Room Temperature In-Situ Synthesis of Inorganic Lead Halide Perovskite Nanocrystals Sol Using Ultraviolet Polymerized Acrylic Monomers as Solvent and Their Composites with High Stability
Abstract
:1. Introduction
2. Experiment
2.1. Materials
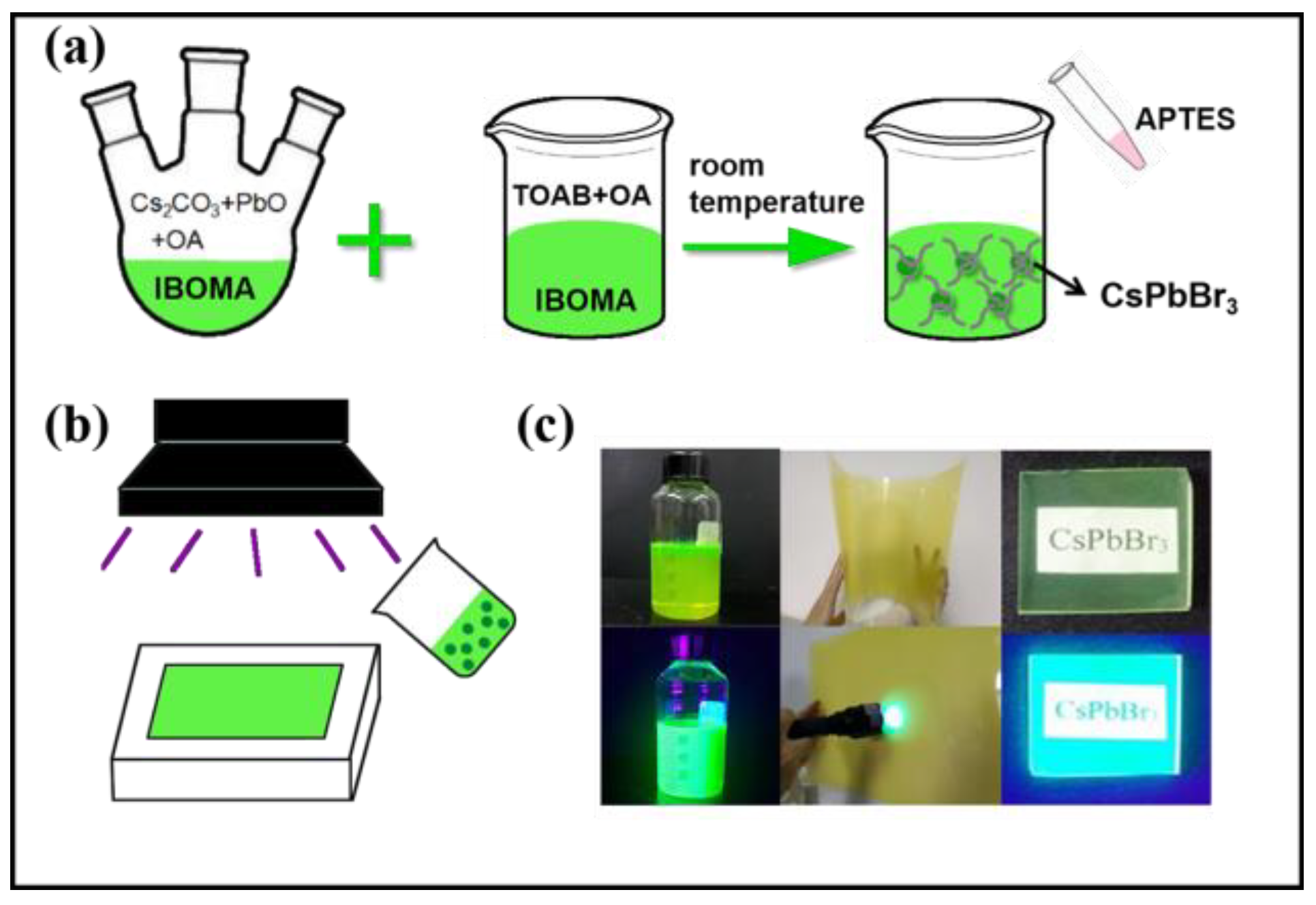
2.2. Synthesis of CsPbBr3 Sol/Composites
2.3. Anion-Exchange Process
2.4. Characterization Methods
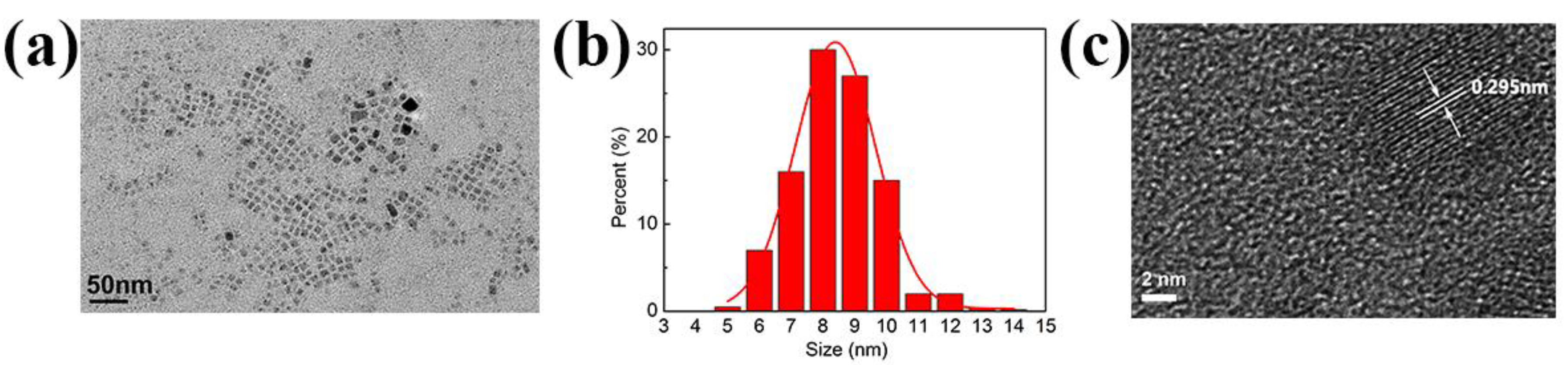
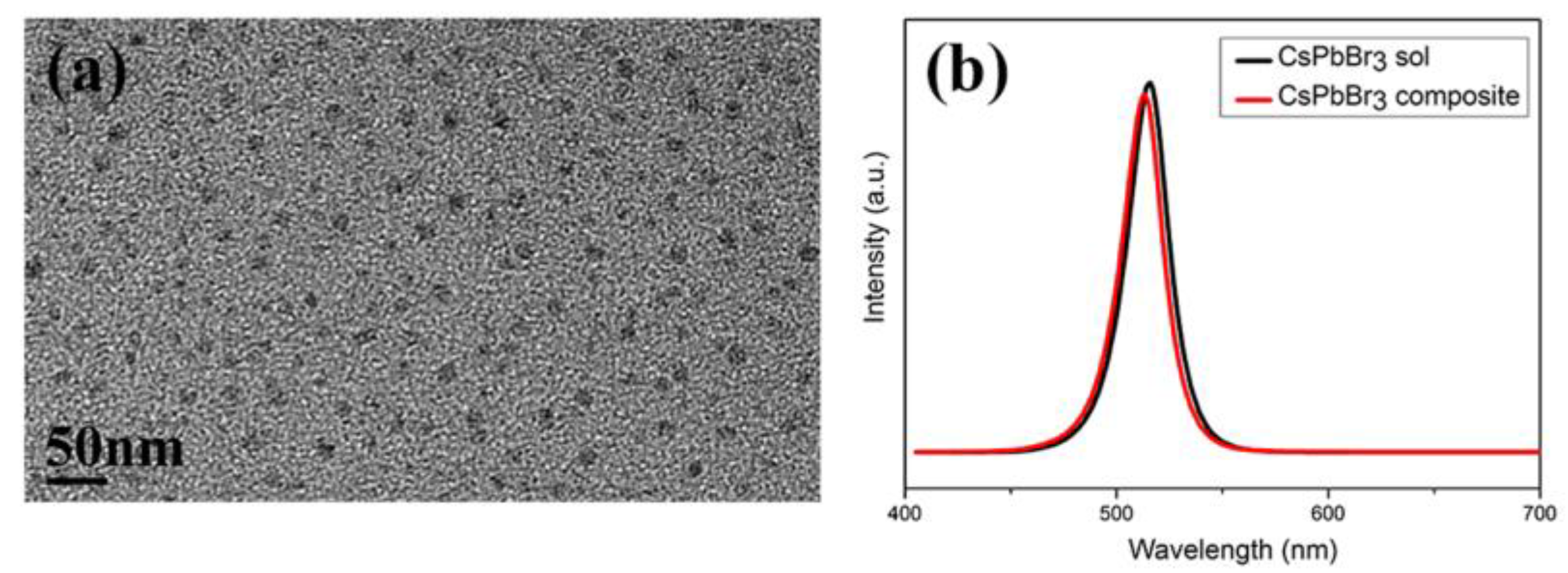
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, H.; Zhao, F.; Liu, L.; Zhang, F.; Wu, X.-G.; Shi, L.; Zou, B.; Pei, Q.; Zhong, H. Emulsion Synthesis of Size-Tunable CH3NH3PbBr3 Quantum Dots: An Alternative Route toward Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2015, 7, 28128–28133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, H.-Z.; Chen, C.; Wu, X.-G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Chulliyil, R.; Ravi, V.K.; Irfanullah, M.; Chowdhury, A.; Nag, A. Colloidal CsPbBr 3 Perovskite Nanocrystals: Luminescence beyond Traditional Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 15424–15428. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Meijerink, A.; Liu, R.-S. Critical Red Components for Next-Generation White LEDs. J. Phys. Chem. Lett. 2016, 7, 495–503. [Google Scholar] [CrossRef]
- Palazon, F.; Di Stasio, F.; Akkerman, Q.A.; Krahne, R.; Prato, M.; Manna, L. Polymer-Free Films of Inorganic Halide Perovskite Nanocrystals as UV-to-White Color-Conversion Layers in LEDs. Chem. Mater. 2016, 28, 2902–2906. [Google Scholar] [CrossRef] [Green Version]
- Beal, R.E.; Slotcavage, D.J.; Leijtens, T.; Bowring, A.R.; Belisle, R.A.; Nguyen, W.H.; Burkhard, G.F.; Hoke, E.T.; McGehee, M.D. Cesium Lead Halide Perovskites with Improved Stability for Tandem Solar Cells. J. Phys. Chem. Lett. 2016, 7, 746–751. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Song, J.; Xiaoming, L.; Zeng, H.; Sun, H. All-Inorganic Colloidal Perovskite Quantum Dots: A New Class of Lasing Materials with Favorable Characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef]
- Ramasamy, P.; Lim, D.-H.; Kim, B.; Lee, S.-H.; Lee, M.-S.; Lee, J.-S. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 2016, 52, 2067–2070. [Google Scholar] [CrossRef]
- Guhrenz, C.; Benad, A.; Ziegler, C.; Haubold, D.; Gaponik, N.; Eychmüller, A. Solid-State Anion Exchange Reactions for Color Tuning of CsPbX3 Perovskite Nanocrystals. Chem. Mater. 2016, 28, 9033–9040. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liao, Q.; Xu, Z.; Li, H.; Zheng, L.-M.; Fu, H. Embedding Perovskite Nanocrystals into a Polymer Matrix for Tunable Luminescence Probes in Cell Imaging. Adv. Funct. Mater. 2017, 27, 1604382. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Y.; Zhang, Y.; Chen, Y.; Li, X.; Wu, D.; Xu, T.; Shan, C.; Du, G. High-Efficiency and Air-Stable Perovskite Quantum Dots Light-Emitting Diodes with an All-Inorganic Heterostructure. Nano Lett. 2016, 17, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Bai, Z.; Lu, W.-G.; Wang, Y.; Zou, B.; Zhong, H.-Z. In Situ Fabrication of Halide Perovskite Nanocrystal-Embedded Polymer Composite Films with Enhanced Photoluminescence for Display Backlights. Adv. Mater. 2016, 28, 9163–9168. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Schlipf, J.; Askar, A.M.; Pantle, F.; Wiltshire, B.D.; Sura, A.; Schneider, P.; Huber, L.; Shankar, K.; Müller-Buschbaum, P. Top-Down Approaches Towards Single Crystal Perovskite Solar Cells. Sci. Rep. 2018, 8, 4906. [Google Scholar] [CrossRef] [Green Version]
- Protesescu, L.; Yakunin, S.; Nazarenko, O.; Dirin, D.N.; Kovalenko, M.V. Low-Cost Synthesis of Highly Luminescent Colloidal Lead Halide Perovskite Nanocrystals by Wet Ball Milling. ACS Appl. Nano Mater. 2018, 1, 1300–1308. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Imran, M.; Zhang, M.; Chang, S.; Wu, X.-G.; Zhang, X.; Tang, J.; Wang, M.; Ali, S.; Li, X.; et al. Efficient Light-Emitting Diodes Based on in Situ Fabricated FAPbBr3 Nanocrystals: The Enhancing Role of the Ligand-Assisted Reprecipitation Process. ACS Nano 2018, 12, 8808–8816. [Google Scholar] [CrossRef]
- Liu, H.W.; Wu, Z.N.; Gao, H.; Shao, J.R.; Zou, H.Y.; Yao, D.; Liu, Y.; Zhang, H.; Yang, B. One-Step Preparation of Cesium Lead Halide CsPbX3 (X = CI, Br, and I) Perovskite Nanocrystals by Microwave Irradiation. ACS Appl. Mater. Interfaces 2017, 9, 42919–42927. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Na Quan, L.; De Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Fang, F.; Chen, W.; Li, Y.; Liu, H.; Mei, M.; Zhang, R.; Hao, J.; Mikita, M.; Cao, W.; Pan, R.; et al. Employing Polar Solvent Controlled Ionization in Precursors for Synthesis of High-Quality Inorganic Perovskite Nanocrystals at Room Temperature. Adv. Funct. Mater. 2018, 28, 1706000. [Google Scholar] [CrossRef]
- Wu, L.; Hu, H.; Xu, Y.; Jiang, S.; Chen, M.; Zhong, Q.; Yang, D.; Liu, Q.; Zhao, Y.; Sun, B.; et al. From Nonluminescent Cs4PbX6 (X = Cl, Br, I) Nanocrystals to Highly Luminescent CsPbX3 Nanocrystals: Water-Triggered Transformation through a CsX-Stripping Mechanism. Nano Lett. 2017, 17, 5799–5804. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.C.; Wu, L.Z.; Tan, Y.S.; Zhong, Q.X.; Chen, M.; Qiu, Y.H.; Yang, D.; Sun, B.Q.; Zhang, Q.; Yin, Y.D. Interfacial Synthesis of Highly Stable CsPbX3/Oxide Janus Nanoparticles. J. Am. Chem. Soc. 2018, 140, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, X.; Wu, H.; Zhang, X.; Sun, C.; Zhang, C.; Zhang, W.; Zheng, W.T.; Yu, W.W.; Rogach, A.L. Water-Assisted Size and Shape Control of CsPbBr3 Perovskite Nanocrystals. Angew. Chem. Int. Ed. 2018, 57, 3337–3342. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Xu, S.; Zhong, H.-Z.; Rogach, A.L.; Bi, W. Aqueous Synthesis of Methylammonium Lead Halide Perovskite Nanocrystals. Angew. Chem. Int. Ed. 2018, 57, 9650–9654. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Ba, Q.; Kim, H.R. Compositional and Dimensional Control of 2D and Quasi-2D Lead Halide Perovskites in Water. Adv. Funct. Mater. 2019, 29, 1900966. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Xiong, Y.; Richter, A.F.; Kershaw, S.V.; Feldmann, J.; Rogach, A.L. Using Polar Alcohols for the Direct Synthesis of Cesium Lead Halide Perovskite Nanorods with Anisotropic Emission. ACS Nano 2019, 13, 8237–8245. [Google Scholar] [CrossRef]
- Tong, J.; Wu, J.; Shen, W.; Zhang, Y.; Liu, Y.; Zhang, T.; Nie, S.; Deng, Z. Direct Hot-Injection Synthesis of Lead Halide Perovskite Nanocubes in Acrylic Monomers for Ultrastable and Bright Nanocrystal–Polymer Composite Films. ACS Appl. Mater. Interfaces 2019, 11, 9317–9325. [Google Scholar] [CrossRef]
- Ma, K.; Du, X.-Y.; Zhang, Y.-W.; Chen, S. In situ fabrication of halide perovskite nanocrystals embedded in polymer composites via microfluidic spinning microreactors. J. Mater. Chem. C 2017, 5, 9398–9404. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Chen, H.; Chen, J.; Zhu, R.; Ma, P.; Towers, A.; Lin, Y.; Gesquiere, A.J.; Wu, S.-T.; et al. Ultrastable, Highly Luminescent Organic-Inorganic Perovskite-Polymer Composite Films. Adv. Mater. 2016, 28, 10710–10717. [Google Scholar] [CrossRef] [PubMed]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, Y.; Sun, H.; Zeng, H. Amino-Mediated Anchoring Perovskite Quantum Dots for Stable and Low-Threshold Random Lasing. Adv. Mater. 2017, 29, 1701185. [Google Scholar] [CrossRef] [PubMed]
- De Roo, J.; Ibáñez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.C.; Van Driessche, I.; Kovalenko, M.V.; Hens, Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef] [Green Version]
- Sarang, S.; Naghadeh, S.B.; Luo, B.; Kumar, P.; Betady, E.; Tung, V.; Scheibner, M.; Zhang, J.Z.; Ghosh, S. Stabilization of the Cubic Crystalline Phase in Organometal Halide Perovskite Quantum Dots via Surface Energy Manipulation. J. Phys. Chem. Lett. 2017, 8, 5378–5384. [Google Scholar] [CrossRef]
- González-Pedro, V.; Veldhuis, S.A.; Begum, R.; Bañuls, M.-J.; Bruno, A.; Mathews, N.; Mhaisalkar, S.G.; Maquieira, Á. Recovery of Shallow Charge-Trapping Defects in CsPbX3 Nanocrystals through Specific Binding and Encapsulation with Amino-Functionalized Silanes. ACS Energy Lett. 2018, 3, 1409–1414. [Google Scholar] [CrossRef]
- Liang, X.; Chen, M.; Wang, Q.; Guo, S.J.; Yang, H. Ethanol-Precipitable, Silica-Passivated Perovskite Nanocrystals Incorporated into Polystyrene Microspheres for Long-Term Storage and Reusage. Angew. Chem. Int. Ed. 2019, 58, 2799–2803. [Google Scholar] [CrossRef]
- Luo, B.; Pu, Y.-C.; Lindley, S.A.; Yang, Y.; Lu, L.; Li, Y.; Li, X.; Zhang, J.Z. Organolead Halide Perovskite Nanocrystals: Branched Capping Ligands Control Crystal Size and Stability. Angew. Chem. Int. Ed. 2016, 55, 8864–8868. [Google Scholar] [CrossRef] [Green Version]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 0903–0922. [Google Scholar] [CrossRef]
- Kim, Y.; Yassitepe, E.; Voznyy, O.; Comin, R.; Walters, G.; Gong, X.; Kanjanaboos, P.; Nogueira, A.F.; Sargent, E. Efficient Luminescence from Perovskite Quantum Dot Solids. ACS Appl. Mater. Interfaces 2015, 7, 25007–25013. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, C.; Ruan, C.; Yin, C.; Wang, X.; Wang, Y.; Yu, W.W. Efficient and Stable White LEDs with Silica-Coated Inorganic Perovskite Quantum Dots. Adv. Mater. 2016, 28, 10088–10094. [Google Scholar] [CrossRef] [PubMed]
- Koolyk, M.; Amgar, D.; Aharon, S.; Etgar, L. Kinetics of cesium lead halide perovskite nanoparticle growth; focusing and de-focusing of size distribution. Nanoscale 2016, 8, 6403–6409. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cai, Z.; Li, F.; Dong, J.; Wang, Y.; Jiang, Y.; Chen, X. Embedding lead halide perovskite quantum dots in carboxybenzene microcrystals improves stability. Nano Res. 2017, 10, 2692–2698. [Google Scholar] [CrossRef]
- Huang, H.; Chen, B.; Wang, Z.; Hung, T.F.; Susha, A.S.; Zhong, H.; Rogach, A.L. Water resistant CsPbX3 nanocrystals coated with polyhedral oligomeric silsesquioxane and their use as solid state luminophores in all-perovskite white light-emitting devices. Chem. Sci. 2016, 7, 5699–5703. [Google Scholar] [CrossRef] [Green Version]
- Meyns, M.; Perálvarez, M.; Heuer-Jungemann, A.; Hertog, W.; Ibáñez, M.; Nafria, R.; Genç, A.; Arbiol, J.; Kovalenko, M.V.; Carreras, J.; et al. Polymer-Enhanced Stability of Inorganic Perovskite Nanocrystals and Their Application in Color Conversion LEDs. ACS Appl. Mater. Interfaces 2016, 8, 19579–19586. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Li, Z.; Wang, B.; Zhu, N.; Zhang, C.; Kongb, L.; Zhang, Q.; Shan, A.; Li, L. Morphology Evolution and Degradation of CsPbBr3 Nanocrystals under Blue Light-Emitting Diode Illumination. ACS Appl. Mater. Interfaces 2017, 9, 7249–7258. [Google Scholar] [CrossRef]
- Wei, S.; Yang, Y.; Kang, X.; Huang, L.; Pan, D.; Wang, L. Room-temperature and gram-scale synthesis of CsPbX 3 (X = Cl, Br, I) perovskite nanocrystals with 50–85% photoluminescence quantum yields. Chem. Commun. 2016, 52, 7265–7268. [Google Scholar] [CrossRef]



| Cl:Br | |||||||
| Ratio | 1.2 | 1.0 | 0.8 | 0.65 | 0.5 | 0.35 | 0 |
| HCL (μL) | 31.5 | 27 | 22.5 | 18 | 13.5 | 9 | 0 |
| I:Br | |||||||
| Ratio | 0 | 0.35 | 0.5 | 0.65 | 0.8 | 1.0 | 1.2 |
| ZnI2 (g) | 0 | 0.016 | 0.024 | 0.032 | 0.04 | 0.048 | 0.056 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Yuan, S.; Cheng, J.; Chen, L.; Liu, C.; Tong, H.; Zeng, H.; Cheng, Q. Room Temperature In-Situ Synthesis of Inorganic Lead Halide Perovskite Nanocrystals Sol Using Ultraviolet Polymerized Acrylic Monomers as Solvent and Their Composites with High Stability. Appl. Sci. 2020, 10, 3325. https://doi.org/10.3390/app10093325
Zhu L, Yuan S, Cheng J, Chen L, Liu C, Tong H, Zeng H, Cheng Q. Room Temperature In-Situ Synthesis of Inorganic Lead Halide Perovskite Nanocrystals Sol Using Ultraviolet Polymerized Acrylic Monomers as Solvent and Their Composites with High Stability. Applied Sciences. 2020; 10(9):3325. https://doi.org/10.3390/app10093325
Chicago/Turabian StyleZhu, Ludan, Shuanglong Yuan, Jun Cheng, Long Chen, Chuanqi Liu, Hua Tong, Huidan Zeng, and Qiling Cheng. 2020. "Room Temperature In-Situ Synthesis of Inorganic Lead Halide Perovskite Nanocrystals Sol Using Ultraviolet Polymerized Acrylic Monomers as Solvent and Their Composites with High Stability" Applied Sciences 10, no. 9: 3325. https://doi.org/10.3390/app10093325
APA StyleZhu, L., Yuan, S., Cheng, J., Chen, L., Liu, C., Tong, H., Zeng, H., & Cheng, Q. (2020). Room Temperature In-Situ Synthesis of Inorganic Lead Halide Perovskite Nanocrystals Sol Using Ultraviolet Polymerized Acrylic Monomers as Solvent and Their Composites with High Stability. Applied Sciences, 10(9), 3325. https://doi.org/10.3390/app10093325





