Seventy percent of Cu leads to segregated Janus (sub-clasters) particles. These simulations show that a segregated (Janus) structure becomes preferred over core@shell architecture when the Cu content increases. In addition, for low Cu concentration, the particles show a bcc phase, at 40% Cu, two phases exist, a bcc phase for Fe, and Cu is mainly ordered as an fcc lattice, high Cu content shows a bcc lattice for Fe and an fcc lattice for Cu. Hexagonal closed-packed (hcp) structures are nucleated only at the Fe−Cu interface because of twins and stacking faults.
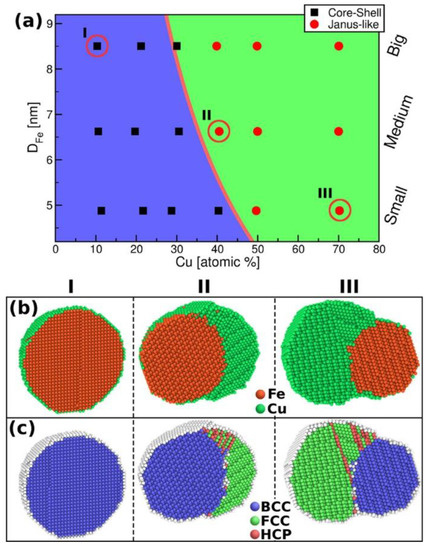
Figure 3.
(a) Cluster structure as a function of size and Cu concentration. (Fe cores (DFe) have diameters 5, 7, and 9 nm). The pink line depicts the core@shell to Janus-like stability transition from the continuous model. Cases I, II, and III are shown below. Cross-sectional views of FeCu particles encircled at (a), showing elemental composition (b), and structural defects (c). Reproduced with permission from ref. [25]. Copyright 2018 American Chemical Society.
Another example of molecular dynamics calculations used to investigate the CuFe system was reported by Kumar [26]. He showed that the FeCu bimetallic nanoparticles can be oriented in random alloy, core-shell, and Janus morphologies, depending on their size, and thermal processing (temperature and cooling rate). At a very slow cooling rate (0.1 K/ps), clear interface Fe-Cu atom is created at a temperature of T = 1200 K and lower. As the temperature decreases further below T = 1200 K, the Fe and Cu atoms crystallize in bcc and fcc crystalline structures, respectively (Figure 4a). With an increasing cooling rate (0.5 and 1 K/ps), the immiscibility between Fe and Cu atoms is not complete (Figure 4b,c), and patchy nanoparticles are created. At a cooling rate of 0.5 K/ps, Fe and Cu atoms crystalized in bcc and fcc structures, respectively (Figure 4b), while at cooling rate of 1 K/ps, both Fe and Cu atoms have bcc structures (Figure 4c). The semi-miscible phase of Cu and Fe atoms is the result of very high cooling rates, 5 and 10 K/ps (Figure 4d,e), which do not allow the atoms to crystallize neither in the fcc structure nor in the bcc structure.
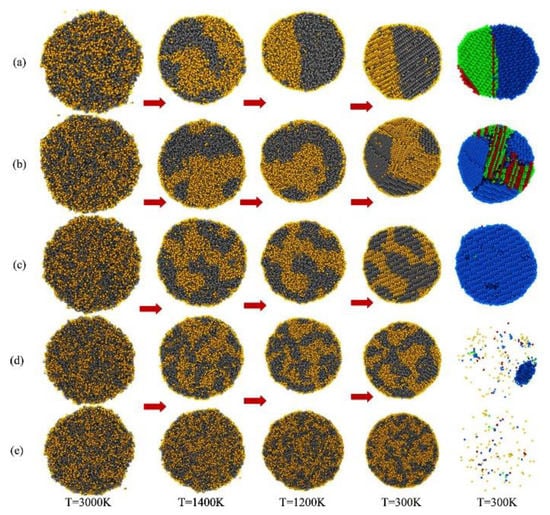
Figure 4.
Snapshots of FeCu bimetallic nanoparticles during cooling from T = 3000 to 10 K at various cooling rates: (a) 0.1 K/ps, (b) 0.5 K/ps, (c) 1 K/ps, (d) 5 K/ps, and (e) 10 K/ps (Colors: ● Cu, ● Fe). The rightmost column depicts the evolution of the various crystal structures of FeCu nanoparticles at 300 K (Colors: ● fcc, ● hcp, and ● bcc atoms). Red arrows show the progress of the cooling process. Reproduced with permission from ref. [26]. Copyright 2019 American Chemical Society.
2.2. Synthesis and Properties
There are two general approaches to the synthesis of nanoparticles, i.e., top-down methods and bottom-up methods. Top-down approach involves the breaking down of the bulk material into nanosized structures or particles. Crystal etching, ball milling and grinding are examples of this approach. Bottom-up approach refers to the build-up of a material from the bottom: atom-by-atom, molecule-by-molecule, or cluster-by cluster. Chemical synthesis of nanoparticles is a typical example, but also self-assembly or nanopatterning can be classified as bottom-up techniques. Since Cu and Fe are immiscible in the whole range of composition in equilibrium, a solid solution of the FeCu system can be obtained only by non-equilibrium techniques, such as mechanical alloying [33,34,35] or vapor deposition [36].
2.2.1. Mechanical Milling
CuFe bimetallic nanoparticles were synthesized by mechanical milling [37,38,39]. Tokada et al. [37] used FeCl3 and CuCl2 with Na to form FeCu nanoparticles by ball milling and studied their magnetic properties. The process was based on two reactions:
FeCl3 + 3Na → Fe + 3NaCl
CuCl2 + 2Na → Cu + 2NaCl
The reactants were milled under Ar atmosphere up to 84 h. With the increase in ball milling duration from 3 h to 84 h, the particle size was found to increase from approximately 16 nm and 50 nm, respectively (Figure 5). They reported the bcc α-Fe and fcc Cu phases formation, and the formation of additional hexagonal Fe(OH)2 phase for the powders milled shorter than 24 h. They also observed that the coercivity (HC) value increased with the grinding time and reached a maximum value of 33.5 kA/m for 48 h of grinding, while further grinding resulted in a decrease in the HC value (Figure 5).
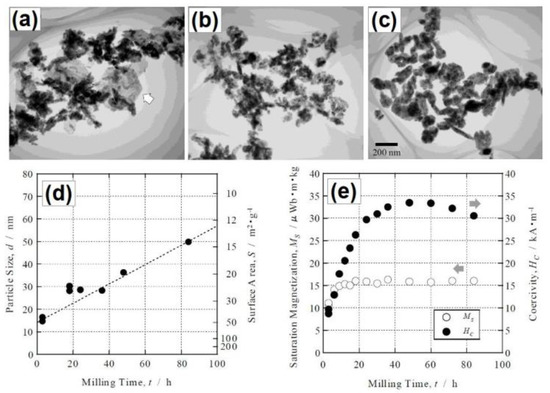
Figure 5.
Transmission electron microscopy (TEM) images (a–c) of the powders for various milling times (18 h, 48 h, 84 h, respectively). Particle sizes of the powders for various milling times (d). Saturation magnetization (MS) and coercivity (HC) values of the as-milled powders for various milling times (e). Reproduced with permission from ref. [37]. Copyright 2002 The Japan Institute of Metals and Materials.
CuFe nanocomposites were obtained by high-energy attrition milling of Fe nanopowder and cuprous oxide (n-Cu2O) in hexane by Sharipova et al. [38]. The authors studied their mechanical properties and found that FeCu nanocomposite has a higher ductility compared to nanostructured Fe (the compressive yield stress of FeCu is 720 MPa, while for Fe nanopowder (30 nm) is 1100 MPa [39]).
Nanocomposites of CuFe/CNT (carbon nanotubes) were synthetized by two-step mechanical milling of a mixture of Cu, Fe, and CNT under argon atmosphere [39]. First, pure Fe and Cu powders in four various proportions were milled for 15 h. Next, the sample showing the maximum solubility of Fe into Cu, i.e., with 20% of Fe in the sample, was chosen, mixed with CNT, and milled for 15 h to synthesize nanocomposite samples. As a result, micron-sized agglomerates consisting of nanoparticles were created. The structural, magnetic, and electrical properties depend on the CNT addition. With the increasing of the CNT content, agglomerates’ size decreased, while coercivity, magnetization, and electrical resistivity of composite samples increased.
2.2.2. Chemical Reduction
FeCu nanoparticles have also been produced by chemical reduction [40,41,42,43,44,45,46,47]. FeCu nanoparticles were synthesized from chemical reduction of Cu and Fe dodecyl sulfate (Fe(DS)2 and Cu(DS)2) by sodium borohydride (NaBH4) and their magnetic properties were studied by Duxin et al. [40]. To prevent oxidation, the synthesis was carried out under a nitrogen atmosphere. The authors found single fcc phase alloys and superparamagnetic behavior above 10 K.
The reduction of FeSO4⋅7H2O and CuSO4⋅5H2O aqueous mixture with sodium borohydride and stirring with the activated carbon was used for the synthesis of C/FeCu. Particles with the average size of 20 nm and a chain-like arrangement with Fe2O3 and Cu2O phases were reported [41].
A two-step reduction method with using FeSO4⋅7H2O stabilized by sodium citrate and CuSO4⋅5H2O with sodium borohydride under N2 protection were used by Feng et al. [42] for the synthesis of Cu doped Fe@Fe2O3 nanoparticles with average size of a dozen of nanometers and different weight ratio Fe/Cu (1:1, 1:2, 3:10, 1:5, 1:10, 1:20 by weight). The effectiveness of arsenic removal from water by these particles was tested (reported in Section 3).
In order to maintain or increase the stability of the particles, stabilizers such as chitosan (CS), green tea (GT), or carboxymethyl cellulose (CMC) are used.
Jiang et al. [43] developed the bimetallic FeCu nanoparticles stabilized by chitosan (CS) with a diameter of 50–100 nm. They chose chitosan due to its biodegradability in the environment and the content of amine and hydroxyl groups, which should strongly bind to nZVI and effectively stabilize nanoparticles. They reported that the concentration of chitosan influences crystal nucleation and aggregation of FeCu particles, and higher chitosan amount results in a more uniform dispersion of FeCu nanoparticles in chitosan. The best amount of chitosan and Cu in CS-CuFe nanocomposites is 2 wt% and 3 wt%, respectively, both in terms of stabilization and catalytic properties.
Green synthesis with green tea (GT) extracts as a reducing agent and stabilizer was used for the preparation of composite of GT and nZVICu nanoparticles with an average size of 60–120 nm [44].
FeCu alloy nanoparticles encapsulation in carbon (FeCu@C) by a combination of a chemical reduction of ferric nitrate Fe(NO3)3⋅9H2O and copper nitrate Cu(NO3)2⋅3H2O in alcohol solution and annealing carbonization (Figure 6) was performed by Xue et al. [45]. The core-shell nanoparticles, with FeCu4 core and amorphous carbon shell, with particles size ranging from 24 nm to 73 nm, and coating thickness of approximately 5 nm were produced. The obtained material presented ultra-soft magnetic properties.
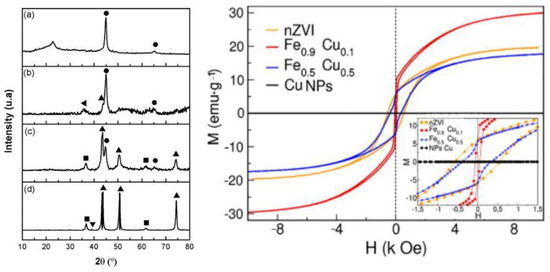
Figure 6.
XRD results of (a) nZVI (nano-scale zero valent iron particles), (b) Fe0.9Cu0.1, (c) Fe0.5Cu0.5, and (d) Cu NPs. Symbols: (●) nZVI, (▲) Cu0, (■) cuprite, (▼) tenorite, and (◄) magnetite. Magnetic hysteresis of the nZVI, Fe0.9Cu0.1, Fe0.5Cu0.5, and Cu particles at room temperature. Reprinted from [49]. Copyright 2018, with permission from Elsevier.
FeCu nanoparticles of various composition (Fe:Cu = 8:2, 5:5, 2:8) prepared by the reduction of iron and copper nitrates were dispersed on the two various media, highly ordered “platelet” graphite nanofibers GNF and amorphous silica [46]. Size of nanoparticles supported on “platelet” GNF is equal 13.1nm, 11.1 nm, 12.7 nm for Fe:Cu = 8:2, 5:5, 2:8, respectively and is twice as large on average as those supported in silica equal to 5.6 nm, 7.6 nm, 8.1 nm for Fe:Cu = 8:2, 5:5, 2:8, respectively. Comparison of catalytic activity and selectivity of those systems shows that the performance of the metal catalysts supported on the graphite nanofibers was better than that on the silica system, what is connected with the nature of the interaction of nanoparticles with the support.
The CuFe bimetallic nanoparticles with Cu/Fe molar ratios of 10.00, 3.00, 0.33 and 0.10 were prepared by a simultaneous reduction of cupric and ferric nitrates with sodium borohydride (NaBH4) in ethylene glycol (EG) under Ar atmosphere by Xiao et al. [47,48]. The nanoparticles were found to be spherical with diameter ranging from 15 to 20 nm and containing FeCu4 and CuFe2O4 phases.
The same procedure was used by Sepúlveda et al. [49] for the synthesis of CuFe nanoparticles with different amounts of Cu, 0.1, and 0.5 in the samples. Structural, crystallographic, magnetic properties were studied (Figure 6). A low amount of Cu in the particles (Fe0.9Cu0.1) favors the core@shell structure while higher amount of Cu (Fe0.5Cu0.5) prefers the segregated (Janus) structure. The authors found CuFe alloy phase accompanied by Fe3O4 phase in the case of Fe0.9Cu0.1, and CuO2 phase in the case of Fe0.5Cu0.5. The magnetic properties are associated with composition changes and with the increasing the Cu concertation, the coercivity decreases, going from 626 Oe for Fe0.9Cu0.1 to 53 Oe for Fe0.5Cu0.5.
Synthesis of CuFe alloy NPs fully covered by graphene layers via high temperature pyrolysis is possible [50]. Using a copper phthalocyanine based precursor and an iron acetylacetonate led to CuFe nanoalloy in which most of the Fe atoms were surrounded by Cu atoms (Figure 7). The main phase of the particles obtained is the CuFe alloy, but a small amount of iron carbide (Fe3C) phase is also observed.
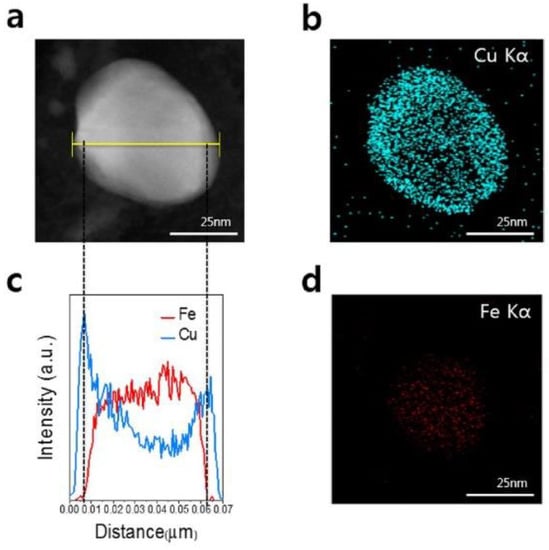
Figure 7.
Structural and elemental analysis of the CuFe alloy. (a) High-angle annular dark field/STEM image of CuFe alloy nanoparticles and EDS elemental mapping of (b) Cu and (d) Fe in the same area. (c) EDS intensity profiles of yellow line in (a). Reproduced with permission from ref. [50]. Copyright 2015 American Chemical Society.
CuFe nanoparticles have also been produced by synthesizing the nZVI using the reduction of ferric chloride (FeCl3.6H2O) by NaBH4 followed by planting Cu onto the surface of nZVI by redox reaction occurring between the Cu2+ and nZVI [51,52,53,54]. Synthesis of bimetallic FeCu nanoparticles with different Cu to Fe ratio of 1:20, 1:10, 1:6.7, and 1:5 (w/w) and average size of 45 nm, 59 nm, 72 nm, and 85 nm, respectively, was reported [52].
Cao et al. [53] proposed method for the synthesis of Fe@Cu core@shell structures with uniform shape and an average diameter of less than 20 nm using monodispersed carboxymethyl cellulose (CMC) as stabilizer (Figure 8).
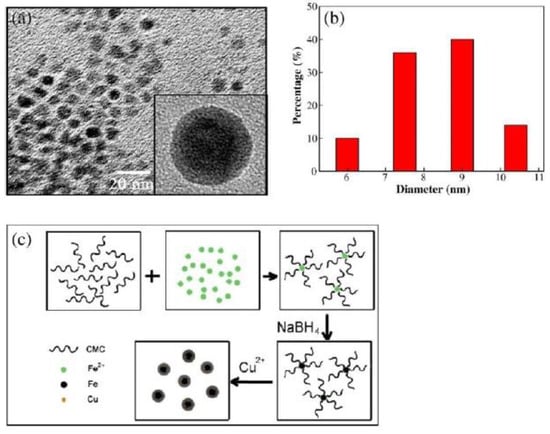
Figure 8.
(a) Transmission electron microscopy (TEM) images of as-prepared carboxymethyl cellulose (CMC)-stabilized Fe@Cu nanoparticles, inset is TEM image of an individual nanoparticle. (b) Size distribution of CMC-stabilized Fe@Cu nanoparticles. (c) The formation process of CMC-stabilized Fe@Cu nanoparticles. Reprinted from [53], Copyright 2011, with permission from Elsevier.
At this point, it should be emphasized that the properties, and in particular the magnetic properties of the synthesized materials, depend not only on the size, shape, structure, but most of all on the composition of the synthesized materials. The presence of iron oxides in nanoparticles influences its magnetic properties and can change saturation magnetization, coercivity, blocking temperature, and relaxation time, and thus may limit their use [55].
3. Applications
As it was shown, the structure depends mainly on the concentration of Cu and Fe in the particles, and mechanical, catalytical, and magnetic properties depend on the structure and composition, and also on the additions such as carbon, graphite, or silicon used for supporting the created nanoparticles. And these properties determinate the applications of bimetallic CuFe nanoparticles. The literature points to applications of CuFe systems in the areas of catalysis and electrochemistry [56,57,58,59], the FeCu nanoparticles are also used as electrocatalysts for energy storage batteries and as photocatalysts and adsorbents for wastewater or water treatment [43,44,47,48,49,50,51,52,53,54,60,61].
3.1. Oxygen Reduction Reaction (ORR)
Energy storage and conversion are very important for the functioning of modern societies. Energy sources such as batteries and fuel cells are becoming the backbone of a conscious low-carbon economy. Vital for energy conversion, especially in the areas of fuel cells and metal-air batteries, is the oxygen reduction reaction (ORR) [62]. During the ORR process, the O2 molecules are reduced by electrons. They are very difficult to break electrochemically, because the bond O = O has an exceptionally strong bond energy of 498 kJ/mol. The energy barrier can be lowered by electrocatalysts that can activate and cleave bonds. ORR in an aqueous electrolyte occurs mainly by two pathways, one is the direct 4-electron reduction pathway from O2 to H2O, and the other is a 2-electron reduction pathway from O2 to hydrogen peroxide (H2O2). In non-aqueous aprotic solvents and/or in alkaline solutions, the 1-electron reduction pathway from O2 to superoxide (O2−) can also occur [62,63].
Since the introduction of the first fuel cell in 1842, platinum has been the most common catalytic material due to its properties, such as chemical inertness and properties in the field of gas adsorption and dissociation. At the same time, due to platinum’s rarity and costliness, alternative catalytic materials are sought after.
Nam et al. [50] show that FeCu bimetallic nanoparticles are very active and stable electrocatalysts for energy storage batteries. ORR activity for the CuFe alloy with graphitic carbon shells was comparable to that of Pt/C electrocatalysts, CuFe showed kinetic current density much higher than that for single Cu and Fe. The reduced “blocking effect” of active sites in the CuFe alloy may reduce the affinity of the hydroxyl groups controlling O2 adsorption for the alloy compared to single Cu or Fe catalysts. Direct pathway of oxygen reduction was deduced from the number of transferred electrons calculation (Figure 9). To demonstrate the practical application of the nanoparticles, scientists created a zinc-air battery in which the air cathode was prepared from a mixture of active carbon CuFe@graphite, and synthetic fluoropolymer binder and the anode was prepared from Zn film. They showed that the CuFe catalyzed Zn-air cell can be used in cars, and that the battery can be recharged. They tested that an air electrode made of CuFe alloy worked for over 100 h without voltage loss, which shows the high stability of the CuFe alloy catalyst.
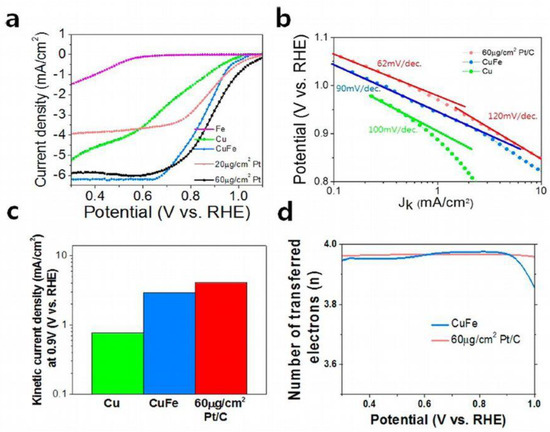
Figure 9.
Electrocatalytic activity of the CuFe alloy. (a) Linear sweep voltammograms of CuFe, Cu, Fe, and Pt/C measured by a RRDE system. (b) Tafel plots of each catalyst. (c) Kinetic current at 0.9 V of each catalyst (except Fe in (b,c) due to low on-set potential of Fe). (d) Calculated number of transferred electrons of CuFe and Pt/C. Reproduced with permission from ref. [50]. Copyright 2015 American Chemical Society.
3.2. Catalysis
It is known that the presence of toxic metals in water poses a serious threat to living organisms. Examples of the most dangerous toxic metal ions found in wastewater are Hg2+, Cr6+, As3+, Cd2+, Pb2+, Mn2+, Ni2+ [64,65]. They cause renal failure, lung impairment, lung cancer, dermatitis, dyspnea, and damage to the reproductive system, liver, and central nervous system [65,66]. In addition, the excess of the above-mentioned metals adversely affects agriculture and the environment [65,66]. CuFe nanoparticles are also examined as materials for the elimination of heavy metals—such as Cr or As—and metalloids from metallic ores in which Cu prevents the oxidation processes of corrosive metals [42,49].
Feng et al. [42] reported that for the efficiency of As3+ removal from smelting wastewater (wastewater produced from smelting, the extraction of metal from its ore by a process involving heating and melting), the copper content in Cu doped Fe@Fe2O3 nanoparticles is crucial and must not exceed 20%. Additionally, it was investigated how pH, temperature and the initial concentration of As3+ affect the efficiency and rate of As3+ removal. It has been shown that the maximum removal capacity of As3+ was achieved at a broad pH range from 3 to 9, suggesting that Cu doped Fe@Fe2O3 particles have the potential to treat As3+ in most surface and groundwater under natural conditions. The removal capacity increased with the temperature increase from 298 K to 308 K, which can be explained by the mobility of the ions as the reaction temperature increased. The adsorption capacity of obtained particles increased with an increase in the initial As3+ concentration, but with an increase in the initial As3+ concentration, the removal rate was almost halved.
Sepúlveda et al. [49] investigated how the morphology of the nanoparticles and copper content influenced the capacity, speed, and intensity of adsorption in As5+ removal. They found that the low content of Cu (Fe0.9Cu0.1) favored the core@shell structure and increased the removal of As5+ compared to Fe0.5Cu0.5 favoring the segregated (Janus) structure. They proposed the mechanism of As5+ removal with the CuFe bimetal, indicating that Cu is responsible for the initial reduction of As5+ to As3+, and the removal of impurities is favored by the transfer of electrons from the Fe0(nZVI) layer to the Cu oxide layer (Figure 10).
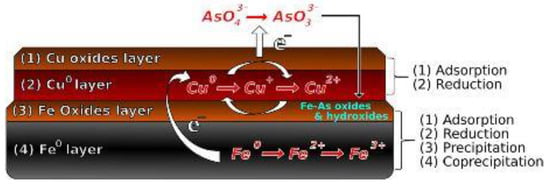
Figure 10.
Proposed mechanism of As5+ removal using bimetallic CuFe materials as the adsorbent. Reprinted with permission from [49]. Copyright 2018, with permission from Elsevier.
It should also be noted that the modification of NP CuFe surface with another material, such as chitosan (CS), improved their adsorption capacity [43]. The authors show that CS-FeCu has the highest Cr6+ removal efficiency in comparison with nZVI, FeCu, and CS-Fe. They reported that the highest stabilization and the reactivity of nZVI, because of the effect of surface coating, were obtained for 2 wt% of chitosan and 3 wt% of Cu in composites. Additionally, the maximum absorption capacities were achieved with pH in the range of 3–9, and CS-FeCu nanoparticles are active even when the initial concentrations of Cr6+ are high.
To remove Cr6+ from water, the composite of GT and nZVICu was also tested. The initial pH, Cr6+ concentration, and reaction temperature have been shown to be important factors influencing the efficiency of Cr6+ removal (Figure 11). Cr6+ removal efficiency increases with a decrease in the initial pH and an increase in temperature [44].
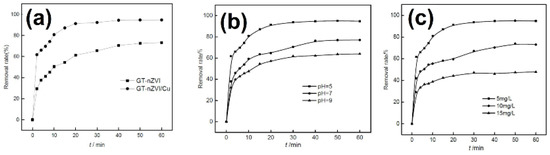
Figure 11.
The removal of Cr6+ by GT- nZVI and GT- nZVICu (a), effect of pH (b), and concentration (c) on the removal of Cr6+. Reprinted from [44], Copyright 2017, with permission from Elsevier.
As can be seen, to maximize the adsorption capacity of CuFe particles, particular attention should be paid to the choice of material for the formation of a heterojunction with CuFe nanoparticles or to modifying its surface, as well as the structure and composition of created nanoparticles and nanocomposites. It would also be worth supplementing the research with the possibility of using CuFe to absorb other toxic metals, such as Pb2+ and Hg2+ or Cd2+.
Bimetallic FeCu nanoparticles can be used to eliminate high concentrations of NO3−-N [51]. These pollutants are produced by agricultural runoff, landfill leachate, leaky septic tanks, municipal storm sewage, animal feed, and industrial waste [67,68] and pose a threat to human health [69]. The reduction of NO3−-N is the most effective while Cu content surrounding nZVI particles is 2.5% (w/w) [51].
The textile industry uses dyes that often pollute the water. Dyes are known to be mutagenic, carcinogenic, and toxic to humans and aquatic animals, and therefore adsorbents are needed to remove them [70,71,72]. CuFe and C/CuFe were used as adsorbents to remove indigo blue. Studies have shown that C/CuFe is more effective in removing indigo blue from aqueous solutions than CuFe [41]. CMC-stabilized Fe-Cu bimetal nanoparticles could effectively dechlorinate 1,2,4-trichlorobenzene (1,2,4-TCB) [53]. Cellulose supported Fe-Cu nanoparticles were also applied for reduction of nitroarenes to arylamines [54]. To increase the catalytic activity of the catalysts, porous materials such as mesoporous silica, activated carbon, mesoporous carbon and carbon nanotubes are also used. Compounds such as phenol, benzoic acid, bisphenol A, 2,4,6-trichlorophenol, imidacloprid, ketoprofen, methylene blue, and methyl orange have been effectively removed from water using CuFe nanoparticles dispersed on mesoporous carbon (CuFe-MC) [60]. The high specific surface area of mesoporous materials increases the absorption of pollutants. Wang et al. [60] indicated the features of a good catalyst:
- the large surface area, and especially a mesoporous structure, favor the rapid diffusion of reagents and products.
- large dispersion of bimetallic iron-copper nanoparticles in the mesoporous carbon matrix significantly increases the number of active centers, which increases absorption and discharge.
- the synergistic effect of iron and copper favored the redox cycles Fe3+/Fe2+ and
- Cu2+/Cu+, increasing the catalytic activity.
- the presence of mesoporous carbon which can also activate H2O2 to produce •OH.
- An additional benefit of using magnetic catalysts is that they can be easily separated by a magnet, which facilitates the removal of contaminants [60].
4. Conclusions
As described in this review, the combination of copper and iron—two well-known and common materials—can provide multi-functional materials for numerous applications. In bulk, copper and iron are slightly soluble in each other, additionally they present a strong tendency to oxidation under atmospheric conditions; therefore, we paid special attention to the synthesis methods of bimetallic CuFe nanoparticles and bimetallic CuFe NPs supported on different materials and the structure of the obtained materials. By mechanical milling, reduction, or two-step reduction, CuFe nanoparticles with alloy, Janus, and core@shell structures were created. Often they were stabilized by carbon, silicon, or cellulose. Structure and properties depend on the size and composition.
Despite the large number of experimental methodologies available, the challenge remains to evolve a simple, efficient, and reliable process for the synthesis of pure CuFe nanoparticles with a defined structure and without any oxide-like impurities. For the challenge of nanoparticle oxidation two techniques seem promising: a combination of a chemical reduction of ferric nitrate and copper nitrate in alcohol solution with annealing carbonization technique, and a high temperature pyrolysis of the copper precursor (chlorophyllin) and the iron precursor (Fe(II) acetylacetonate). While the reduction of copper and iron sulfides with sodium borohydride, and reduction of cupric and ferric nitrates with sodium borohydride in ethylene glycol under Ar seem to be less effective.
CuFe nanoparticles have a great application potential in energy storage, fuel cells, and pollution treatment. CuFe nanoparticles have a great potential for applications in energy storage, fuel cells, and water purification. Therefore, it is important to take advantage of their magnetic and catalytic properties, low cost, and abundance and move them from the laboratory to large-scale applications.
Funding
This research received no external funding.
Data Availability Statement
Not applicable for a review paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, X.; Wang, D.; Li, Y. Synthesis and catalytic properties of bimetallic nanomaterials with various architectures. Nano Today 2012, 7, 448–466. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Zhao, D. Lab on upconversion nanoparticles: Optical properties and applications engineering via designed nanostructure. Chem. Soc. Rev. 2015, 44, 1346–1378. [Google Scholar] [CrossRef]
- Liu, F.; Hou, Y.; Gao, S. Exchange-coupled nanocomposites: Chemical synthesis, characterization and applications. Chem. Soc. Rev. 2014, 43, 8098–8113. [Google Scholar] [CrossRef] [PubMed]
- Srinoi, P.; Chen, Y.-T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Pellegrino, T.; Manna, L. Synthesis, properties and perspectives of hybrid nanocrystal structures. Chem. Soc. Rev. 2006, 35, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble metal-based bimetallic nanoparticles: The effect of the structure on the optical, catalytic and photocatalytic properties. Adv. Colloid Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef]
- Ismail, A.M.; Samu, G.F.; Balog, Á.; Csapó, E.; Janáky, C. Composition-Dependent Electrocatalytic Behavior of Au–Sn Bimetallic Nanoparticles in Carbon Dioxide Reduction. ACS Energy Lett. 2019, 4, 48–53. [Google Scholar] [CrossRef]
- Wang, H.; Hao, H.; Li, Y. Size-Dependent Electrochemistry and Electrocatalysis at Single Au@Pt Bimetallic Nanoparticles. J. Phys. Chem. C 2020, 124, 24740–24746. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Ahn, C.H.; Choi, J.W.; Cho, H.J. Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2004; Volume 6, p. 815. [Google Scholar]
- Gul, S.; Khan, S.B.; Rehman, I.U.; Khan, M.A. A Comprehensive Review of Magnetic Nanomaterials Modern Day Theranostics. Front. Mater. 2019, 6, 179. [Google Scholar] [CrossRef]
- Peng, S.; Wang, C.; Xie, J.; Sun, S. Synthesis and Stabilization of Monodisperse Fe Nanoparticles. J. Am. Chem. Soc. 2006, 128, 10676–10677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X. Nanoscale Iron Particles for Environmental Remediation: An Overview. J. Nanoparticle Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, X.; Wang, J.; Fan, J. Highly reactive and stable nanoscale zero-valent iron prepared within vesicles and its high-performance removal of water pollutants. Appl. Catal. B Environ. 2018, 221, 610–617. [Google Scholar] [CrossRef]
- Eljamal, R.; Eljamal, O.; Khalil, A.M.; Saha, B.B.; Matsunaga, N. Improvement of the chemical synthesis efficiency of nano-scale zero-valent iron particles. J. Environ. Chem. Eng. 2018, 6, 4727–4735. [Google Scholar] [CrossRef]
- Kanel, S.R.; Manning, B.; Charlet, L.; Choi, H. Removal of Arsenic(III) from Groundwater by Nanoscale Zero-Valent Iron. Environ. Sci. Technol. 2005, 39, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Jiang, R. Novel electrocatalysts for direct methanol fuel cells. Solid State Ionics 2002, 148, 591–599. [Google Scholar] [CrossRef]
- He, H.; Lei, Y.; Xiao, C.; Chu, D.; Chen, R.; Wang, G. Molecular and Electronic Structures of Transition-Metal Macrocyclic Complexes as Related to Catalyzing Oxygen Reduction Reactions: A Density Functional Theory Study. J. Phys. Chem. C 2012, 116, 16038–16046. [Google Scholar] [CrossRef]
- Ahn, C.H.; Choi, J.W.; Cho. H., J. Catalysts for oxygen reduction from heat-treated carbon-supported iron phenantroline complexes. J. Appl. Electrochem. 2002, 32, 211–216. [Google Scholar]
- Wang, H.; Côté, R.; Faubert, G.; Guay, D.; Dodelet, J.P. Effect of the Pre-Treatment of Carbon Black Supports on the Activity of Fe-Based Electrocatalysts for the Reduction of Oxygen. J. Phys. Chem. B 1999, 103, 2042–2049. [Google Scholar] [CrossRef]
- Lefèvre, M.; Dodelet, J.P.; Bertrand, P. O2Reduction in PEM Fuel Cells: Activity and Active Site Structural Information for Catalysts Obtained by the Pyrolysis at High Temperature of Fe Precursors. J. Phys. Chem. B 2000, 104, 11238–11247. [Google Scholar] [CrossRef]
- Lefèvre, M.; Dodelet, J.P.; Bertrand, P. Molecular Oxygen Reduction in PEM Fuel Cells: Evidence for the Simultaneous Presence of Two Active Sites in Fe-Based Catalysts. J. Phys. Chem. B 2002, 106, 8705–8713. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Rojas-Nunez, J.; Gonzalez, R.I.I.; Bringa, E.M.; Allende, S.; Sepúlveda, P.; Arancibia-Miranda, N.; Baltazar, S.E. Toward Controlled Morphology of FeCu Nanoparticles: Cu Concentration and Size Effects. J. Phys. Chem. C 2018, 122, 8528–8534. [Google Scholar] [CrossRef]
- Kumar, S. Structural Evolution of Iron–Copper (Fe–Cu) Bimetallic Janus Nanoparticles during Solidification: An Atomistic Investigation. J. Phys. Chem. C 2019, 124, 1053–1063. [Google Scholar] [CrossRef]
- Akbarzadeh, H.; Abbaspour, M.; Mehrjouei, E.; Ramezanzadeh, S. Ag–Au nanoparticles encapsulated inside porous hollow carbon nanospheres: A molecular dynamics study. New J. Chem. 2018, 42, 13619–13628. [Google Scholar] [CrossRef]
- Abbaspour, M.; Akbarzadeh, H.; Salemi, S.; Lotfi, S. Investigation of Possible Formation of Au@M (M = Cu, Ir, Pt, and Rh) Core–Shell Nanoclusters in a Condensation–Coalescence Process Using Molecular Dynamics Simulations. Ind. Eng. Chem. Res. 2018, 57, 14837–14845. [Google Scholar] [CrossRef]
- Tran, D.T.; Roy, L.J. Theoretical Study of Cu38−n Aun Clusters Using a Combined Empirical Potential−Density Functional Approach. Phys. Chem. Chem. Phys. 2009, 11, 10340–10349. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, C.C.; Tran, V.H. Molecular dynamics study of microscopic structures, phase transitions and dynamic crystallization in Ni nanoparticles. RSC Adv. 2017, 7, 25406–25413. [Google Scholar] [CrossRef]
- Guisbiers, G.; Cruz, R.M.; Díaz, L.B.; Salazar, J.J.V.; Perez, R.M.; Torres, J.A.R.; Lopez, J.-L.R.; Carrizales, J.M.M.; Whetten, R.L.; Yacama’n, M.J. Electrum, the Gold−Silver Alloy, from the Bulk Scale to the Nanoscale: Synthesis, Properties, and Segregation Rules. ACS Nano 2015, 10, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Guisbiers, G.; Khanal, S.; Zepeda, F.R.; Puente, J.R.; José-Yacaman, M. Cu−Ni Nano-alloy: Mixed, Core−shell or Janus Nano-particle? Nanoscale 2014, 6, 14630–14635. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Holzer, J.C.; Krill, C.E.; Johnson, W.L. Mechanically driven alloying and grain size changes in nanocrystalline Fe-Cu powders. J. Appl. Phys. 1993, 73, 2794–2802. [Google Scholar] [CrossRef]
- Hong, L.; Fultz, B. Two-phase coexistence in Fe–Cu alloys synthesized by ball milling. Acta Mater. 1998, 46, 2937–2946. [Google Scholar] [CrossRef]
- Yavari, A.R.; Des, P.J. Ordering and Disordering in Alloys; Yavari, A.R., Ed.; Elsevier: London, UK, 1992; p. 414. [Google Scholar]
- Sumiyama, K.; Yoshitake, T.; Nakamura, Y. Magnetic Properties of Metastable bcc and fcc Fe-Cu Alloys Produced by Vapor Quenching. J. Phys. Soc. Jpn. 1984, 53, 3160–3165. [Google Scholar] [CrossRef]
- Todaka, Y.; McCormick, P.G.; Tsuchiya, K.; Umemoto, M. Synthesis of Fe-Cu Nanoparticles by Mechanochemical Processing Using a Ball Mill. Mater. Trans. 2002, 43, 667–673. [Google Scholar] [CrossRef]
- Sharipova, A.F.; Psakhie, S.G.; Gotman, I.; Gutmanas, E.Y. Smart Nanocomposites Based on Fe–Ag and Fe–Cu Nanopowders for Biodegradable High-Strength Implants with Slow Drug Release. Phys. Mesomech. 2020, 23, 128–134. [Google Scholar] [CrossRef]
- Vishlaghi, M.B.; Ataie, A. Investigation on solid solubility and physical properties of Cu–Fe/CNT nano-composite prepared via mechanical alloying route. Powder Technol. 2014, 268, 102–109. [Google Scholar] [CrossRef]
- Duxin, N.; Brun, N.; Colliex, C.; Pileni, M.P. Synthesis and Magnetic Properties of Elongated Fe−Cu Alloys. Langmuir 1998, 14, 1984–1989. [Google Scholar] [CrossRef]
- Trujillo-Reyes, J.; Sánchez-Mendieta, V.; Colín-Cruz, A.; Morales-Luckie, R.A. Removal of Indigo Blue in Aqueous Solution Using Fe/Cu Nanoparticles and C/Fe–Cu Nanoalloy Composites. Water Air Soil Pollut. 2009, 207, 307–317. [Google Scholar] [CrossRef]
- Feng, H.; Tang, L.; Tang, J.; Zeng, G.; Dong, H.; Deng, Y.; Wang, L.; Liu, Y.; Ren, X.; Zhou, Y. Cu-Doped Fe@Fe2O3 core–shell nanoparticle shifted oxygen reduction pathway for high-efficiency arsenic removal in smelting wastewater. Environ. Sci. Nano 2018, 5, 1595–1607. [Google Scholar] [CrossRef]
- Jiang, D.; Huang, D.; Lai, C.; Xu, P.; Zeng, G.; Wan, J.; Tang, L.; Dong, H.; Huang, B.; Hu, T. Difunctional chitosan-stabilized Fe/Cu bimetallic nanoparticles for removal of hexavalent chromium wastewater. Sci. Total Environ. 2018, 644, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Ma, S.; Liu, T.; Deng, X. Green synthesis of nano zero-valent iron/Cu by green tea to remove hexavalent chromium from groundwater. J. Clean. Prod. 2018, 174, 184–190. [Google Scholar] [CrossRef]
- Xue, J.; Xiang, H.K.; Ding, H.Q.; Pang, S.L.; Wang, X.H.; Cao, H. Preparation and Magnetic Properties of Carbon Encapsulated Fe-Cu Alloy Nanoparticles. Adv. Mater. Res. 2010, 177, 673–676. [Google Scholar] [CrossRef]
- Carneiro, O.C.; Anderson, P.E.; Rodriguez, N.M.; Baker, R.T.K. Decomposition of CO−H2 over Graphite Nanofiber-Supported Iron and Iron−Copper Catalysts. J. Phys. Chem. B 2004, 108, 13307–13314. [Google Scholar] [CrossRef]
- Xiao, K.; Bao, Z.; Qi, X.; Wang, X.; Zhong, L.; Lin, M.; Fang, K.; Sun, Y. Unsupported CuFe bimetallic nanoparticles for higher alcohol synthesis via syngas. Catal. Commun. 2013, 40, 154–157. [Google Scholar] [CrossRef]
- Xiao, K.; Qi, X.; Bao, Z.; Wang, X.; Zhong, L.; Fang, K.; Lin, M.; Sun, Y. CuFe, CuCo and CuNi nanoparticles as catalysts for higher alcohol synthesis from syngas: A comparative study. Catal. Sci. Technol. 2013, 3, 1591–1602. [Google Scholar] [CrossRef]
- Sepúlveda, P.; Rubio, M.A.; Baltazar, S.E.; Rojas-Nunez, J.; Llamazares, J.S.; Garcia, A.G.; Arancibia-Miranda, N. As(V) removal capacity of FeCu bimetallic nanoparticles in aqueous solutions: The influence of Cu content and morphologic changes in bimetallic nanoparticles. J. Colloid Interface Sci. 2018, 524, 177–187. [Google Scholar] [CrossRef]
- Nam, G.; Park, J.; Choi, M.; Oh, P.; Park, S.; Kim, M.G.; Park, N.; Cho, J.; Lee, J.-S. Carbon-Coated Core–Shell Fe–Cu Nanoparticles as Highly Active and Durable Electrocatalysts for a Zn–Air Battery. ACS Nano 2015, 9, 6493–6501. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Ataie-Ashtiani, B.; Kholghi, M. Nitrate reduction by nano-Fe/Cu particles in packed column. Desalination 2011, 276, 214–221. [Google Scholar] [CrossRef]
- Zin, M.T.; Borja, J.; Hinode, H.; Kurniawan, W. Synthesis of Bimetallic Fe/Cu Nanoparticles with Different Copper Loading Ratios. Int. Sch. Sci. Res. Innov. 2013, 7, 1031–1035. [Google Scholar]
- Cao, J.; Xu, R.; Tang, H.; Tang, S.; Cao, M. Synthesis of monodispersed CMC-stabilized Fe–Cu bimetal nanoparticles for in situ reductive dechlorination of 1, 2, 4-trichlorobenzene. Sci. Total. Environ. 2011, 409, 2336–2341. [Google Scholar] [CrossRef]
- Karami, S.; Zeynizadeh, B.; Shokri, Z. Cellulose supported bimetallic Fe–Cu nanoparticles: A magnetically recoverable nanocatalyst for quick reduction of nitroarenes to amines in water. Cellulose 2018, 25, 3295–3305. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef]
- Han, Z.; Dong, Y.; Dong, S. Copper–iron bimetal modified PAN fiber complexes as novel heterogeneous Fenton catalysts for degradation of organic dye under visible light irradiation. J. Hazard. Mater. 2011, 189, 241–248. [Google Scholar] [CrossRef]
- Lam, F.L.Y.; Hu, X. pH-Insensitive Bimetallic Catalyst for the Abatement of Dye Pollutants by Photo-Fenton Oxidation. Ind. Eng. Chem. Res. 2013, 52, 6639–6646. [Google Scholar] [CrossRef]
- Xia, M.; Long, M.; Yang, Y.; Chen, C.; Cai, W.; Zhou, B. A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution. Appl. Catal. B Environ. 2011, 110, 118–125. [Google Scholar] [CrossRef]
- Lin, H.; Zhong, X.; Ciotonea, C.; Fan, X.; Mao, X.; Li, Y.; Deng, B.; Zhang, H.; Royer, S. Efficient degradation of clofibric acid by electro-enhanced peroxydisulfate activation with Fe-Cu/SBA-15 catalyst. Appl. Catal. B Environ. 2018, 230, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Zhao, G. Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2015, 164, 396–406. [Google Scholar] [CrossRef]
- Chun, C.L.; Baer, D.R.; Matson, D.W.; Amonette, J.E.; Penn, R.L. Characterization and Reactivity of Iron Nanoparticles prepared with added Cu, Pd, and Ni. Environ. Sci. Technol. 2010, 44, 5079–5085. [Google Scholar] [CrossRef]
- Ray, A.; Mukhopadhyay, I.; Pati, R.K. (Eds.) Electrocatalysts for Fuel Cells and Hydrogen Evolution—Theory to Design; IntechOpen: London, UK, 2018. [Google Scholar]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Hiscock, K.; Lloyd, J.; Lerner, D. Review of natural and artificial denitrification of groundwater. Water Res. 1991, 25, 1099–1111. [Google Scholar] [CrossRef]
- Joekar-Niasar, V.; Ataie-Ashtiani, B. Assessment of nitrate contamination in unsaturated zone of urban areas: The case study of Tehran, Iran. Environ. Earth Sci. 2008, 57, 1785–1798. [Google Scholar] [CrossRef]
- Nolan, B.T.; Ruddy, B.C.; Hitt, K.J.; Helsel, D.R. Risk of Nitrate in Groundwaters of the United StatesA National Perspective. Environ. Sci. Technol. 1997, 31, 2229–2236. [Google Scholar] [CrossRef]
- Ayazi, Z.; Khoshhesab, Z.M.; Amani-Ghadim, A. Synthesis of nickel ferrite nanoparticles as an efficient magnetic sorbent for removal of an azo-dye: Response surface methodology and neural network modelling. Nanochem. Res. 2018, 3, 109–123. [Google Scholar]
- Mushtaq, M.W.; Kanwal, F.; Imran, M.; Ameen, N.; Batool, M.; Batool, A.; Bashir, S.; Abbas, S.M.; Rehman, A.U.; Riaz, S.; et al. Synthesis of surfactant-coated cobalt ferrite nanoparticles for adsorptive removal of acid blue 45 dye. Mater. Res. Express 2018, 5, 035058. [Google Scholar] [CrossRef]
- Hassani, A.; Çelikdağ, G.; Eghbali, P.; Sevim, M.; Karaca, S.; Metin, Ö. Heterogeneous sono-Fenton-like process using magnetic cobalt ferrite-reduced graphene oxide (CoFe2O4-rGO) nanocomposite for the removal of organic dyes from aqueous solution. Ultrason. Sonochem. 2018, 40, 841–852. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).