Nickel-Based Structured Catalysts for Indirect Internal Reforming of Methane
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. XRD and Textural Characterization of Ni-SmDC Catalyst
3.2. Morphological Characterization of Uncoated Foam
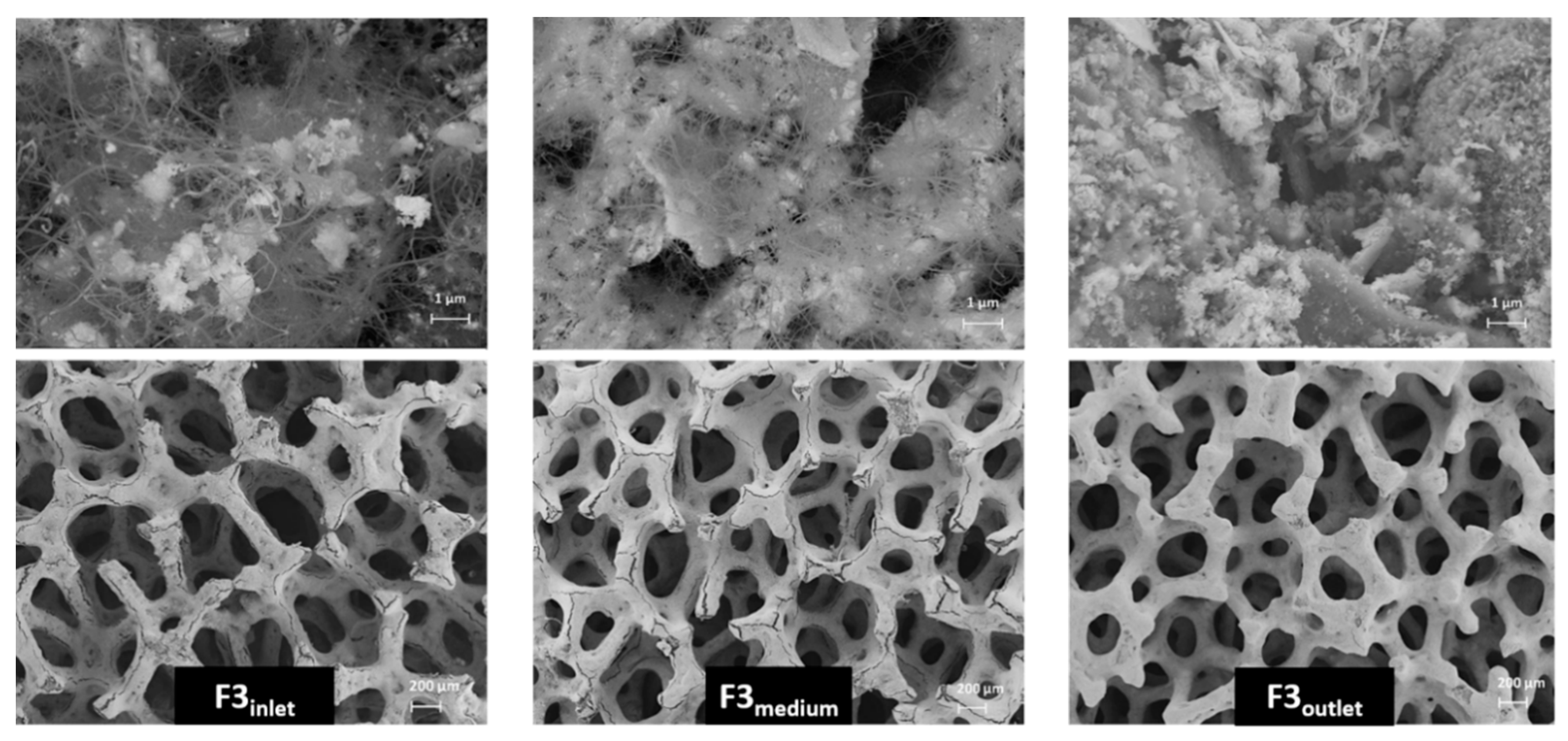
3.3. Morphological Characterization of Structured Catalyst
3.4. Raman Characterization
3.5. Temperature Programmed Reduction (H2-TPR)
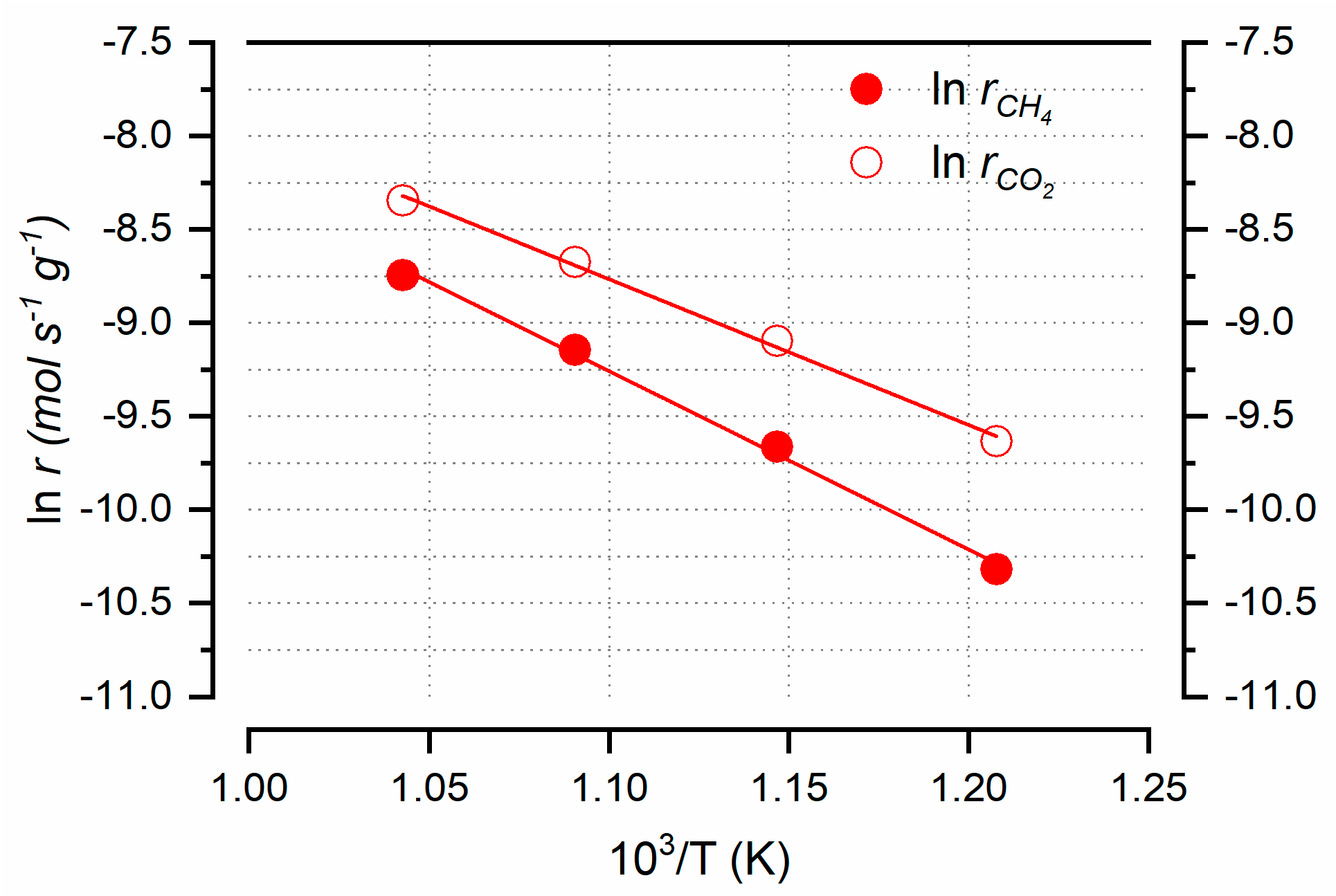
3.6. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A Review on Catalyst Development for Dry Reforming of Methane to Syngas: Recent Advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A Review on Dry Reforming of Methane in Aspect of Catalytic Properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A Review of CH4–CO2 Reforming to Synthesis Gas over Ni-Based Catalysts in Recent Years (2010–2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst Design for Dry Reforming of Methane: Analysis Review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Lanzini, A.; Leone, P.; Guerra, C.; Smeacetto, F.; Brandon, N.P.; Santarelli, M. Durability of Anode Supported Solid Oxides Fuel Cells (SOFC) under Direct Dry-Reforming of Methane. Chem. Eng. J. 2013, 220, 254–263. [Google Scholar] [CrossRef]
- Shiratori, Y.; Ijichi, T.; Oshima, T.; Sasaki, K. Internal Reforming SOFC Running on Biogas. Int. J. Hydrogen Energy 2010, 35, 7905–7912. [Google Scholar] [CrossRef]
- Lo Faro, M.; Antonucci, V.; Antonucci, P.L.; Aricó, A.S. Fuel Flexibility: A Key Challenge for SOFC Technology. Fuel 2012, 102, 554–559. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A. Ni Supported on γ-Al2O3 Promoted by Ru for the Dry Reforming of Methane in Packed and Monolithic Reactors. Fuel Process. Technol. 2017, 158, 130–140. [Google Scholar] [CrossRef]
- Sarno, C.; Luisetto, I.; Zurlo, F.; Licoccia, S.; Di Bartolomeo, E. Lanthanum Chromite Based Composite Anodes for Dry Reforming of Methane. Int. J. Hydrogen Energy 2018, 43, 14742–14750. [Google Scholar] [CrossRef]
- Klein, J.M.; Bultel, Y.; Georges, S.; Pons, M. Modeling of a SOFC Fuelled by Methane: From Direct Internal Reforming to Gradual Internal Reforming. Chem. Eng. Sci. 2007, 62, 1636–1649. [Google Scholar] [CrossRef]
- Balzarotti, R.; Italiano, C.; Pino, L.; Cristiani, C.; Vita, A. Ni/CeO2-Thin Ceramic Layer Depositions on Ceramic Monoliths for Syngas Production by Oxy Steam Reforming of Biogas. Fuel Process. Technol. 2016, 149, 40–48. [Google Scholar] [CrossRef]
- Chai, R.; Fan, S.; Zhang, Z.; Chen, P.; Zhao, G.; Liu, Y.; Lu, Y. Free-Standing NiO-MgO-Al2O3 Nanosheets Derived from Layered Double Hydroxides Grown onto FeCrAl-Fiber as Structured Catalysts for Dry Reforming of Methane. ACS Sustain. Chem. Eng. 2017, 5, 4517–4522. [Google Scholar] [CrossRef]
- Fukuhara, C.; Hyodo, R.; Yamamoto, K.; Masuda, K.; Watanabe, R. A Novel Nickel-Based Catalyst for Methane Dry Reforming: A Metal Honeycomb-Type Catalyst Prepared by Sol-Gel Method and Electroless Plating. Appl. Catal. A Gen. 2013, 468, 18–25. [Google Scholar] [CrossRef]
- Pegios, N.; Schroer, G.; Rahimi, K.; Palkovits, R.; Simeonov, K. Design of Modular Ni-Foam Based Catalysts for Dry Reforming of Methane. Catal. Sci. Technol. 2016, 6, 6372–6380. [Google Scholar] [CrossRef]
- Sadykov, V.; Mezentseva, N.; Fedorova, Y.; Lukashevich, A.; Pelipenko, V.; Kuzmin, V.; Simonov, M.; Ishchenko, A.; Vostrikov, Z.; Bobrova, L.; et al. Structured Catalysts for Steam/Autothermal Reforming of Biofuels on Heat-Conducting Substrates: Design and Performance. Catal. Today 2015, 251, 19–27. [Google Scholar] [CrossRef]
- Benito, P.; Monti, M.; Bersani, I.; Basile, F.; Fornasari, G.; Scavetta, E.; Tonelli, D.; Vaccari, A. Coating of FeCrAlloy Foam with Rh Catalysts: Optimization of Electrosynthesis Parameters and Catalyst Composition. Catal. Today 2012, 197, 162–169. [Google Scholar] [CrossRef]
- Yanxia, L.; Chaoming, L.; Zhongliang, L.; Lixia, S. Catalytic Combustion of CH4/Air Mixtures over Metal Foam Monoliths. Phys. Procedia 2015, 66, 249–252. [Google Scholar] [CrossRef][Green Version]
- Sinn, C.; Pesch, G.R.; Thöming, J.; Kiewidt, L. Coupled Conjugate Heat Transfer and Heat Production in Open-Cell Ceramic Foams Investigated Using CFD. Int. J. Heat Mass Transf. 2019, 139, 600–612. [Google Scholar] [CrossRef]
- Kim, S.; Lee, C.-W. A Review on Manufacturing and Application of Open-Cell Metal Foam. Procedia Mater. Sci. 2014, 4, 305–309. [Google Scholar] [CrossRef]
- Walther, G.; Klöden, B.; Büttner, T.; Weissgärber, T.; Kieback, B.; Böhm, A.; Naumann, D.; Soberi, S.; Timberg, L. A New Class of High Temperature and Corrosion Resistant Nickel-Based Open-Cell Foams. Adv. Eng. Mater. 2008, 10, 803–811. [Google Scholar] [CrossRef]
- Ho, P.H.; De Nolf, W.; Ospitali, F.; Beton, D.; Torkuhl, L.; Fornasari, G.; Vaccari, A.; Benito, P. Insights into Coated NiCrAl Open-Cell Foams for the Catalytic Partial Oxidation of CH4. React. Chem. Eng. 2019, 4, 1768–1778. [Google Scholar] [CrossRef]
- Meille, V. Review on Methods to Deposit Catalysts on Structured Surfaces. Appl. Catal. A Gen. 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Cristiani, C.; Ferreira, C.; Kohler, S. Methods for the Catalytic Activation of Metallic Structured Substrates. Catal. Sci. Technol. 2014, 4, 2846–2870. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 Catalysts for the Dry Reforming of Methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Di Bartolomeo, E. Co and Ni Supported on CeO2 as Selective Bimetallic Catalyst for Dry Reforming of Methane. Int. J. Hydrogen Energy 2012, 37, 15992–15999. [Google Scholar] [CrossRef]
- Düdder, H.; Kähler, K.; Krause, B.; Mette, K.; Kühl, S.; Behrens, M.; Scherer, V.; Muhler, M. The Role of Carbonaceous Deposits in the Activity and Stability of Ni-Based Catalysts Applied in the Dry Reforming of Methane. Catal. Sci. Technol. 2014, 4, 3317–3328. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G. Ni-Based Catalysts for Reforming of Methane with CO2. Int. J. Hydrogen Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Jiang, S.P.; Ye, Y.; He, T.; Ho, S.B. Nanostructured Palladium-La0.75Sr0.25Cr0.5Mn0.5O3/Y2O3-ZrO2 Composite Anodes for Direct Methane and Ethanol Solid Oxide Fuel Cells. J. Power Sources 2008, 185, 179–182. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E. Dry Reforming of Methane over Ni Supported on Doped CeO2: New Insight on the Role of Dopants for CO2 Activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Akri, M.; Pronier, S.; Chafik, T.; Achak, O.; Granger, P.; Simon, P.; Trentesaux, M.; Batiot-Dupeyrat, C. Development of Nickel Supported La and Ce-Natural Illite Clay for Autothermal Dry Reforming of Methane: Toward a Better Resistance to Deactivation. Appl. Catal. B Environ. 2017, 205, 519–531. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Makri, M.M.; Djinović, P.; Erjavec, B.; Pintar, A.; Efstathiou, A.M. Dry Reforming of Methane over 5 Wt% Ni/Ce1-XPrxO2-δ Catalysts: Performance and Characterisation of Active and Inactive Carbon by Transient Isotopic Techniques. Appl. Catal. B Environ. 2016, 197, 168–183. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Djinović, P.; Davlyatova, L.F.; Pintar, A.; Efstathiou, A.M. Origin and Reactivity of Active and Inactive Carbon Formed during DRM over Ni/Ce0.38Zr0.62O2-δ Studied by Transient Isotopic Techniques. Catal. Today 2018, 299, 201–211. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Damaskinos, C.M.; Kyprianou, K.K.; Kollia, M.; Efstathiou, A.M. The Effect of Pt on the Carbon Pathways in the Dry Reforming of Methane over Ni-Pt/Ce0.8Pr0.2O2-δ Catalyst. Catal. Today 2019. [Google Scholar] [CrossRef]
- Liu, B.; Liu, R.; Li, Q.J.; Yao, M.G.; Zou, B.; Cui, T.; Liu, B.B.; Liu, J. Study of High Pressure Structural Stability of CeO2 Nanoparticles. Chin. Phys. C 2013, 37. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W. Provisional international union of pure and applied chemistry commission on colloid and surface chemistry subcommittee on reporting gas adsorption data * Reporting physisorption data for gas/solid systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar]
- Kim, D.H.; Yu, B.Y.; Cha, P.R.; Yoon, W.Y.; Byun, J.Y.; Kim, S.H. A Study on FeCrAl Foam as Effective Catalyst Support under Thermal and Mechanical Stresses. Surf. Coat. Technol. 2012, 209, 169–176. [Google Scholar] [CrossRef]
- Kvernes, I.A.; Kofstad, P. The Oxidation Behavior of Some Ni-Cr-Al Alloys at High Temperatures. Metall. Trans. 1972, 3, 1511–1519. [Google Scholar] [CrossRef]
- Seraffon, M.; Simms, N.J.; Sumner, J.; Nicholls, J.R. Oxidation Behaviour of NiCrAl and NiCoCrAl Bond Coatings under Industrial Gas Turbine Conditions. Oxid. Met. 2014, 81, 203–215. [Google Scholar] [CrossRef]
- Kim, H.G.; Lim, S.H. Microstructural Characterization of Oxide Layers Formed on Ni–20Cr–8Al Alloy Foam Using Transmission Electron Microscopy. Surf. Interface Anal. 2017, 49, 880–884. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Jeurgens, L.P.H.; Sloof, W.G. Promoting Exclusive α-Al2O3 Growth upon High-Temperature Oxidation of NiCrAl Alloys: Experiment versus Model Predictions. Acta Mater. 2005, 53, 1643–1653. [Google Scholar] [CrossRef]
- Ptak, M.; Maczka, M.; G, A.; Pikul, A.; Macalik, L.; Hanuza, J. Temperature-Dependent XRD, IR, Magnetic, SEM and TEM Studies of Jahn–Teller Distorted NiCr2O4 Powders. J. Solid State Chem. 2013, 201, 270–279. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Van Der Pers, N.M.; Sloof, W.G. Oxide Phase Development upon High Temperature Oxidation of γ-NiCrAl Alloys. Mater. Corros. 2006, 57, 269–275. [Google Scholar] [CrossRef]
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al Alloys Between 1000 ° and 1200 °C. J. Electrochem. Soc. 1971, 118, 1782. [Google Scholar] [CrossRef]
- Guan, S.W.; Smeltzer, W.W. Oxygen Solubility and a Criterion for the Transition from Internal to External Oxidation of Ternary Alloys. Oxid. Met. 1994, 42, 375–391. [Google Scholar] [CrossRef]
- Shim, S.-H.; Duffy, T.S.; Jeanloz, R.; Yoo, C.-S.; Iota, V. Raman Spectroscopy and X-Ray Diffraction of Phase Transitions in Cr2O3 to 61 GPa. Phys. Rev. B Condens. Matter Mater. Phys. 2004, 69, 1–12. [Google Scholar] [CrossRef]
- Mohammadtaheri, M.; Yang, Q.; Li, Y.; Corona-Gomez, J. The Effect of Deposition Parameters on the Structure and Mechanical Properties of Chromium Oxide Coatings Deposited by Reactive Magnetron Sputtering. Coatings 2018, 8, 111. [Google Scholar] [CrossRef]
- Wang, Z.; Saxena, S.K.; Lazor, P.; O’Neill, H.S.C. An in Situ Raman Spectroscopic Study of Pressure Induced Dissociation of Spinel NiCr2O4. J. Phys. Chem. Solids 2003, 64, 425–431. [Google Scholar] [CrossRef]
- Li, L.; Chen, F.; Lu, J.Q.; Luo, M.F. Study of Defect Sites in Ce1-Xmxo2-δ (x = 0.2) Solid Solutions Using Raman Spectroscopy. J. Phys. Chem. A 2011, 115, 7972–7977. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Zedan, A.F.; AlKetbi, M.; Stephen, S.; Ather, M.; Katsiotis, M.S.; Arvanitidis, J.; Christofilos, D.; Isakovic, A.F.; AlHassan, S. Tailoring the Efficiency of an Active Catalyst for CO Abatement through Oxidation Reaction: The Case Study of Samarium-Doped Ceria. J. Environ. Chem. Eng. 2018, 6, 266–280. [Google Scholar] [CrossRef]
- Storaro, L.; Ganzerla, R.; Lenarda, M.; Zanoni, R.; Jiménez López, A.; Olivera-Pastor, P.; Rodríguez Castellón, E. Catalytic Behavior of Chromia and Chromium-Doped Alumina Pillared Clay Materials for the Vapor Phase Deep Oxidation of Chlorinated Hydrocarbons. J. Mol. Catal. A Chem. 1997, 115, 329–338. [Google Scholar] [CrossRef]
- Hari Krishna Charan, P.; Ranga Rao, G. Investigation of Chromium Oxide Clusters Grafted on SBA-15 Using Cr-Polycation Sol. J. Porous Mater. 2013, 20, 81–94. [Google Scholar] [CrossRef]
- Ilieva, L.I.; Andreeva, D.H. Investigation of the Chromium Oxide System by Means of Temperature-Programmed Reduction. Thermochim. Acta 1995, 265, 223–231. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Cheng, L.; Shin, E.W.; Men, Y. Three-Dimensionally Ordered Macroporous Spinel-Type MCr2O4 (M = Co, Ni, Zn, Mn) Catalysts with Highly Enhanced Catalytic Performance for Soot Combustion. Catal. Sci. Technol. 2015, 5, 4594–4601. [Google Scholar] [CrossRef]
- Luo, J.W.; Song, J.D.; Jia, W.Z.; Pu, Z.Y.; Lu, J.Q.; Luo, M.F. Catalytic Dehydrofluorination of 1,1,1,3,3-Pentafluoropropane to 1,3,3,3-Tetrafluoropropene over Fluorinated NiO/Cr2O3 Catalysts. Appl. Surf. Sci. 2018, 433, 904–913. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, J.; Xie, S.; Yang, H.; Zhao, X.; Zhang, K.; Lin, H.; Dai, H.; Guo, G. Mesoporous Cr2O3-Supported Au-Pd Nanoparticles: High-Performance Catalysts for the Oxidation of Toluene. Microporous Mesoporous Mater. 2016, 224, 311–322. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Fabiano, C.; Laganà, M.; Pino, L. Influence of Ce-Precursor and Fuel on Structure and Catalytic Activity of Combustion Synthesized Ni/CeO2 Catalysts for Biogas Oxidative Steam Reforming. Mater. Chem. Phys. 2015, 163, 337–347. [Google Scholar] [CrossRef]
- Tang, C.; Li, J.; Yao, X.; Sun, J.; Cao, Y.; Zhang, L.; Gao, F.; Deng, Y.; Dong, L. Mesoporous NiO-CeO2 Catalysts for CO Oxidation: Nickel Content Effect and Mechanism Aspect. Appl. Catal. A Gen. 2015, 494, 77–86. [Google Scholar] [CrossRef]
- Giordano, F.; Trovarelli, A.; De Leitenburg, C.; Giona, M. A Model for the Temperature-Programmed Reduction of Low and High Surface Area Ceria. J. Catal. 2000, 193, 273–282. [Google Scholar] [CrossRef]
- Yang, P.; Meng, Z.; Yang, S.; Shi, Z.; Zhou, R. Highly Active Behaviors of CeO2-CrOx Mixed Oxide Catalysts in Deep Oxidation of 1,2-Dichloroethane. J. Mol. Catal. A Chem. 2014, 393, 75–83. [Google Scholar] [CrossRef]
- Seo, M.; Kim, S.Y.; Kim, Y.D.; Park, E.D.; Uhm, S. Highly Stable Barium Zirconate Supported Nickel Oxide Catalyst for Dry Reforming of Methane: From Powders toward Shaped Catalysts. Int. J. Hydrogen Energy 2018, 43, 11355–11362. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Isotopic and Kinetic Assessment of the Mechanism of Reactions of CH4 with CO2 or H2O to Form Synthesis Gas and Carbon on Nickel Catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, Y.; Sakurai, M.; Kameyama, H. Study of Porous Anodic Alumina Supported Plate-Type Catalysts during Daily Start-up and Shut-down Operation of Methane Steam Reforming. Appl. Catal. A Gen. 2009, 364, 101–107. [Google Scholar] [CrossRef]
- Li, D.; Atake, I.; Shishido, T.; Oumi, Y.; Sano, T.; Takehira, K. Self-Regenerative Activity of Ni/Mg(Al)O Catalysts with Trace Ru during Daily Start-up and Shut-down Operation of CH4 Steam Reforming. J. Catal. 2007, 250, 299–312. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An Overview on Dry Reforming of Methane: Strategies to Reduce Carbonaceous Deactivation of Catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Chai, Y.; Fu, Y.; Feng, H.; Kong, W.; Yuan, C.; Pan, B.; Zhang, J.; Sun, Y. A Nickel-Based Perovskite Catalyst with a Bimodal Size Distribution of Nickel Particles for Dry Reforming of Methane. ChemCatChem 2018, 10, 2078–2086. [Google Scholar] [CrossRef]
- Sokolov, S.; Kondratenko, E.V.; Pohl, M.M.; Barkschat, A.; Rodemerck, U. Stable Low-Temperature Dry Reforming of Methane over Mesoporous La 2O3-ZrO2 Supported Ni Catalyst. Appl. Catal. B Environ. 2012, 113–114, 19–30. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A. Ni/CeO2-Al2O3 Catalysts for the Dry Reforming of Methane: The Effect of CeAlO3 Content and Nickel Crystallite Size on Catalytic Activity and Coke Resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Leimert, J.; Karl, J.; Dillig, M. Dry Reforming of Methane Using a Nickel Membrane Reactor. Processes 2017, 5, 82. [Google Scholar] [CrossRef]


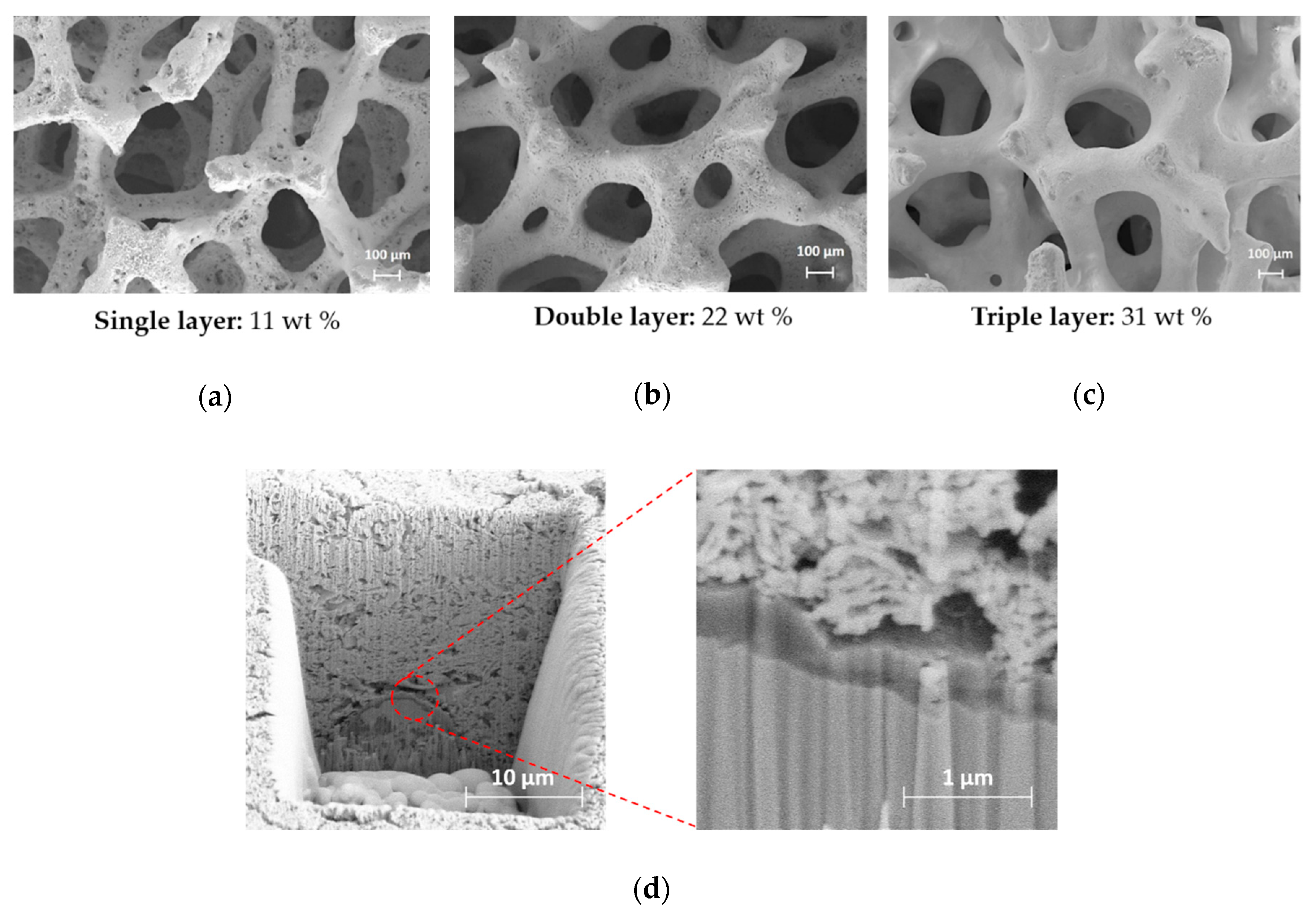
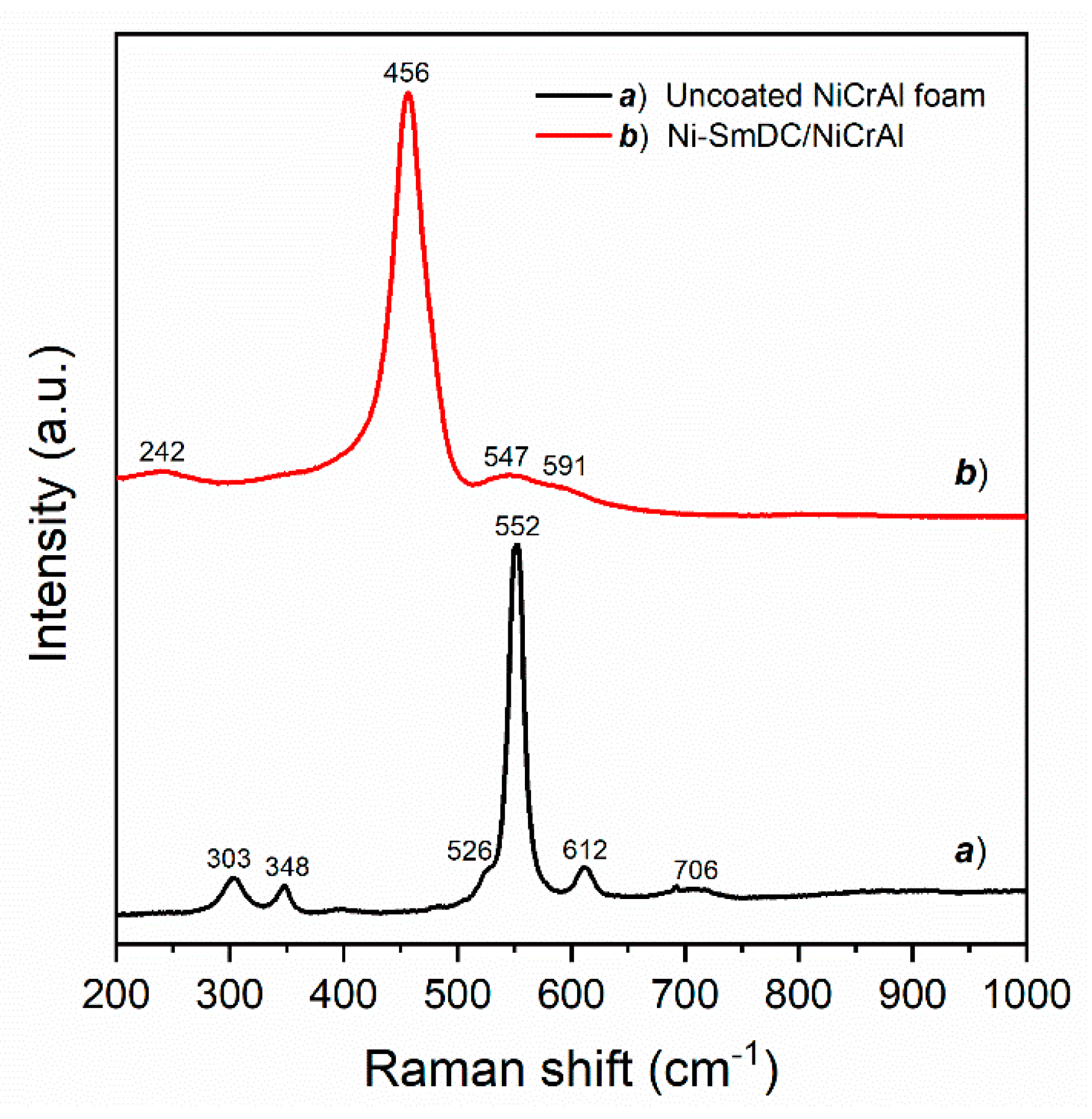


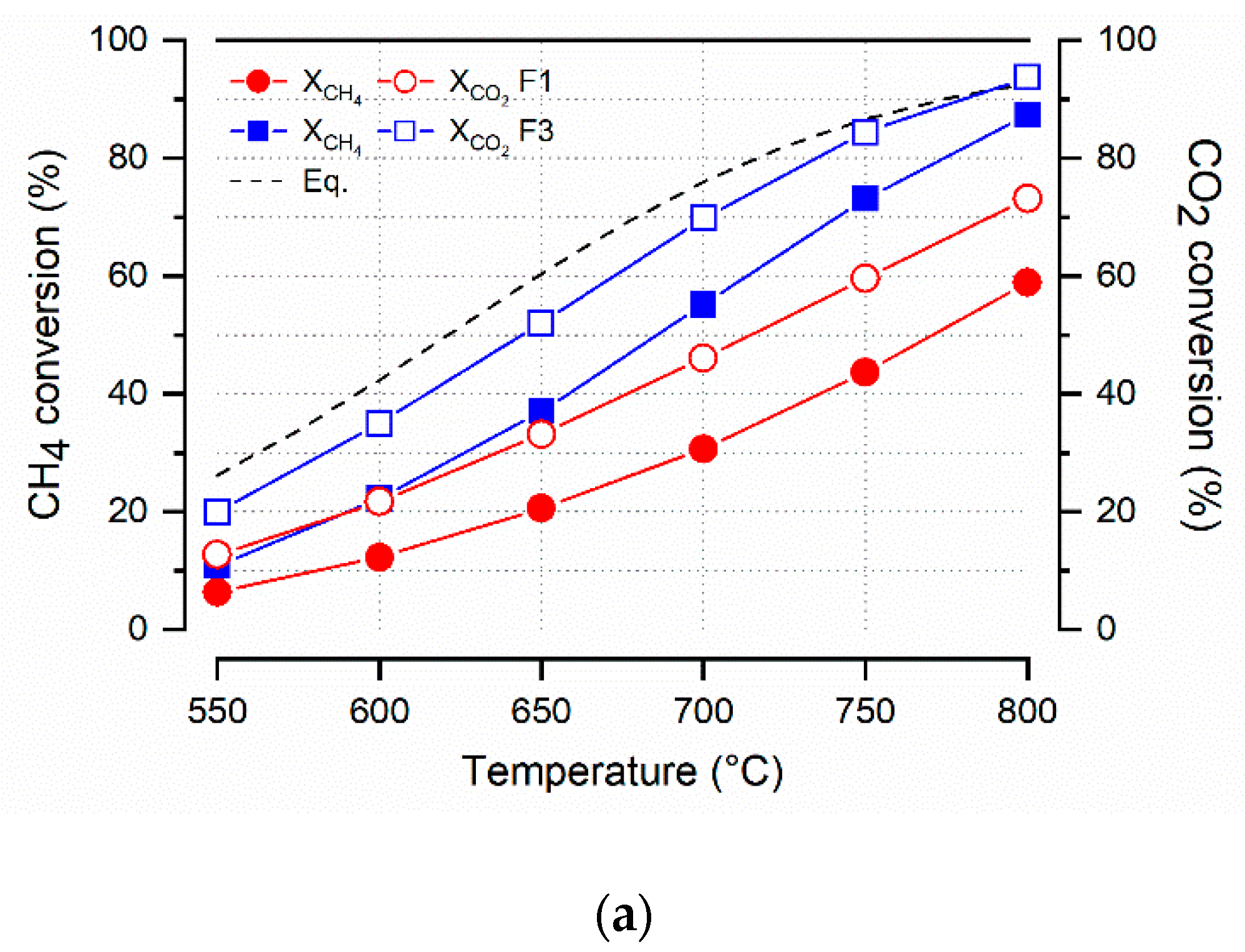
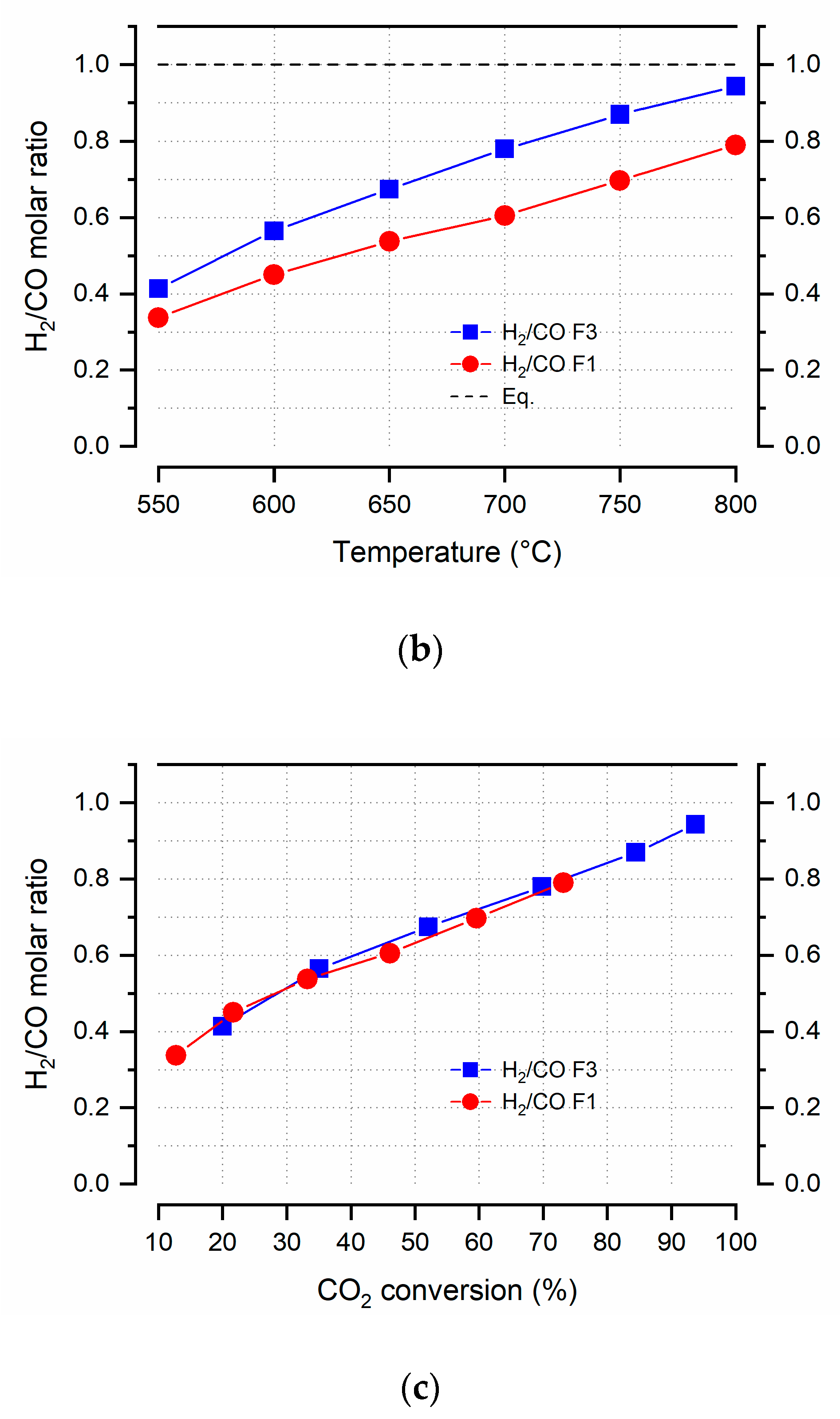

| Sample | Crystallites Size (nm) | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | ||
|---|---|---|---|---|---|---|
| SmDC | NiO | Ni0 | ||||
| Ni-SmDC | 18 | 14 | - | 31 | 0.15 | 18 |
| Ni-SmDC_r (*) | 20 | - | 20 | 15 | 0.07 | 23 |
| Element | Weight (%) | |||
|---|---|---|---|---|
| Spectrum 1 | Spectrum 2 | Spectrum 3 | Spectrum 4 | |
| O | 2.8 | 3.6 | - | 10.6 |
| Al | 3.3 | 2.2 | 3.8 | 18.4 |
| Cr | 26.9 | 52.3 | 30.0 | 60.4 |
| Ni | 67.0 | 41.9 | 66.2 | 10.6 |
| Sample | H2-Consumption (mmol g−1) |
|---|---|
| NiCrAl | 0.11 |
| Ni-SmDC | 1.33 |
| Ni-SmDC/NiCrAl | 0.36 |
| 1.20 (*) |
| XCH4 (%) | XCO2 (%) | H2/CO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 700 °C | 750 °C | 800 °C | 700 °C | 750 °C | 800 °C | 700 °C | 750 °C | 800 °C | |
| Run 1 | 55.2 | 73.1 | 87.4 | 70.0 | 84.4 | 93.7 | 0.78 | 0.87 | 0.94 |
| Run 2 | 52.0 | 68.9 | 82.1 | 67.2 | 81.6 | 90.7 | 0.76 | 0.84 | 0.91 |
| Run 3 | 59.1 | 76.7 | 88.7 | 72.7 | 86.7 | 94.3 | 0.81 | 0.89 | 0.95 |
| Run 4 | 63.4 | 79.4 | 90.2 | 75.7 | 88.1 | 94.5 | 0.84 | 0.91 | 0.97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, M.; Luisetto, I.; Tuti, S.; Licoccia, S.; Romano, C.; Notargiacomo, A.; Di Bartolomeo, E. Nickel-Based Structured Catalysts for Indirect Internal Reforming of Methane. Appl. Sci. 2020, 10, 3083. https://doi.org/10.3390/app10093083
Santoro M, Luisetto I, Tuti S, Licoccia S, Romano C, Notargiacomo A, Di Bartolomeo E. Nickel-Based Structured Catalysts for Indirect Internal Reforming of Methane. Applied Sciences. 2020; 10(9):3083. https://doi.org/10.3390/app10093083
Chicago/Turabian StyleSantoro, Mariarita, Igor Luisetto, Simonetta Tuti, Silvia Licoccia, Claudia Romano, Andrea Notargiacomo, and Elisabetta Di Bartolomeo. 2020. "Nickel-Based Structured Catalysts for Indirect Internal Reforming of Methane" Applied Sciences 10, no. 9: 3083. https://doi.org/10.3390/app10093083
APA StyleSantoro, M., Luisetto, I., Tuti, S., Licoccia, S., Romano, C., Notargiacomo, A., & Di Bartolomeo, E. (2020). Nickel-Based Structured Catalysts for Indirect Internal Reforming of Methane. Applied Sciences, 10(9), 3083. https://doi.org/10.3390/app10093083









