Abstract
Fucoidans constitute a family of fucose-rich sulfated polysaccharides, which possess multiple characteristics, including antioxidant, antitumor, antivirus, anticoagulant, and anti-inflammatory properties. In addition, the incidence of colon cancer has risen rapidly worldwide. In the present study, fucoidan extracts were extracted from the Sargassum glaucescens (SG) pretreated by compressional-puffing, and four fucoidans (SG1-SG4) were obtained with different puffing conditions. It was found that SG4 possessed the highest extraction yield, relatively high cytotoxicity against human colon carcinoma HT-29 cells, and relatively low cytotoxicity to normal cells, as compared to the other extracted fucoidans. Moreover, SG4 caused cell cycle arrest of HT-29 cells at sub-G1, S, and G2/M phases. SG4 also induced HT-29 cellular apoptosis, as evidenced by the loss of mitochondrial membrane potential (MMP), increased cytochrome c release, activation of caspase-9 and -3, increased DNA fragmentation, and increased early and late apoptotic cells visualized by annexin V/propidium iodide (PI) assay. Additional biological experiments revealed that the Akt/mammalian target of rapamycin (mTOR)/S6 pathway is involved in SG4-induced apoptosis of HT-29 cells. These results clearly indicate that SG4 showed anti-colon cancer potential via the induction of cell cycle arrest and apoptosis, and thus may have a possible application as an adjuvant therapeutic agent in colon cancer treatment.
1. Introduction
Fucoidan, mostly extracted from brown algae, is a family of fucose-rich sulfated polysaccharides [1,2]. Fucoidan has been reported to possess a variety of important pharmacological activities, including anticancer [3], immunomodulatory [4], antiviral [5], antithrombotic and anticoagulant [6], antiadhesive [7], neuroprotective [8,9], and antioxidant effects [10]. Due to the diversity of brown algae species, growing conditions of seaweeds, extraction methods of fucoidan, compositional and structural differences of fucoidan, and varying molecular weights of fucoidan, there is still research interest associated with isolation of fucoidan, identification of potential bioactive components in fucoidan, and explication of the biological mechanisms involved at the molecular level.
The occurrence of human cancer cells can be triggered by free radicals [11]. Thus, the application of natural antioxidants as chemopreventive agents for the treatment of cancer has gained in popularity. Previous investigations have demonstrated that fucoidan acts as a potential ROS scavenger [3] and is capable of preventing oxidative damage [12]. In addition, there is growing evidence showing that fucoidan exhibits anticancer activity in cell models [2,13] and can induce inhibition of tumor growth in mice [14]. Thus, fucoidan is considered to have potential as a novel and natural agent for auxiliary therapy in the treatment of cancer due to its antioxidant and anticancer properties.
The global incidence rates of colorectal cancer have risen steadily in recent years. In Taiwan, dietary habits have gradually become more westernized over the past three decades. The incidence of colon cancer has risen sharply and is currently the third most frequent cause of cancer-related mortality [15,16]. Previous investigations suggested there are drawbacks such as side effects and toxicity in regularly used anti-colorectal cancer drugs such as cisplatin [17]. Thus, there is a requirement for agents derived from natural sources with no or minimal side effects and no toxicities. Recently, natural bioactive compounds have drawn the attention of researchers due to their possible therapeutic and cancer-preventive activities at non-toxic levels and recent efforts have sought to gain a better understanding of their mechanisms of action, particularly with regard to their chemopreventive qualities [18]. In addition, both HT-29 cells and Caco-2 cells are considered useful in in vitro models for monitoring anti-colon cancer effects [18,19]. However, in a study conducted by González-Ballesteros et al., in general, extract from brown algae Cystoseira baccata or extract containing gold nanoparticles exerted a stronger cytotoxic effect against HT-29 than that against Caco-2 [20]. Moreover, we previously reported the growth inhibitory effect of HT-29 cells exerted by crude and purified fucoidans obtained from S. cristaefolium [3]. Thus, in this study, we opted to use HT-29 cells to study the growth inhibitory effects of SG1-SG4 and the related signaling pathways.
The present study extends our previous investigation, in which we prepared four fucoidan extracts from S. glaucescens (SG), namely SG1 (without compressional-puffing), SG2 (compressional-puffed at 140 °C), SG3 (compressional-puffed at 180 °C), and SG4 (compressional-puffed at 220 °C) [10]. In this study, the anticancer activity of SG1-SG4 against human colon carcinoma HT-29 cells was examined with respect to cytotoxicity, cell cycle analysis, mitochondrial membrane potential (MMP), cytochrome c, expression of caspase-9 and -3, DNA fragmentation, and annexin V/propidium iodide (PI) staining and the underlying mechanisms involved. This report, to the authors’ best knowledge, is the first to evaluate the possible mechanism of anti-colon cancer activity of crude fucoidans extracted from SG by compressional-puffing pretreatment. Additionally, we planned to explore the potential application of fucoidan from SG as a natural and safe chemopreventive agent for the prevention and treatment of cancer, especially colon cancer.
2. Materials and Methods
2.1. Materials
S. glaucescens, a type of brown algae, which was collected from Penghu, Taiwan, was washed with fresh water, dried, and then kept at 4 °C until use. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), and 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Trypsin/EDTA, fetal bovine serum (FBS), RPMI-1640 culture medium, DMEM culture medium, penicillin, and streptomycin were obtained from Gibco (Grand Island, NY, USA). Tetramethylrhodamine ethyl ester (TMRE) was purchased from Molecular Probes, Invitrogen Corp., (Carlsbad, CA, USA). Antibodies including FITC (fluorescein isothiocyanate)-labelled anti-cytochrome c antibody, FITC-LEHD-FMK, FITC-DEVD-FMK, FITC-conjugated anti-BrdU antibody, FITC-conjugated anti-phospho-Akt (Ser473) antibody, PE-conjugated anti-phospho-mTOR (Ser2448) antibody, PerCP-eFluor 710-conjugated anti-phospho-S6 (Ser235, Ser236) antibody, and APC (allophycocyanin)-conjugated anti-Akt1 antibody were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Annexin V-FITC and PI were purchased from BD PharMingen (San Diego, CA, USA).
2.2. Compressional-Puffing Procedure
The dried algal sample was compressional-puffed according to Huang et al. [10]. Four puffing conditions (non-puffing, puffing at 140 °C, puffing at 180 °C, puffing at 220 °C) were performed to pretreat the algal samples. After the compressional-puffing process, the algae samples were pulverized into fine particles and stored at 4 °C for the following experimental use.
2.3. Water Extraction Procedure
The extraction of crude fucoidan from brown seaweeds was done according to Huang et al. [10]. Briefly, the puffed algal sample was pre-rinsed with 95% ethanol for the removal of proteins, lipid, and pigments. The residue was collected and extracted the polysaccharides by 85 °C water for 1 h. After centrifugation, the collected supernatant was serially treated with 20% ethanol (to precipitate alginic acid) and 50% ethanol (to precipitate fucoidan). After the extraction process, four crude extracts of fucoidan were obtained, namely SG1 (without compressional-puffing), SG2 (compressional-puffed at 140 °C), SG3 (compressional-puffed at 180 °C), and SG4 (compressional-puffed at 220 °C). The obtained fucoidans were then recovered by centrifugation, drying, and stored at 4 °C for further use.
2.4. Cell Culture
HT-29 (human colon cancer cell line, BCRC 60157), SV-HUC-1 (human normal uroepithelial cells, BCRC 60358), and HEK 293 (human normal embryonic kidney cells, BCRC 60019) were purchased from the Bioresource Collection and Research Center (BCRC) (Hsinchu, Taiwan), and BEAS-2B (human bronchial epithelial cells, ATCC CRL-9609) was obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). HT-29 was cultured in RPMI 1640 medium, and SV-HUC-1, HEK 293, and BEAS-2B were maintained in DMEM medium. All culture media were supplemented with FBS (10%) and penicillin-streptomycin (100 units/mL and 100 µg/mL, respectively). All cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. The cells were passaged every 2–3 days with 0.05% trypsin and were sub-cultured at an initial density of 1 × 105 cells/mL per 10-cm tissue dish.
2.5. Evaluation of Cytotoxic Activity
The cytotoxicity assay of the samples was determined by the MTT colorimetric assay. Briefly, cells were plated in 96-well culture plates (1 × 104 cells/well) with 100 μL growth medium and after 24 h incubation at 37 °C under a humidified 5% CO2-containing air atmosphere. The fucoidan extract was prepared as a 20 mg/mL stock solution by thoroughly dissolving fucoidan powder in phosphate-buffered saline (PBS). The medium was then removed, and the cells were treated with various concentrations of fucoidan extracts. Final concentrations of 0, 50, 100, 200, 300, 400, and 500 μg/mL were obtained by diluting the stock solution with serum-free medium to prevent the fucoidan extract potentially losing its potency in the presence of serum. The MTT solution was prepared as a 1 mg/mL stock in PBS, and the MTT solution was filtered through a 0.22 μm filter. After 48 h treatment, cells were rinsed with PBS, and 100 μL MTT solution (diluted with serum-free medium to a final concentration of 0.1 mg/mL) was added to each well. After 2 h incubation, when the cell-formazan crystal complexes had formed, 100 μL DMSO was added into each well, and the solution was mixed completely by repeated pipetting in order to dissolve the staining dye. Then, the absorbance values were recorded at wavelength of 570 nm using a PowerWave 340 microplate spectrophotometer (Bio-Tek Instruments Inc., Winooski, VT, USA). The viable cells (%) are presented as a percentage of the control values [21].
2.6. DAPI-Staining Analysis
Cells (4 × 104 cells/mL) were incubated with 200 μg/mL SG4 for 48 h, and then, cells were rinsed two times with PBS to gain cell samples. Next, cell samples were fixed with 3.7% paraformaldehyde in PBS for 10 min at room temperature (RT). After fixation, cell samples were stained with DAPI solution (PBS containing 0.5 μg/ml DAPI) for 10 min at RT. Finally, cells were rinsed with PBS twice and examined using a Zeiss fluorescence microscope (Oberkochen, Germany).
2.7. Flow Cytometry-Based Analyses
In all flow cytometry-based analyses, cells (4 × 104 cells/mL) were incubated with 200 μg/mL SG4 for 48 h, and then, cells were de-attached by trypsin and rinsed two times in cold PBS to gain cell samples. Then, each flow cytometry-based analysis was performed as the following protocols.
For cell cycle analysis, it was conducted by following up the method of Yang et al. [8]. Briefly, HT-29 cells were collected, washed twice with PBS, and re-suspended in 70% (v/v) ethanol and stored at 4 °C for at least 2 h. The cells were then washed with staining buffer twice and stained with 25 μg/mL RNase A. After staining with RNase A for 15 min, the cells were stained with 50 μg/mL PI solution and flow analysis was carried out.
For MMP analysis, it was assayed by following up the method of Yang et al. [8]. Briefly, HT-29 cells were collected and adjusted the cell density to 1 × 106 cells/mL with staining buffer. The cells were then incubated with TMRE (100 nM) at 37 °C for 20 min. After the reaction, the TMRE was removed, and the cells were re-suspended in staining buffer for flow analysis.
For cytochrome c release analysis, the procedure of this experiment was conducted by following up the method of Huang et al. [9]. Briefly, HT-29 cells were collected and re-suspended in 100 μL PBS. Cells were fixed using 100 μL fixation buffer at 37 °C for 20 min under darkness. Next, the cells were washed, re-suspended in 100 μL permeabilization buffer, followed by incubation with FITC-labelled anti-cytochrome c antibody (1:10, v/v) at RT for 60 min under darkness. After rinsing with permeabilization buffer, the cells were re-suspended in staining buffer for flow analysis.
For activated caspase -9 and -3 analyses, the activated caspase analyses were conducted by following up the method of Huang et al. [9]. Briefly, HT-29 cells were harvested and adjusted the cell density to 1 × 106 cells/mL with complete medium. The cells were then treated with fluorescent probe FITC-LEHD-FMK (for detecting caspase-9 activity) or fluorescent probe FITC-DEVD-FMK (for detecting caspase-3 activity) at 37 °C for 60 min under darkness. The cells were washed with wash buffer and re-suspended in staining buffer for flow analysis.
For quantitation of DNA fragmentation, the procedure of this experiment was performed by following up the method of Huang et al. [9]. Briefly, HT-29 cells were harvested and fixed with 4% paraformaldehyde, washed, and then incubated with 70% ice-cold ethanol at −20 °C overnight. Cells were washed with wash buffer; BrdU was added, and then, they were incubated with FITC-conjugated anti-BrdU antibody at RT for 30 min under darkness. After staining, the cells were re-suspended in staining buffer for flow analysis.
For annexin V- FITC/PI staining analysis, it was conducted by annexin V-FITC apoptosis detection kit according to the method of Yang et al. [8]. Briefly, HT-29 cells were harvested and adjusted the cell density to 1 × 106 cells/mL with binding buffer. Cells were incubated with annexin V-FITC (1:20, v/v) and PI (1:20, v/v) at 25 °C for 15 min under darkness. After staining, the cells were washed and re-suspended in staining buffer for flow analysis.
For phosphorylated Akt, mTOR, and S6 analyses, the assay of phosphorylated Akt, mTOR, and S6 was completed by following up the method of Huang et al. [22]. In brief, HT-29 cells were harvested and re-suspended in 100 μL PBS; 100 μL fixation buffer was added, and then they were stored at 37 °C for 20–60 min under darkness. Next, the cells were rinsed in staining buffer, re-suspended with 100 μL staining buffer, and incubated with APC (allophycocyanin)-conjugated anti-Akt1 antibody (1:50, v/v), FITC-conjugated anti-phospho-Akt (Ser473) antibody (1:20, v/v), PE-conjugated anti-phospho-mTOR (Ser2448) antibody (1:20, v/v), or PerCP-eFluor 710-conjugated anti-phospho-S6 (Ser235, Ser236) antibody (1:20, v/v) at RT for 60 min under darkness. After staining, the cells were washed with staining buffer and re-suspended in staining buffer for flow analysis.
All flow cytometric analyses listed above were completed using a BD Accuri C6 flow cytometer (San Jose, CA, USA). To estimate the percentage of cells in each phase of the cell cycle analysis, the percentage of cells in each phase of the annexin V-FITC/PI staining analysis, or the percentage of cells with high fluorescence intensity (mean channel fluorescence values above the marker border), the data were analyzed by BD Accuri C6 software.
2.8. Data Analysis
The statistical differences were examined using one-way analysis of variance (ANOVA) followed by the Student’s t-test or Duncan multiple range test. Statistical significance was considered a value of p < 0.05 and quantitative data were presented as mean ± SD (n = 3).
3. Results and Discussion
3.1. SG1-SG4 Exhibit Cytotoxicity to Colon Cancer Cells
Four crude extracts of fucoidan (SG1-SG4) were produced according to the procedures reported in our previous investigation [10]. The purpose of high temperature puffing pretreatment (up to 220 °C) is to disrupt the cellular structure of the algal sample and facilitate the extraction of fucoidan by warm water [10]. The extracted fucoidans SG1-SG4 exhibited characteristics of fucoidan as illustrated by TLC, monosaccharide composition, and FTIR analyses [10]. Moreover, SG1-SG4 had similar molecular weight distributions, monosaccharide compositions, and FTIR spectra. In addition, the total sugar contents of SG1, SG2, SG3, and SG4 were 53.72% ± 0.97%, 66.42% ± 2.76%, 80.37% ± 3.00%, and 78.38% ± 1.72%, respectively, indicating they had distinct total sugar contents and carbohydrates comprised more than 50% of the composition of SG1-SG4 [10]. Furthermore, the amounts of fucose, sulfate, and uronic acids in SG1-SG4 varied [10]. Of note, the extract with the most impurities (alginate, protein, and polyphenols) was SG1 (6.26 + 3.78 + 2.70 = 12.74, g/100 g, dry basis), followed by SG2 (4.08 + 3.75 + 2.56 = 10.39, g/100 g, dry basis), and SG3 (1.80 + 2.76 + 2.02 = 6.58, g/100 g, dry basis), and the lowest impurity content was found in SG4 (0.64 + 2.97 + 1.07 = 4.68 g/100 g) [10]. These results are consistent with previous findings that demonstrated certain impurities such as polyuronic acids, proteins, and phenolic compounds are usually coextracted with fucoidan [23]. Since SG1-SG4 have different compositions, their biological activities such as anti-cancer activity warrant further elucidation. In our preliminary experiments, we treated HT-29 cells with SG1-SG4 at a concentration of 500 ug/mL and different time courses (24, 48, and 72 h) and found that treatment duration of 48 h was optimal for inducing cytotoxicity of HT-29 cells. In order to ensure consistency across all cellular experiments, the duration of treatment of cells was thus set at 48 h. The results of HT-29 cells treated with different concentrations of SG1-SG4 for 48 h and the cytotoxicity of SG1-SG4 against HT-29 cells are shown in Figure 1a. All of the fucoidans (SG1-SG4) showed cytotoxicity to HT-29 cells with IC50 of 93.3 ± 8.5 μg/mL for SG1, 297.3 ± 5.2 μg/mL for SG2, 331.7 ± 51.4 μg/mL for SG3, and 272.0 ± 13.4 μg/mL for SG4, respectively (Figure 1b). Among SG1-SG4, SG1 showed the highest cytotoxicity to HT-29 cells. In addition, among SG2-SG4, their cytotoxicities to HT-29 cells were not significantly different (p > 0.05) (Figure 1b). Interestingly, SG1 also possessed a higher antioxidant activity as compared to other fucoidans extracted [10]. The high cytotoxicity to cells and high antioxidant activity exhibited by SG1 may be attributed to the higher polyphenols content present in SG1 in the form of impurities or other structural factors. However, further research is needed to elucidate this issue. Our previous studies suggested that SG4 had the highest extraction yield of fucoidan (9.83%±0.11%), followed by SG3 (8.27%±0.01%) and SG2 (6.57%±0.02%), and then SG1 (2.02%±0.02%) [10]. Although SG1 showed slightly more potent cytotoxicity to HT-29 cells as compared to the other SGs, the extraction yield for SG1 was very low. From a cost perspective, SG4 is thus a better choice for further development as an anti-colon cancer agent. Additionally, we performed a similar experiment utilizing three commonly used human epithelial normal cells from different tissues (e.g., SV-HUC-1 cells derived from ureter, HEK-293 cells derived from embryonic kidney, and BEAS-2B cells derived from lung) to examine the toxic effects exerted by SG1 and SG4 on normal cells. As shown in Figure 1c, SG1 and SG4 showed cytotoxicities to three normal cells with an IC50 value more than 500 μg/mL. In addition, SG1 also showed more toxic effects on these three normal cells as compared to SG4. A possible reason for this may be the different polyphenols contents in SG1 and SG4 or other composition and structural variances. Interestingly, HT-29 cells showed a survival rate of less than 60%, and three normal cells had a survival rate of more than 90%, following treatment with SG4 for 48 h at a concentration of 200 μg/mL. Therefore, the anticancer activity and signaling cascade of SG4 against HT-29 cells were further investigated using SG4 at a concentration of 200 μg/mL for 48 h. Previous studies reported the inactivation of PI3K (an upstream molecule of Akt) and Akt may be triggered by fucoidan, leading to cellular apoptosis [24]. Overexpression of protein kinase B (PKB)/Akt is frequently described in many types of human cancers [25]. Moreover, apoptosis induced by the inhibition of PKB/Akt predominantly occurs in cancer cells rather than in normal cells [26]. The reason that SG4 is more cytotoxic to cancer cells than to normal cells may be attributed to the differential expressions of PKB/Akt between cancer cells and normal cells. However, further experiments are still needed to elucidate the precise mechanism. In summary, SG4 was found to be a better candidate as an anti-colon cancer agent due to its relatively high cytotoxicity to HT-29 cells, low cytotoxicity to normal cells, and high production yield.
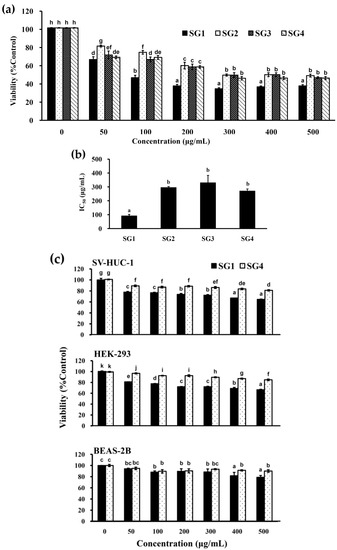
Figure 1.
Effects of crude extracts of fucoidans (SG1-SG4) on cell viabilities of HT-29, SV-HUC-1, HEK-293, and BEAS-2B cells: (a) HT-29 cells were treated with varying concentrations of SG1-SG4 for 48 h, and cell viability was assessed; (b) the IC50 values of SG1-SG4 to HT-29 cells are presented as determined for (a). (c) SV-HUC-1, HEK-293, and BEAS-2B cells were coincubated with varying concentrations of SG1-SG4 for 48 h, and cell viability was evaluated. The experiments were run in triplicate. Differences existing between columns are labeled with different letters, p < 0.05.
3.2. SG4 Induced Cell Cycle Arrest of HT-29 Cells
The effect of SG4 on cell cycle profile of HT-29 cells was examined using flow cytometry-based analysis. As shown in Figure 2a,b, when HT-29 cells were treated with 200 μg/mL of SG4, SG4 exhibited a higher percentage of cells in the sub-G1 phase (13.9% ± 0.1%) as compared to the control (3.0% ± 0.1%). Previous investigations suggested that DNA fragmentation as well as small fragments of DNA can be detected when cells are treated with an apoptosis-inducing agent [3,27]. Cells that have lost DNA possess less PI fluorescence intensity, and this reduction in DNA content is represented by the so-called sub-G1 peak which appears to the left of the G1 peak. The increased sub-G1 population is directly proportional to the induction of DNA fragmentation, which is a manifestation of apoptosis [27]. Thus, SG4 might cause DNA fragmentation (also known as sub-G1 cell cycle arrest) of HT-29 cells. The retardation of cell growth can be visualized by examining the cell cycle phase distribution [3]. In Figure 2b, a significant increase (p < 0.001) of the S phase population was found after treatment with SG4 (17.4% ± 0.2%) as compared to that of the untreated cells (16.1% ± 0.1%). Moreover, a significant increase (p < 0.01) of the G2/M population was also detected after treatment with SG4 (20.2% ± 0.4%) as compared to that of the untreated cells (17.7% ± 0.5%). These effects were accompanied by a significant decrease (p < 0.001) of the G0/G1 population after treatment with SG4 (48.5% ± 0.3%) as compared to that of the untreated cells (63.2% ± 0.4%). The results clearly show that the accumulation of cells in the S phase and G2/M phase might account for the induction of cell cycle arrest in HT-29 cells by SG4. In summary, SG4 retarded the growth of HT-29 cells via induction of sub-G1, S, and G2/M cell cycle arrest. Previous studies have shown that the induction of cell cycle arrest is not a separate event; indeed, apoptotic cell death is usually preceded by the arrest of cell cycle [28]. Therefore, we conducted experiments involving cell cycle arrest in order to further elucidate the possible mechanisms underlying SG4-induced apoptosis.
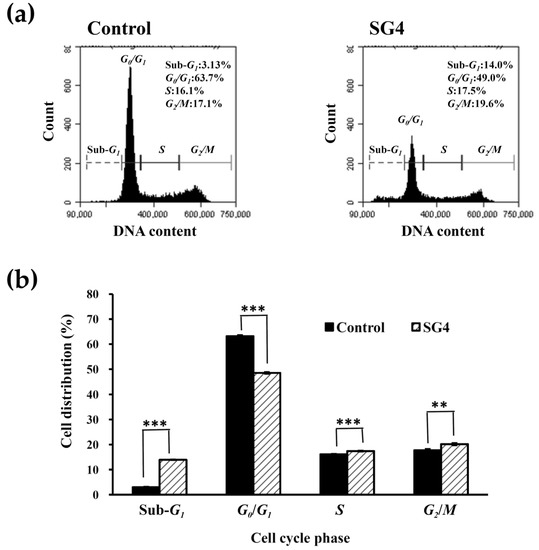
Figure 2.
The cell cycle profile of HT-29 cells treated with 200 μg/mL SG4 for 48 h: (a) In the histograms, x axis and y axis denote DNA content and cell counts, respectively. The cell cycle distribution (sub-G1, G0/G1, S, and G2/M) is indicated in each histogram; (b) the percentage of sub-G1, G0/G1, S, and G2/M cells is presented as determined for (a). The experiments were performed in triplicate. ** p < 0.01; *** p < 0.001.
3.3. SG4 Induced Apoptosis of Ht-29 Cells Via a Mitochondria-Mediated Signaling Pathway
The functions of mitochondria may be mediated by the mitochondrial ATP-sensitive K channels and the mitochondrial permeability transition pore. The irretrievable opening of the mitochondrial permeability transition pore is an indicator of early apoptosis and is fatal to cells [29]. MMP is an essential component in the production of energy (ATP) and in the maintenance of cellular homeostasis [30]. As a result, the loss of MMP is directly linked to the induction of apoptosis [31]. A potentiometric TMRE dye can be adopted to quantify loss of MMP [8]. TMRE may bind to active mitochondria (possessing negative charge) due to its ability to permeate cells as well as its positively charged properties. Thus, the malfunction of mitochondrial results in a decrease of TMRE accumulation in mitochondria. Figure 3a,b suggests that the percentage of cells with high TMRE intensity in the control was 88.7% ± 0.3%. When HT-29 cells were treated with 200 μg/mL SG4 for 48 h, the percentage of cells with high TMRE intensity was significantly decreased to 37.4% ± 1.3% (p < 0.001), which suggests that SG4-mediated mitochondrial dysfunction occurs. Release of cytochrome c from mitochondria is indicative of early apoptosis and is often an upstream signal of the mitochondria-dependent apoptotic pathway [32,33]. In the present study, we applied an immunodetection method to quantify the release of cytochrome c in cells by flow cytometry. As shown in Figure 3c,d, when HT-29 cells were treated with 200 μg/mL SG4 for 48 h, the high fluorescent cell population significantly decreased from 89.7% ± 0.6% (control) to 76.5% ± 1.0% (p < 0.001), indicating SG4-mediated cytochrome c release from mitochondria occurs. In summary, these results suggest SG4 induced mitochondria-dependent apoptotic effects, as visualized by the loss of MMP and release of cytochrome c.
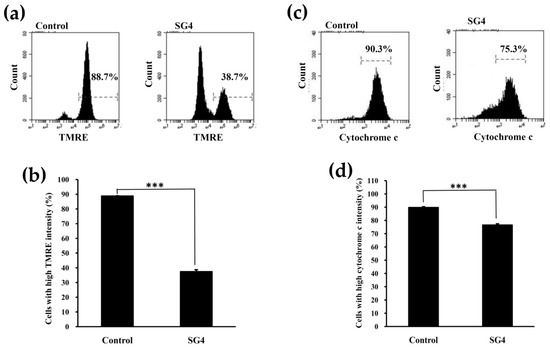
Figure 3.
The MMP and cytochrome c analyses of HT-29 cells treated with 200 μg/mL SG4 for 48 h: (a) In the histograms, x axis and y axis denote TMRE content and cell counts, respectively. The percentage of cells with mean channel fluorescence values above the marker border is indicated in each histogram; (b) the percentage of cells with high fluorescence of TMRE is presented as determined for (a). (c) In the histograms, x axis and y axis denote cytochrome c content and cell counts, respectively. The percentage of cells with mean channel fluorescence values above the marker border is indicated in each histogram; (d) the percentage of cells with high fluorescence of cytochrome c is presented as determined for (c). The experiments were performed in triplicate. *** p < 0.001.
3.4. SG4 Induced Activation of Caspase-9 and Caspase-3 in HT-29 Cells
There are two fundamental pathways, the extrinsic pathway (death receptor pathway) and the intrinsic pathway (the mitochondria pathway), in cell apoptosis [34]. The intrinsic pathway may operate as a consecutive process of loss of mitochondrial transmembrane potential, release of cytochrome c into the cytoplasm, formation of a complex composed of cytochrome c, the cytoplasmic protein Apaf-1, and pro-caspase-9 (known as an apoptosome), which induces the activation of caspase-3. The effector caspases may cleave various proteins leading to the various morphological and biochemical processes involved in apoptosis [35,36]. Here, the activation of caspase-9 and -3 in cells was measured by a flow cytometry. As shown in Figure 4a,b, when HT-29 cells were treated with 200 μg/mL SG4 for 48 h, the high fluorescent cell population in the caspase-9 group significantly increased from 5.30% ± 0.14% (control) to 16.3% ± 0.8% (p < 0.001), indicating an increase of active caspase-9. Moreover, as shown in Figure 4c,d, when HT-29 cells were treated with 200 μg/mL SG4 for 48 h, the high fluorescent cell population in the caspase-3 group significantly increased from 5.43% ± 0.33% (control) to 17.1% ± 0.5% (p < 0.001), indicating an increase of active caspase-3. These results clearly show that SG4 induced activation of caspase-9 and -3 in HT-29 cells.
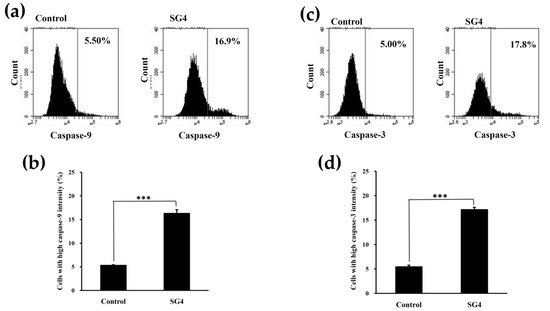
Figure 4.
The expression of active caspase-9 and -3 in HT-29 cells treated with 200 μg/mL SG4 for 48 h: (a) In the histograms, x axis and y axis denote caspase-9 content and cell counts, respectively. The percentage of cells with mean channel fluorescence values above the marker border is indicated in each histogram; (b) the percentage of cells with high fluorescence of caspase-9 is presented as determined for (a). (c) In the histograms, x axis and y axis denote caspase-3 content and cell counts, respectively. The percentage of cells with mean channel fluorescence values above the marker border is indicated in each histogram; (d) the percentage of cells with high fluorescence of caspase-3 is presented as determined for (c). The experiments were performed in triplicate. *** p < 0.001.
3.5. SG4 Increased DNA Fragmentation and Annexin V Binding to Membranes in HT-29 Cells
The activation of caspase-3 seems to play a critical role in inducing apoptosis and DNA fragmentation (an indicator of late-stage apoptosis) [37]. Here, the detection of chromatin condensation and DNA fragmentation in cells was performed by DAPI staining and flow cytometric-based TUNEL staining, respectively. The results of DAPI staining in Figure 5a suggested that treatment of HT-29 cells with 200 μg/mL SG4 for 48 h resulted in nuclear condensation (bright blue), indicating that they were undergoing apoptosis (white arrows in Figure 5a). In addition, as shown in Figure 5b,c, when HT-29 cells were treated with 200 μg/mL SG4 for 48 h, the high fluorescent cell population significantly increased from 3.17% ± 0.25% (control) to 21.4% ± 0.5% (p < 0.001), indicating SG4-mediated DNA fragmentation occurs. Previous investigations showed that, when inducing apoptosis, cells lose plasma membrane asymmetry, and the phosphatidylserine (PS) residues become exposed at the outer plasma membrane [38]. Annexin V specifically binds to PS, and this can be utilized to detect apoptosis by monitoring the loss of plasma membrane integrity [38]. Here, we used an annexin V-FITC/PI staining analysis for determining the percentage of dead cells (cells in early-stage or late-stage apoptosis, as well as in necrosis) by flow cytometry. As shown in Figure 6a,b, exposure of HT-29 cells to 200 μg/mL SG4 for 48 h resulted in an increase from 0.03% ± 0.02% (control) to 9.42% ± 0.02% (p < 0.001) in the percentages of early apoptotic cells, an increase from 0.08% ± 0.02% (control) to 25.3% ± 0.5% (p < 0.001) in the percentages of late apoptotic cells, an increase from 0.73% ± 0.13% (control) to 9.90% ± 0.23% (p < 0.001) in the percentages of necrotic cells, and a reduction from 99.1% ± 0.2% (control) to 55.4% ± 0.3% (p < 0.001) in the percentages of live cells. These findings clearly suggest that SG4 causes HT-29 cell death (consisting of apoptosis and necrosis) and apoptosis is predominantly involved. Taken together, SG4 induced colon cancer cell apoptosis as revealed by DNA fragmentation and annexin V-FITC/PI staining, and therefore, SG4 may have potential for use as a natural agent in a preventive therapy for colon cancer.
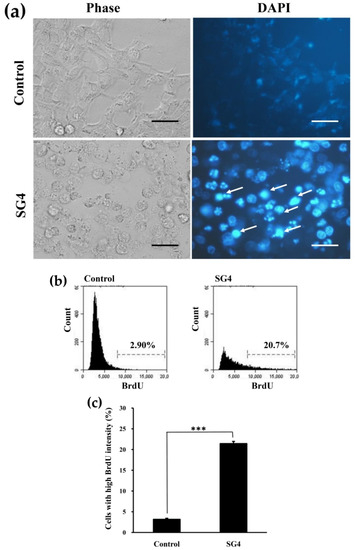
Figure 5.
The induction of chromatin condensation and DNA fragmentation in HT-29 cells treated with 200 μg/mL SG4 for 48 h: (a) Morphological and DAPI-staining assessments of HT-29 cells treated with 200 μg/mL SG4 for 48 h. Arrows in the graph indicate nuclear condensation (bright blue) (scale bar, 50 μm). (b) In the histograms, x axis and y axis denote BrdU content and cell counts, respectively. The percentage of cells with mean channel fluorescence values above the marker border is indicated in each histogram; (c) the percentage of cells with high fluorescence of BrdU is presented as determined for (b). The experiments were performed in triplicate. *** p < 0.001.
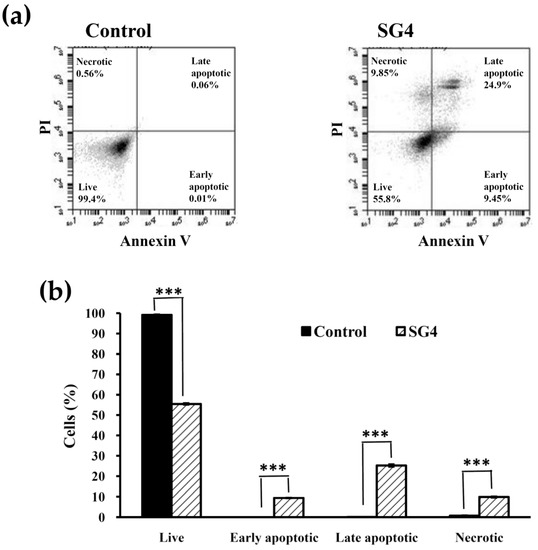
Figure 6.
The annexin V-FITC/PI staining analysis of HT-29 cells treated with 200 μg/mL SG4 for 48 h: (a) In the histograms, annexin-V fluorescence is displayed on the x axis and PI fluorescence on the y axis. The percentage of cells on each boundary (live, early apoptotic, late apoptotic, and necrotic) is indicated in each histogram; (b) the percentage of live, early apoptotic, late apoptotic, and necrotic cells is presented as determined for (a). The experiments were performed in triplicate. *** p < 0.001.
3.6. Phosphorylation of Akt, mTOR, and S6 is Involved in the SG4-Induced Apoptosis of HT-29 Cells
Previous studies suggest that phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is a critical factor involved in the occurrence of human cancers. This pathway is also related to numerous cellular functions including proliferation, migration, adhesion, invasion, metabolism, angiogenesis, and survival [39]. A flow cytometric method was performed to determine the expressions of p-Akt, Akt1, p-mTOR, and p-S6 in cells. As shown in Figure 7a,b, when HT-29 cells were treated with 200 μg/mL SG4 for 48 h, the high fluorescent cell population in p-Akt, p-mTOR, and p-S6 groups significantly decreased (p < 0.05) from 44.7% ± 0.8% (control) to 29.1% ± 2.3%, 53.3% ± 1.2% (control) to 28.7% ± 1.4%, and 53.0% ± 1.6% (control) to 32.2% ± 1.1%, respectively. These results indicate the occurrence of SG4-mediated dephosphorylation of Akt, mTOR, and S6. Moreover, it was found that the expression of Akt1 (total Akt) was not varied with respect to SG4 treatment (98.6% ± 0.7% (control) to 96.7% ± 0.3%). Therefore, our results show that dephosphorylation of Akt, mTOR, and S6 is involved in the SG4-induced cytotoxicity of HT-29 cells. These data were similar to previous findings, which suggested that fucoidan decreased p-Akt of DU-145 human prostate cancer cells in a concentration-dependent manner [40]. Further elucidation of the precise molecular mechanism as well as signaling cascade, especially using in vivo models, is warranted.
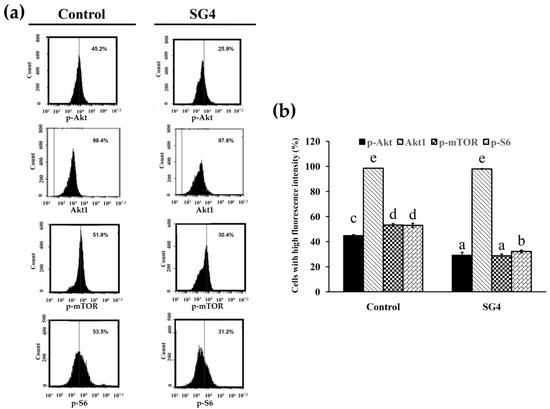
Figure 7.
The expression of p-Akt, Akt1, p-mTOR, and p-S6 in HT-29 cells treated with 200 μg/mL SG4 for 48 h: (a) In the histograms, x axis and y axis denote p-Akt, Akt1, p-mTOR, or p-S6 content and cell counts, respectively. The percentage of cells with mean channel fluorescence values above the marker border is indicated in each histogram; (b) the percentage of cells with high fluorescence of p-Akt, Akt1, p-mTOR, and p-S6 is presented as determined for (a). The experiments were performed in triplicate. Differences existing between columns are labeled with different letters, p < 0.05.
4. Conclusions
In this paper, four fucoidan extracts (SG1-SG4) were extracted from SG pretreated by compressional-puffing. Among SG1-SG4, SG4 possessed the highest extraction yield. SG4 also showed high cytotoxicity against HT-29 cells, and low cytotoxicity to normal cells. In addition, SG4 caused cell cycle arrest of HT-29 cells and induced HT-29 cellular apoptosis via loss of MMP, increased cytochrome c release, activation of caspase-9 and -3, increased DNA fragmentation, and increased early and late apoptotic cells. Additional biological experiments revealed that the Akt/mTOR/S6 pathway is involved in SG4-induced apoptosis of HT-29 cells. These results indicate that SG4 may have potential applications as an adjuvant therapeutic agent in colon cancer treatment. Further in vivo studies on the anti-colon cancer effects of SG4 are needed.
Author Contributions
Conceptualization, W.-C.S. and C.-H.K.; methodology, Y.-H.T.; software, S.-L.H.; validation, A.-W.K., Y.-H.H., and C.-Y.H.; formal analysis, A.-W.K. and S.-L.H.; investigation, Y.-H.T. and Y.H.H.; resources, C.H.K. and S.-L.H.; data curation, A.-W.K. and W.-C.S.; writing—original draft preparation, W.-C.S. and C.-H.K.; writing—review and editing, Y.-H.H. and C.-Y.H.; supervision, Y.-H.H; project administration, C.-Y.H.; funding acquisition, W.-C.S., Y.-H.H., and C.-Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Yuan’s General Hospital, Taiwan, grant number YUAN-IACR-20-02 to Wei-Cheng Shiao, as well as by the Ministry of Science and Technology, Taiwan, grant number MOST 107-2320-B-992-001, which was awarded to Chun-Yung Huang. The authors thank the Ministry of Education, Taiwan, for supporting this study (grant number MOE-RSC-108RSN0005), awarded to Yong-Han Hong.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bilan, M.I.; Grachev, A.A.; E Ustuzhanina, N.; Shashkov, A.S.; E Nifantiev, N.; I Usov, A. Structure of a fucoidan from the brown seaweed Fucus evanescens C. Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Wu, T.-C.; Hsieh, S.-L.; Tsai, Y.-H.; Yeh, C.-W.; Huang, C.-Y. Antioxidant activity and growth inhibition of human colon cancer cells by crude and purified fucoidan preparations extracted from Sargassum cristaefolium. J. Food Drug Anal. 2015, 23, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, H.R.B.; Srinivasan, P.; Rekha, S. Immunomodulatory activity of fucoidan against aspirin-induced gastric mucosal damage in rats. Int. Immunopharmacol. 2011, 11, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ponce, N.M.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, S.; Wang, J.; Li, F.; Chen, A.; Li, B. A comparative study of antithrombotic and antiplatelet activities of different fucoidans from Laminaria japonica. Thromb. Res. 2012, 129, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Yang, W.-N.; Chen, P.-W.; Huang, C.-Y. Compositional characteristics and in vitro evaluations of antioxidant and neuroprotective properties of crude extracts of fucoidan prepared from compressional puffing-pretreated Sargassum crassifolium. Mar. Drugs 2017, 15, 183. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Kuo, C.-H.; Chen, P.-W. Compressional-puffing pretreatment enhances neuroprotective effects of fucoidans from the brown seaweed Sargassum hemiphyllum on 6-hydroxydopamine-induced apoptosis in SH-SY5Y cells. Molecules 2017, 23, 78. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Wu, S.-J.; Yang, W.-N.; Kuan, A.-W.; Chen, C.-Y. Antioxidant activities of crude extracts of fucoidan extracted from Sargassum glaucescens by a compressional-puffing-hydrothermal extraction process. Food Chem. 2016, 197, 1121–1129. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Chandini, S.K.; Ganesan, P.; Bhaskar, N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem. 2008, 107, 707–713. [Google Scholar] [CrossRef]
- Ermakova, S.; Sokolova, R.; Kim, S.-M.; Um, B.-H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS ONE 2012, 7, 43483. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Hsu, C.P.; Chen, C.C.; Liao, T.Z.; Chiu, C.F.; Lester, P.J.L.; Shih, Y.T. Anti-proliferation and radiation-sensitizing effect of an anthocyanidin-rich extract from purple-shoot tea on colon cancer cells. J. Food Drug Anal. 2012, 20, 329. [Google Scholar]
- Ministry of Health and Welfare. Taiwan, Statistics of Causes of Death. Available online: http://dep.mohw.gov.tw/DOS/lp-4472-113.html (accessed on 14 March 2020).
- Rabik, C.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2006, 33, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Park, S.Y.; Lee, J.-Y.; Park, J.H.Y. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.; Chitra, J. In vitro anticancer activity of fucoidan extracted from Sargassum cinereum against Caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef]
- Ballesteros, N.G.; López, S.P.; Rodríguez-González, J.; Lastra, M.; Rodríguez-Argüelles, M. Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: Its activity in colon cancer cells. Colloids Surf. B Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Kuo, C.-H.; Lee, C.-H. Antibacterial and antioxidant capacities and attenuation of lipid accumulation in 3T3-L1 adipocytes by low-molecular-weight fucoidans prepared from compressional-puffing-pretreated Sargassum crassifolium. Mar. Drugs 2018, 16, 24. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Wu, T.-C.; Hong, Y.-H.; Hsieh, S.-L.; Guo, H.-R.; Huang, R.-H. Enhancement of cell adhesion, cell growth, wound healing, and oxidative protection by gelatins extracted from extrusion-pretreated tilapia (Oreochromis sp.) fish scale. Molecules 2018, 23, 2406. [Google Scholar] [CrossRef]
- Imbs, T.I.; Skriptsova, A.; Zvyagintseva, T.N. Antioxidant activity of fucose-containing sulfated polysaccharides obtained from Fucus evanescens by different extraction methods. J. Appl. Phycol. 2014, 27, 545–553. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.-S.; Kim, E. Fucoidan from seaweed Fucus vesiculosus inhibits migration and invasion of human lung cancer cell via PI3K-Akt-mTOR pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Mukherjee, S.; Ray, D.; Raha, S. Involvement of the Akt/PKB signaling pathway with disease processes. Mol. Cell. Biochem. 2003, 253, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Troussard, A.A.; McDonald, P.C.; Wederell, E.D.; Mawji, N.M.; Filipenko, N.R.; Karen, G.; E Kucab, J.; Dunn, S.E.; Emerman, J.T.; Bally, M.; et al. Preferential dependence of breast cancer cells versus normal cells on integrin-linked kinase for protein kinase B/Akt activation and cell survival. Cancer Res. 2006, 66, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Qin, C.; Wang, M.; Gan, D.; Cao, L.; Ye, H.; Zeng, X. Preparation, preliminary characterization and inhibitory effect on human colon cancer HT-29 cells of an acidic polysaccharide fraction from Stachys floridana Schuttl. ex Benth. Food Chem. Toxicol. 2013, 60, 269–276. [Google Scholar] [CrossRef]
- Jakubikova, J.; Bao, Y.; Sedlak, J. Isothiocyanates induce cell cycle arrest, apoptosis and mitochondrial potential depolarization in HL-60 and multidrug-resistant cell lines. Anticancer Res. 2005, 25, 3375–3386. [Google Scholar]
- Crompton, M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999, 341, 233–249. [Google Scholar] [CrossRef]
- Tang, X.-Q.; Feng, J.-Q.; Chen, J.; Chen, P.-X.; Zhi, J.-L.; Cui, Y.; Guo, R.-X.; Yu, H.-M. Protection of oxidative preconditioning against apoptosis induced by H2O2 in PC12 cells: Mechanisms via MMP, ROS, and Bcl-2. Brain Res. 2005, 1057, 57–64. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.B.; Paim, B.A.; Cosso, R.G.; Castilho, R.; Rottenberg, H.; Vercesi, A.E. Method for monitoring of mitochondrial cytochrome c release during cell death: Immunodetection of cytochrome c by flow cytometry after selective permeabilization of the plasma membrane. Cytom. Part A J. Int. Soc. Anal. Cytol. 2006, 69, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Tschoeke, S.K.; Hellmuth, M.; Hostmann, A.; Robinson, Y.; Ertel, W.; Oberholzer, A.; Heyde, C.-E. Apoptosis of human intervertebral discs after trauma compares to degenerated discs involving both receptor-mediated and mitochondrial-dependent pathways. J. Orthop. Res. 2008, 26, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.; Aubert, M. Flow Cytometric Detection of Activated Caspase-3. In Apoptosis and Cancer: Methods and Protocols, 2008th ed.; Mor, G., Alvero, A.B., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2008; pp. 47–56. [Google Scholar]
- Robertson, J.D.; Orrenius, S.; Zhivotovsky, B. Review: Nuclear events in apoptosis. J. Struct. Biol. 2000, 129, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, P.; Widlak, P.; Zou, H.; Luo, X.; Garrard, W.T.; Wang, X. The 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis. Proc. Natl. Acad. Sci. USA 1998, 95, 8461–8466. [Google Scholar] [CrossRef] [PubMed]
- Van Engeland, M.; Nieland, L.J.W.; Ramaekers, F.C.S.; Schutte, B.; Reutelingsperger, C.P.M. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Choo, G.-S.; Lee, H.-N.; Shin, S.-A.; Kim, H.-J.; Jung, J.-Y. Anticancer effect of fucoidan on DU-145 prostate cancer cells through inhibition of PI3K/Akt and MAPK pathway expression. Mar. Drugs 2016, 14, 126. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).