Prediction of Health Care Costs by Dental Health Care Costs and Periodontal Status
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. Measuring Salivary Levels of Lactate Dehydrogenase (LD)
2.4. Data Collection on Health Care Costs
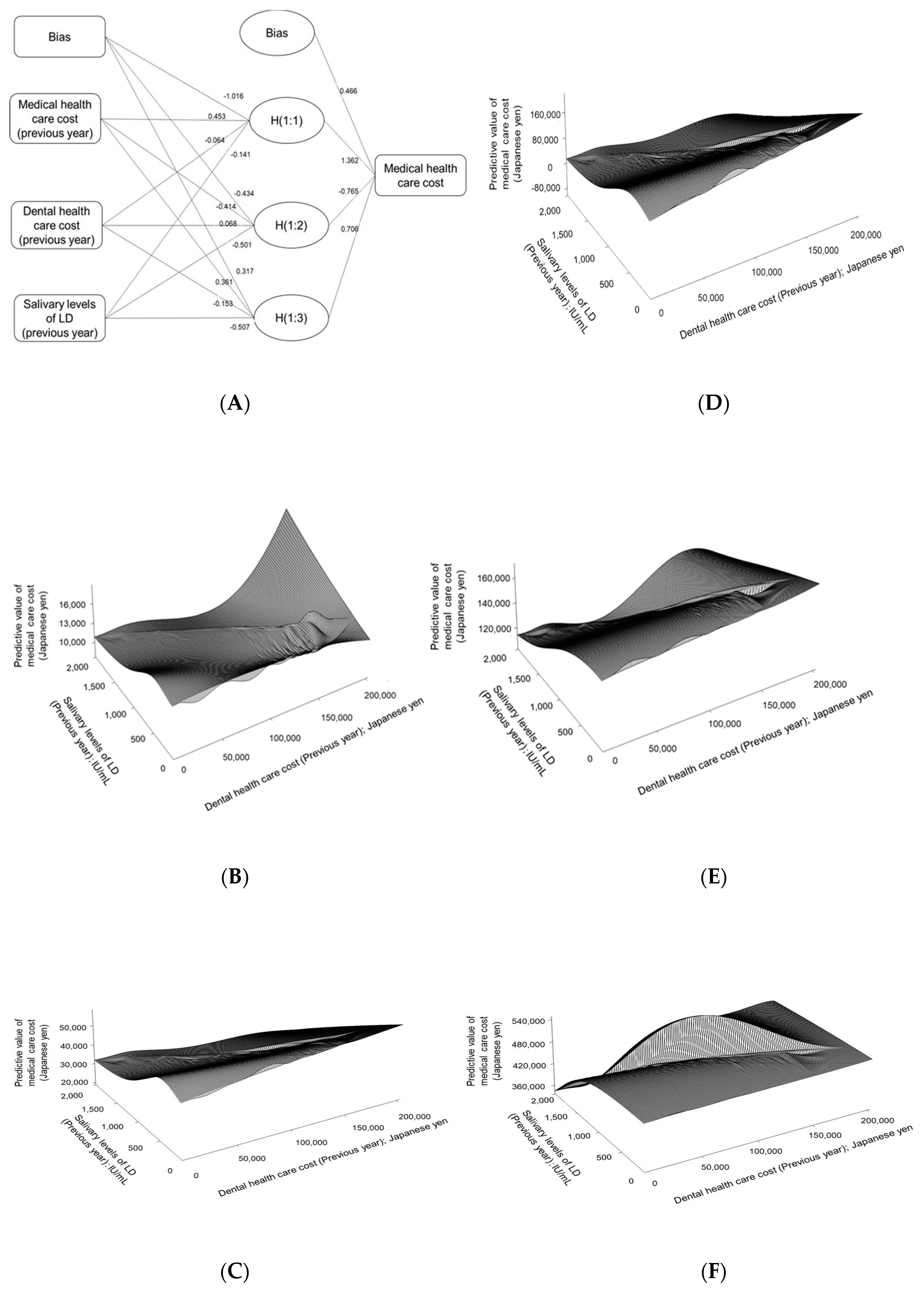
2.5. Statistical Method
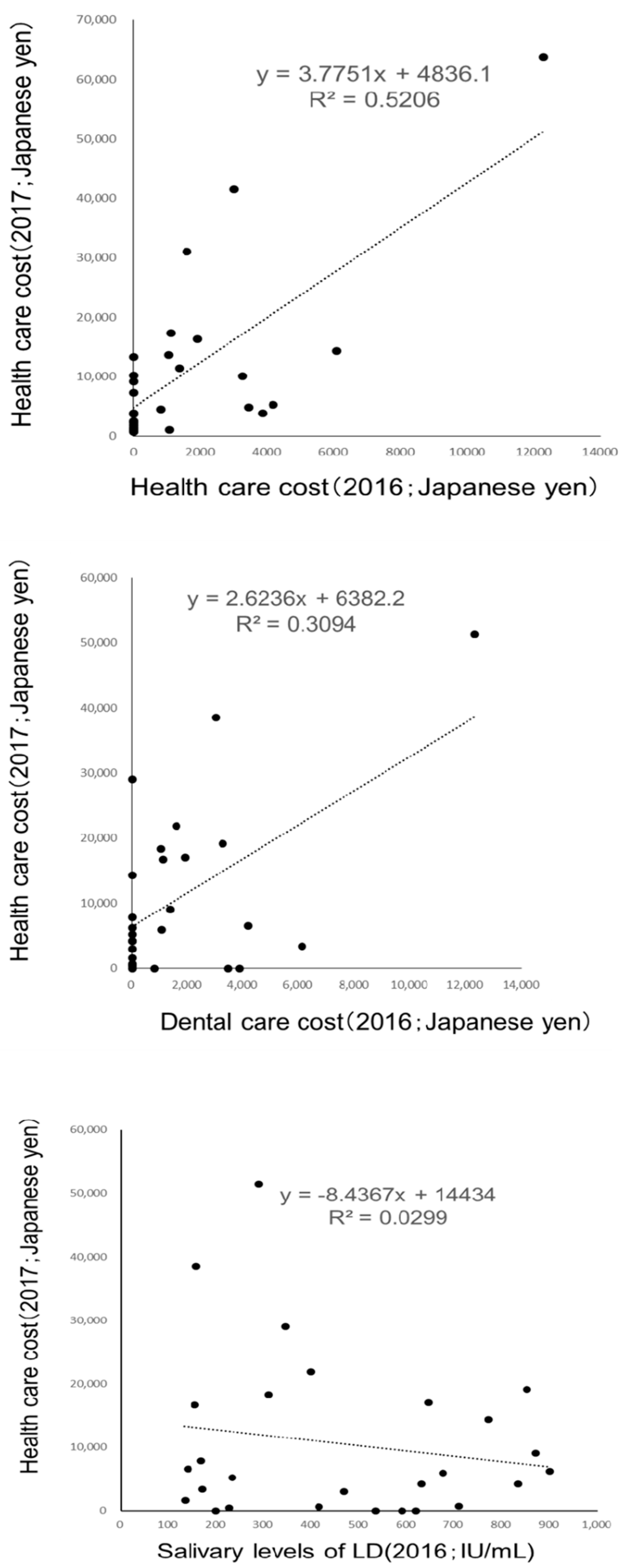
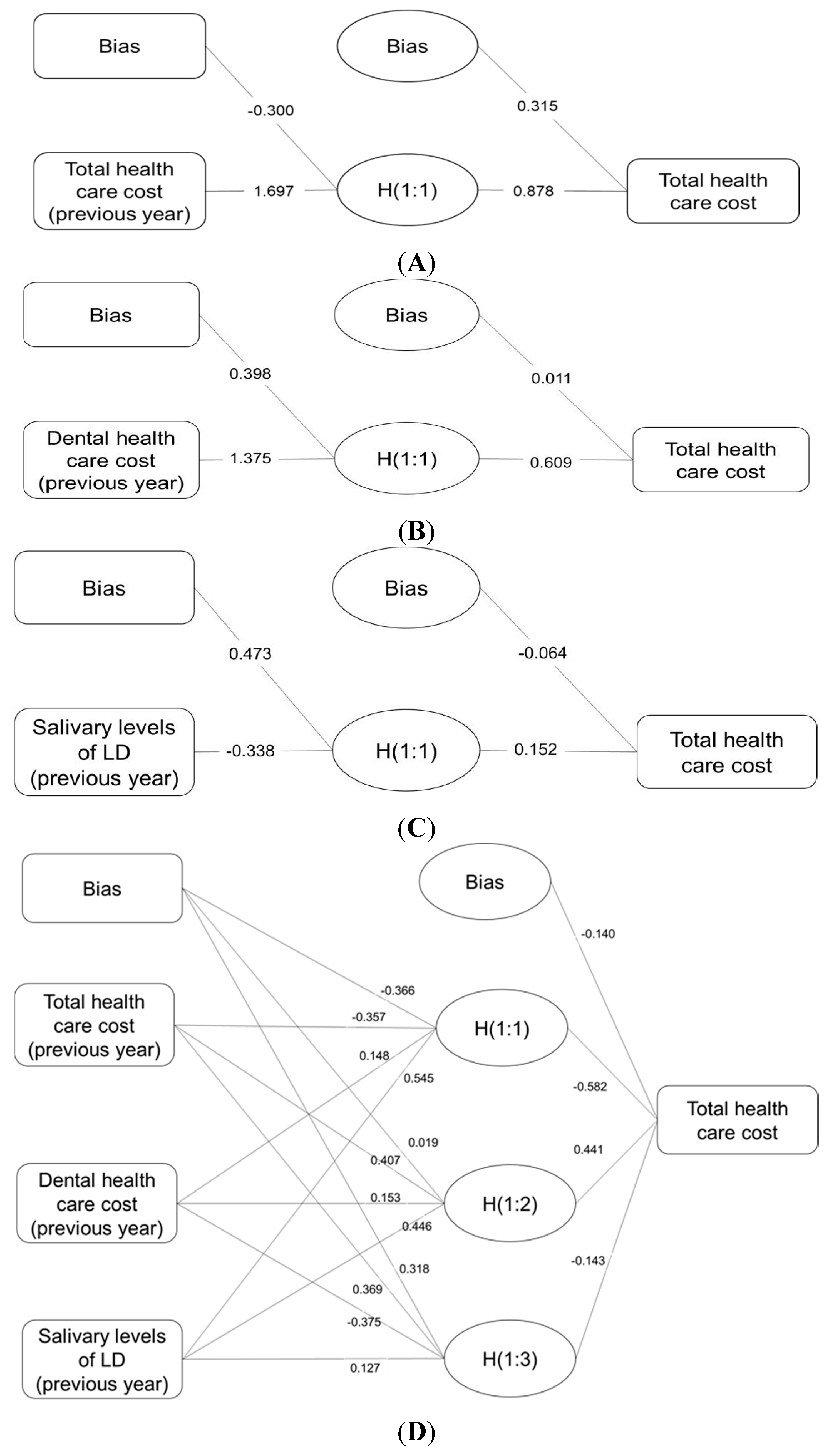
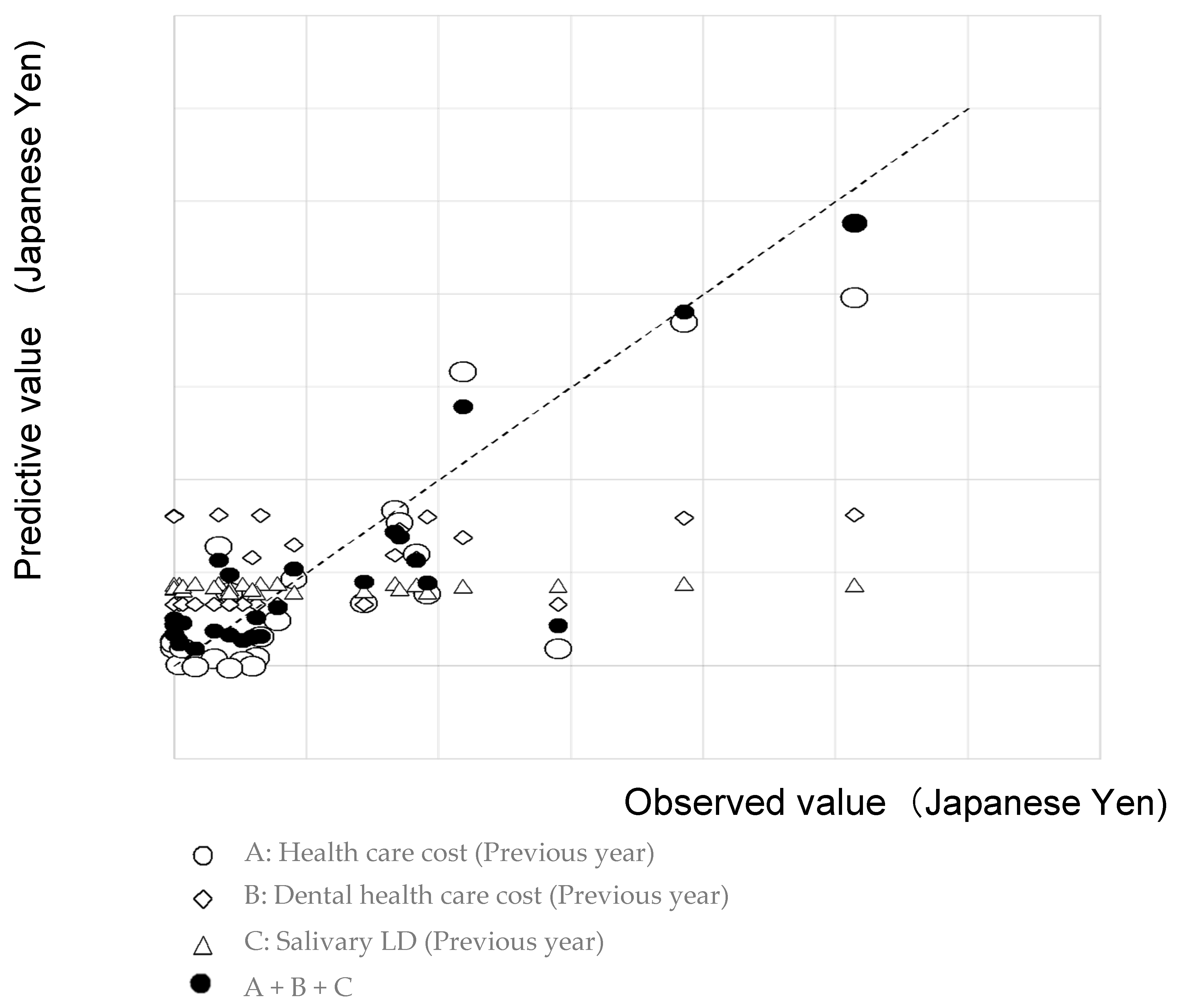
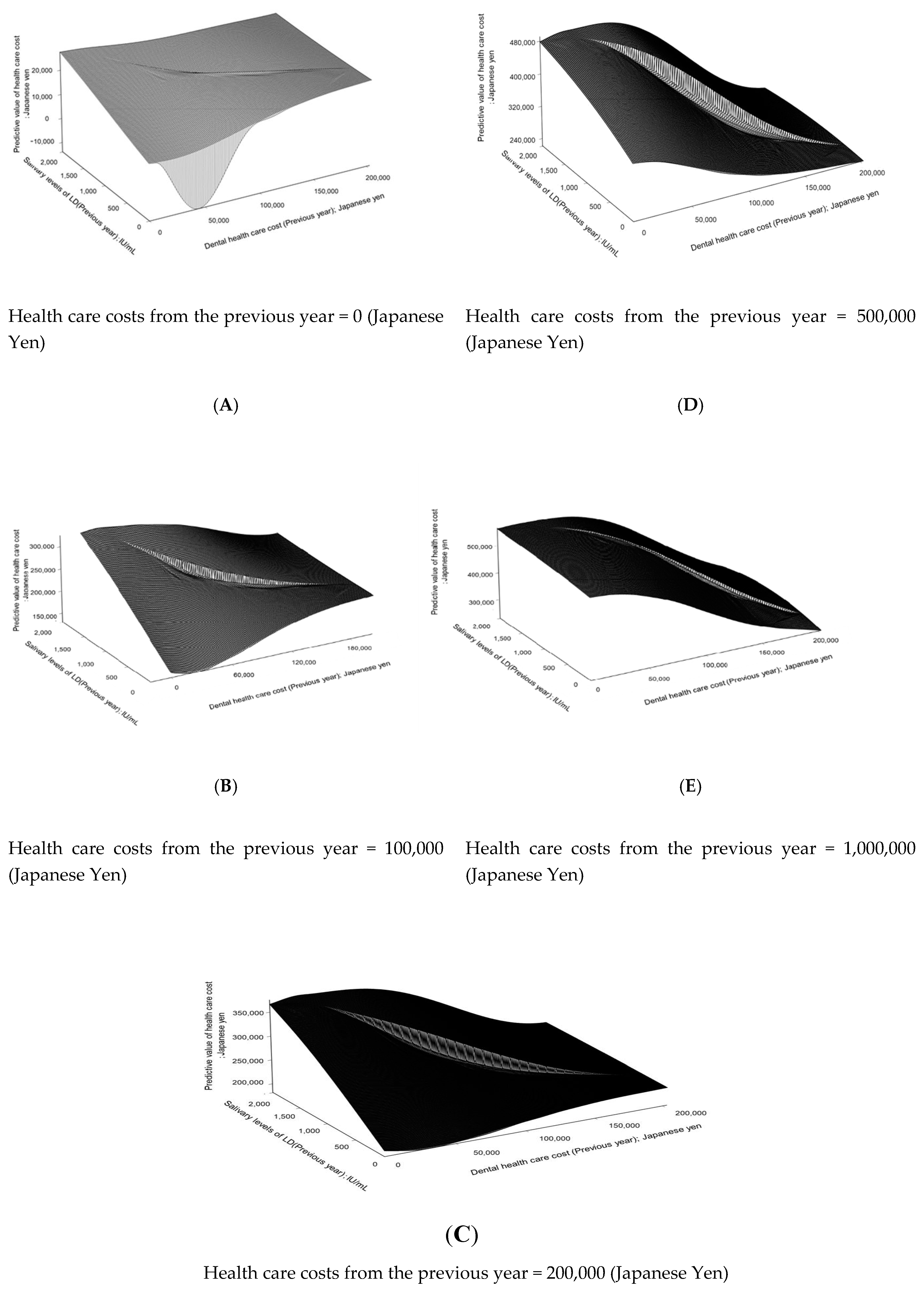
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethics Approval and Consent to Participate
Availability of Data and Material
Abbreviations
References
- Ministry of Health, Labour and Welfare Japan. Estimates of National Medical Care Expenditure 2017. Available online: https://www.mhlw.go.jp/toukei/list/37-21.html (accessed on 15 March 2019).
- Hirotomi, T.; Yoshihara, A.; Ogawa, H.; Miyazaki, H. Number of teeth and 5-year mortality in an elderly population. Community Dent Oral Epidemiol. 2015, 43, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, K.; Tomata, Y.; Aida, J.; Watanabe, T.; Kakizaki, M.; Tsuji, I. Tooth loss and mortality in elderly Japanese adults: Effect of oral care. J. Am. Geriatr. Soc. 2013, 61, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Holmlund, A.; Holm, G.; Lind, L. Number of teeth as a predictor of cardiovascular mortality in a cohort of 7674 subjects followed for 12 years. J. Periodontol. 2010, 81, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.J.; Lamster, I.B.; Greenspan, J.S.; Pitts, N.B.; Scully, C.; Warnakulasuriya, S. Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Herzberg, M.C.; Taubman, M.A.; Van Dyke, T.E. Recent advances in host defense mechanisms/therapies against oral infectious diseases and consequences for systemic disease. Adv. Dent. Res. 2014, 26, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Enwonwu, C.O.; Salako, N. The periodontal disease-systemic health-infectious disease axis in developing countries. Periodontol. 2000 2012, 60, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare Japan, A Basic Direction for Comprehensive Implementation of National Health Promotion. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000047330.pdf (accessed on 15 March 2018).
- Sullan, R.M.; Li, J.K.; Crowley, P.J.; Brady, L.J.; Dufrêne, Y.F. Binding forces of Streptococcus mutans P1 adhesin. ACS Nano 2015, 9, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.P.; Oliveira, F.A.; Silva, P.G.; Alves, A.P.; Mota, M.R.; Montenegro, R.C.; Burbano, R.M.R.; Seabra, A.D.; Lobo Filho, J.G.; Lima, D.L.F.; et al. Molecular analysis of oral bacteria in dental biofilm and atherosclerotic plaques of patients with vascular disease. Int. J. Cardiol. 2014, 174, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Kesavalu, L.; Lucas, A.R.; Verma, R.K.; Liu, L.; Dai, E.; Sampson, E.; Progulske-Fox, A. Increased atherogenesis during Streptococcus mutans infection in ApoE-null mice. J. Dent. Res. 2012, 91, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Mang-de la Rosa, M.R.; Castellanos-Cosano, L.; Romero-Perez, M.J.; Cutando, A. The bacteremia of dental origin and its implications in the appearance of bacterial endocarditis. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e67–e74. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Okada, A.; Kakuta, E.; Gunji, T.; Kajiura, S.; Hanada, N. A new screening method for periodontitis: An alternative to the community periodontal index. BMC Oral Health 2016, 1, 64. [Google Scholar] [CrossRef]
- Nomura, Y.; Shimada, Y.; Hanada, N.; Numabe, Y.; Kamoi, K.; Sato, T.; Gomi, K.; Arai, T.; Inagaki, K.; Fukuda, M.; et al. Salivary biomarkers for predicting the progression of chronic periodontitis. Arch. Oral Biol. 2012, 57, 413–420. [Google Scholar] [CrossRef]
- Nomura, Y.; Tamaki, Y.; Tanaka, T.; Arakawa, H.; Tsurumoto, A.; Kirimura, K.; Sato, T.; Hanada, N.; Kamoi, K. Screening of periodontitis with salivary enzyme tests. J. Oral Sci. 2006, 48, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Said, M.M.; Otomaru, T.; Sumita, Y.; Leung, K.C.M.; Khan, Z.; Taniguchi, H. Systematic review of literature: Functional outcomes of implant-prosthetic treatment in patients with surgical resection for oral cavity tumors. J. Investig. Clin. Dent. 2017, 8, e12207. [Google Scholar] [CrossRef]
- Kakuta, E.; Nomura, Y.; Naono, Y.; Koresawa, K.; Shimizu, K.; Hanada, N. Correlation between health-care costs and salivary tests. Int. Dent. J. 2013, 63, 249–253. [Google Scholar] [CrossRef]
- Karlsson, G.; Teiwik, A.; Lundström, A.; Ravald, N. Costs of periodontal and prosthodontic treatment and evaluation of oral health in patients after treatment of advanced periodontal disease. Community Dent. Oral Epidemiol. 1995, 23, 159–164. [Google Scholar] [CrossRef]
- Millar, W.J.; Locker, D. Dental insurance and use of dental services. Health Rep. 1999, 11, 55–67. [Google Scholar]
- Ramraj, C.; Quiñonez, C.R. Self-reported cost-prohibitive dental care needs among Canadians. Int. J. Dent. Hyg. 2013, 11, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.; Cooney, P.; Lawrence, H.; Ravaghi, V.; Quiñonez, C. The potential oral health impact of cost barriers to dental care: Findings from a Canadian population-based study. BMC Oral Health 2014, 14, 78. [Google Scholar] [CrossRef] [PubMed]
| Mean ± SD | Median (25th–75th Percentile) | ||
|---|---|---|---|
| 2016 | |||
| Health care costs (USD) | 1035.00 | ±1300.27 | 572.45 (220.36–1255.09) |
| Medical health care costs (USD) | 877.27 | ±248.55 | 482.36 (126.91–1161.73) |
| Dental health care costs (USD) | 157.73 | ±2.39 | 84.55 (0–279.55) |
| Salivary levels of LD (IU/mL) | 4.09 | ±2.39 | 3.70 (1.73–5.94) |
| 2017 | |||
| Health care costs (USD) | 994.00 | ±1172.27 | 550.45 (122–1577.27) |
| Medical health care costs (USD) | 775.55 | ±975.55 | 42.65 (105.45–1165.73) |
| Dental health care costs (USD) | 218.36 | ±402.64 | 0 (0–455.91) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, Y.; Sato, T.; Kamoshida, Y.; Suzuki, S.; Okada, A.; Otsuka, R.; Kakuta, E.; Hanada, N. Prediction of Health Care Costs by Dental Health Care Costs and Periodontal Status. Appl. Sci. 2020, 10, 3140. https://doi.org/10.3390/app10093140
Nomura Y, Sato T, Kamoshida Y, Suzuki S, Okada A, Otsuka R, Kakuta E, Hanada N. Prediction of Health Care Costs by Dental Health Care Costs and Periodontal Status. Applied Sciences. 2020; 10(9):3140. https://doi.org/10.3390/app10093140
Chicago/Turabian StyleNomura, Yoshiaki, Tetsuro Sato, Yoshinori Kamoshida, Syunsuke Suzuki, Ayako Okada, Ryoko Otsuka, Erika Kakuta, and Nobuhiro Hanada. 2020. "Prediction of Health Care Costs by Dental Health Care Costs and Periodontal Status" Applied Sciences 10, no. 9: 3140. https://doi.org/10.3390/app10093140
APA StyleNomura, Y., Sato, T., Kamoshida, Y., Suzuki, S., Okada, A., Otsuka, R., Kakuta, E., & Hanada, N. (2020). Prediction of Health Care Costs by Dental Health Care Costs and Periodontal Status. Applied Sciences, 10(9), 3140. https://doi.org/10.3390/app10093140






