1. Introduction
The treatment of fresh extractions sockets is a common challenge in dental practice. Following tooth extraction, the soft and hard alveolar tissues show dimensional, structural, and volumetric changes [
1]. These changes to the alveolar ridge consist in the highest amount of horizontal bone loss with a concomitant vertical ridge height loss, which has been recorded to be more prominent at the vestibular site.
The findings from a recent systematic review evidenced that the horizontal bone loss, ranging between 29% and 63%, was higher than the vertical bone loss, ranging between 11% and 22%, at 6 months after dental extraction [
2]. Multiple key factors seem to be involved in this tissue shrinkage, and the most important role seems to be played by bundle bone resorption and blood clot stabilization [
2,
3,
4]. These three-dimensional tissue changes can result in difficulties in performing a prosthetically-driven implant placement leading to prosthetic compromises. An adequate architecture of the alveolar ridge volume seems to be one of the most important key factors to obtain successful esthetic implant-supported restorations [
2,
5]. Consequently, in-depth knowledge of post-extraction healing patterns and contour changes can be essential for a correct implant treatment planning.
It has been well determined that the soft tissue morphology around implants is related to the underlying bone topography; moreover, the buccal bone plate can affect the stability of peri-implant soft tissues around implant restorations [
6,
7]. Furthermore, a reduced thickness of the vestibular bone plate might induce midfacial recession of soft tissue, which is considered a very common cause of esthetic failure in implant-supported rehabilitation [
8].
The ridge preservation technique includes all procedures that maintain the alveolar volume within the soft and hard tissue socket existing at the time of extraction [
9]. This procedure is suggested when implant insertion is not possible at the time of extraction, when implant primary stability cannot be achieved or when there are absolute contraindications to implant treatment. The preservation of the alveolar volume using bone substitutes in fresh extraction sockets, minimizes bone resorption and facilitates subsequent insertion of a dental implant. This improves esthetic and functional criteria, reducing the need of additional bone grafting surgeries at the time of implant placement [
10].
Several techniques and biomaterials have been proposed over time, including fresh extraction socket grafting with a bone substitute, occluding the access to the socket with a resorbable barrier, overbuilding of the vestibular bony wall, or a combination of some of them, with or without primary intention healing [
4]. However, no significant clinical or histomorphometrical differences have been evidenced between the various bone substitutes, although collagen alone has been demonstrated to be unable to reduce tissue changes after tooth extraction [
1,
4,
11]. Resorbable collagen membranes and bovine-derived xenografts is one of the most common combinations for treatment, since it has been found to be effective for bone regeneration around implants, alveolar ridge augmentation, and alveolar ridge preservation [
12,
13,
14,
15,
16]. However, some randomized controlled clinical trials have also found promising outcomes for calcium phosphate alloplastic graft materials in terms of new bone formation and preservation of the alveolar ridge volume [
16,
17,
18].
Synthetic calcium phosphate ceramics are widely used because they are similar to human bone, which is principally composed of calcium phosphate hydroxyapatite [
1,
16]. Calcium phosphate-based bone grafts are osteoconductive and free of any infection risk compared to animal-derived xenograft or allograft. Synthetic calcium phosphates are composed of resorbable beta-tricalcium phosphates (β-TCPs), non-resorbable hydroxyapatites (HA), and combinations of these two biomaterials, which are called biphasic calcium phosphates (BCPs).
An ideal bone substitute should be resorbable, for the purpose of allowing substitution with newly formed bone, while balancing resorption with volumetric preservation. The objective of combining the insoluble HA with β-TCPs is that hydroxyapatite would maintain the space, without being fully resorbable, while the Beta-tricalcium phosphates would resorb, therefore, promoting the bone regeneration [
16].
However, the substitution of β-TCPs with new bone does not occur at a 1:1 ratio, leading to a lower amount of new bone volume than the volume of tricalcium phosphate absorbed [
19]. Moreover, synthetic BCPs have been further coated with poly-lactic acid (PLA) that allows the material to form a solid block in the defect (GUIDOR
easy-graft) [
20]. It has already been shown that this in situ hardening of grafts can be successfully used for ridge preservation procedures in intact extraction sockets without the use of a membrane [
21].
Therefore, the primary aim of this prospective single cohort study was to evaluate the histological and histomorphometric outcomes of a synthetic BCP (60% HA and 40% β-TCP ) and a synthetic poly-lactic acid (PLA) membrane (GUIDOR easy-graft CRYSTAL and GUIDOR bioresorbable matrix barrier, Sunstar Suisse SA, Etoy, Switzerland ) used to graft fresh extraction socket sites with a full or partial resorption of the buccal bone plate.
2. Materials and Methods
A single cohort prospective study was designed. Patients requiring tooth extraction in the esthetic area having a vestibular bone plate reduction (>5mm) without any soft tissue defect (type II socket by Juodzbalys et al. 2008) and having been scheduled for an implant-supported prosthesis, were considered eligible for this study [
22].
Patients were excluded from this study, if they matched any of the following criteria:
systemic contraindication to oral surgical treatment,
oncological treatment with irradiation in the head and/or neck area,
long-term drug therapy (non-steroidal anti-inflammatory),
bisphosphonate therapy
lack of adjacent teeth,
the tooth located in molar area,
periodontal disease (severe untreated),
unwillingness to return for the follow-up examination,
smokers (>10 cigarettes/day).
The Helsinki Declaration was followed. The protocol was authorized by the Ethical Committee (Comité Etico de Experimentacion) of the University of Seville, Spain (protocol approved 9 September 2011). Patients were recruited from September 2016 to September 2017 at the Department of Stomatology of the University of Seville and at the Department of Surgery and Translational Medicine, Oral Surgery unit, University of Florence, Italy.
Accurate explanations and a written informed consent form were provided to being enrolled in the trial. Patients who were included in the study were accurately evaluated clinically and radiographically (panoramic/periapical radiographs); moreover, data were collected for each patient such as gender, age, smoking habits, indications for tooth extraction, position of tooth, and presence of adjacent teeth.
After the informed consent was signed, one session of oral hygiene was provided to all patients before surgical extraction procedures to prepare a more favorable oral environment to wound healing.
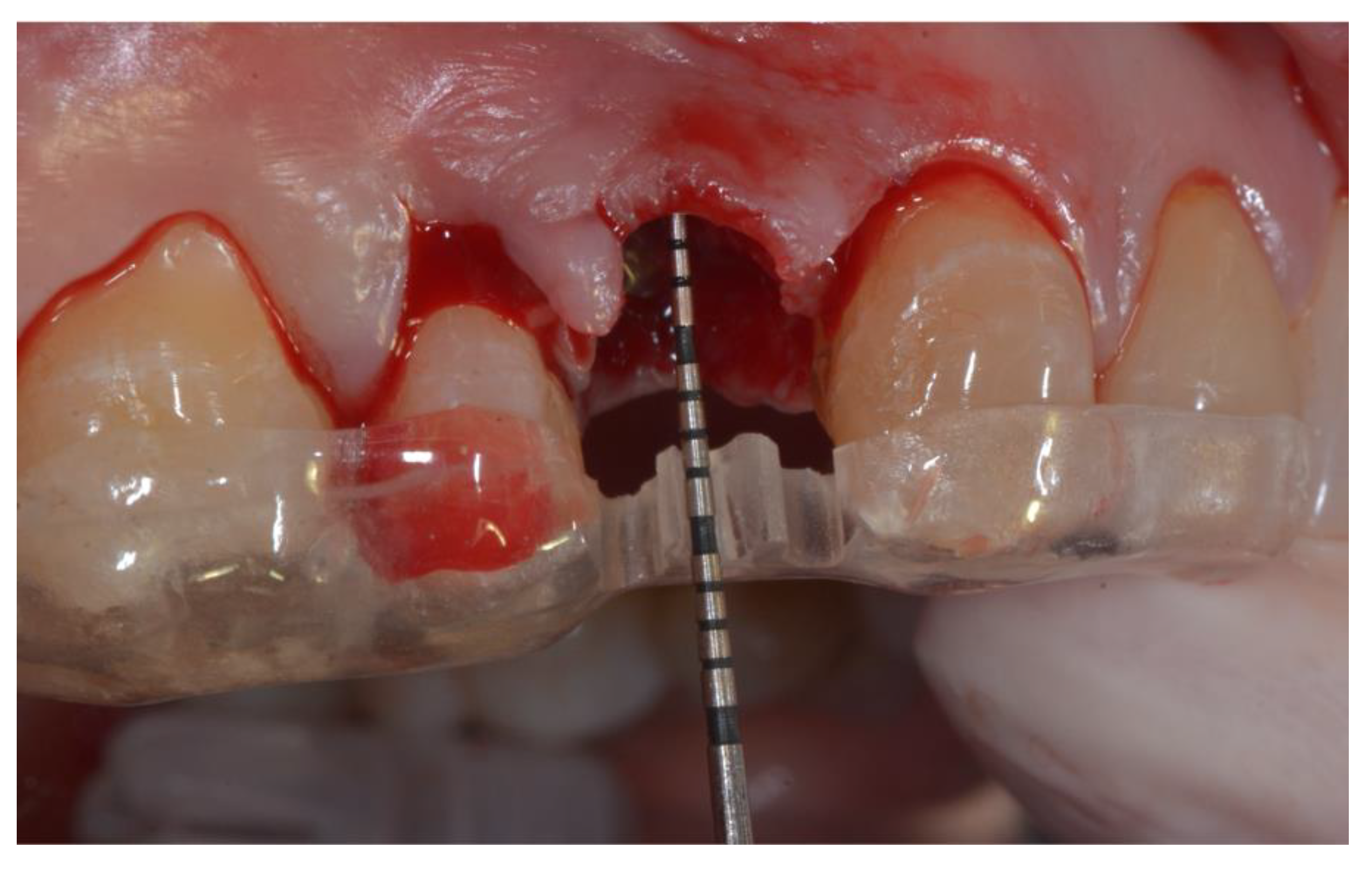
A surgical template was fabricated for clinical measurement at T0 (ridge preservation surgery) and T1 (six-month re-entry surgery, before biopsy and implant placement). It was realized including one tooth anterior and posterior to the hopeless tooth, to serve as a fixed reference guide.
All patients rinsed for one minute with chlorhexidine mouthwash 0.2% before the surgery (and two times a day for the following three weeks). Under local anesthesia using lidocaine (adrenaline 1:50,000), patients were operated with the same surgical approach consisting of minimally invasive tooth extraction, without raising a muco-periosteal flap. Great attention was taken to reduce the damage to the vestibular bone plate and to maintain the integrity of the alveolar bone morphology. The extraction sockets were thoroughly debrided to remove all soft tissue, subsequently, the acrylic custom-made template was used to take the horizontal and vertical bone measurements. The surgical template was stabilized on the adjacent teeth to allow reproducible horizontal and vertical measurements at the different time points of the study. The vertical measurements were performed taking the surgical stent as reference point, as shown in
Figure 1.
Extraction sockets were grafted with biphasic calcium phosphate (60% HA and 40% β-TCP, GUIDOR® easy-graft Crystal, Sunstar Group, Etoy, Switzerland).
A bioresorbable matrix (GUIDOR® bioresorbable matrix barrier, Sunstar Group, Etoy, Switzerland) was then used to cover the buccal dehiscence, which was cut into the shape of “ice cream cone” to regenerate the bone plate. The membrane was placed over the residual vestibular bone plate between the periosteum and the remaining bone and was slipped under the flap to separate the vestibular gingival tissues from the graft. Subsequently, alveolar extraction sites were overfilled and slightly condensed with hardening HA/β-TCP. The grafting material was compacted, taking care not to bend or damage the membrane. After grafting the fresh extraction socket, the coronal part of the membrane was folded to seal the socket and the top of the socket was covered with a collagen sponge (Collafleece®, Botiss Biomaterials, Berlin, Germany) and then sutured with silk suture without achieving a complete soft tissue closure.
One hour before surgical treatment, a prophylactic antibiotic therapy of 2 g of amoxicillin (allergic to penicillin: 600 mg clindamycin) was prescribed to all patients. Post-operatively, 1g amoxicillin (allergic to penicillin: 300 mg clindamycin) twice a day for four days after surgery was prescribed. Patients were instructed to continue with prophylactic antibiotic therapy, and ibuprofen 600 mg tablets were prescribed as an anti-inflammatory to be taken twice a day as long as required. Removable prostheses, if present, were permitted for use after 3 weeks from surgery and after they had been adjusted and refitted.
All surgeries were performed by two calibrated surgeons using similar and standardized procedures. Intra-oral digital periapical radiographs were taken (7 mA, 70 KVp) using a parallel cone technique with digital sensor (Schick Technologies, Long Island City, NY, USA.) before tooth extraction, 4 weeks after grafting procedure, before implant placement, and thereafter in the follow-up period. A paralleling tool and custom-made bite blocks of polyvinyl siloxane impression material (Flexi-time, Heraeus-Kulzer, Hanau, Germany) were used for the standardization of the X-ray.
After 6 months of healing, the surgical re-entry procedure was performed: full-thickness flaps were elevated to allow access to the alveolar ridges; vertical and horizontal measurements were re-evaluated. The custom-made templates were used to perform clinical measurements; subsequently, a bone biopsy was harvested from the grafted sockets according to the implant position planning. Thereafter, the implant was inserted, and the flaps were sutured. Patients received the same drug prescription as after the first surgery. The bone cores were coded and sent for analysis to the Laboratory.
Outcome evaluations were:
- -
Histological and histomorphometrical analyses of bone biopsies: percentage of new bone (NB), biomaterial graft particles (P), soft tissue (ST).
- -
Alveolar horizontal bone loss (ΔAHB): alveolar horizontal bone changes at the mid-crestal level, between buccal and lingual/palatal buccal plates. Measurements were taken with a periodontal probe using a custom-made acrylic template at T0 and T1.
- -
Alveolar vertical bone loss (ΔAVB): alveolar vertical bone changes between the custom-made acrylic template and the bone ridge. Measurements were taken with a periodontal probe at T0 and T1.
- -
Any biological complications at the extraction socket sites.
2.1. Histological Analysis
Immediately after surgeries, the biopsies were fixed by immersion in buffered formalin (10%) and processed (Precise-1-Automated System; Assing, Rome, Italy) to obtain thin ground sections. Ascending series of alcohol rinses dehydrated the specimens, and subsequently, these were embedded in glycol-methacrylate resin (Technovit-7200-VLC; Kulzer, Wehrheim, Germany).
After polymerization, the specimens were sectioned, along their longitudinal axis, with a high precision diamond disk at about 150 µm and ground down to about 30 µm with a specially designed grinding machine Precise-1-Automated System. Three slices were obtained from each specimen, subsequently stained with acid fuchsin and toluidine blue before the analysis. Histological analysis was carried out using a light microscope (Laborlux S, Leitz, Wetzlar, Germany) connected to a high-resolution video camera (3CCD, JVCKY-F55B, JVC, Yokohama, Japan) and interfaced with a monitor and personal computer (Intel-Pentium III 1200 MMX, Intel, Santa Clara, CA, USA). This optical system was associated with a digitizing pad (Matrix-Vision-GmbH, Oppenweiler, Germany) and a histomorphometry software package with image capturing capabilities (Image Pro-Plus-4.5, Media Cybernetics Inc., Immagini & Computer S.n.c., Milan, Italy).
A single expert examiner (GI), who was not involved in the surgical treatment, evaluated the histomorphometrical results. Outcome measures were as follows: percentages of new bone, marrow spaces, and residual graft particles.
2.2. Statistical Analysis
Alveolar horizontal and vertical bone loss between T0 and T1 were identified by the Wilcoxon signed rank test (Statistical Package for the Social Sciences for Windows; S.P.S.S. Inc., Chicago, USA). A p-value of 0.05 or less was set to be considered statistically significant.
3. Results
Thirteen subjects, five males and eight females, were included in this study. Three of them smoked less than 10 cigarettes/day. Each patient was treated at one site in the esthetic upper or lower jaw with a ridge preservation procedure; after healing, each patient received an implant-supported prosthesis.
All of the extracted teeth were judged hopeless, mostly because of vertical tooth fracture (9/13 cases), some others for periodontal reasons (2/13 cases), or periapical lesions (2/13 cases) (
Table 1).
All sites have a complete or partial (>5mm) buccal bone plate deficiency without any soft tissue deficiencies (type II socket). None of the sites had acute infection, but most of the sites had a mild amount of granulation tissue, which did not contraindicate the application of this synthetic biphasic calcium phosphates grafting material.
The healing of all sites was by secondary intention wound, which was uneventful without any signs of infection for 11 patients. In 2 cases, a biological complication with prominent signs of inflammatory response was recorded during early healing phases. In those two cases, the twice-daily 0.2% chlorhexidine mouthwash therapy was protracted for the following 6 weeks after surgery.
After 6 months of healing, all 13 patients received the surgical re-entry procedure for biopsies of the sites and implant insertion.
Dental implants (Sweden & Martina, Due Carrare, Padova, Italy) were placed in all preserved sites obtaining initial stability with an insertion torque of 20/35 Ncm. Three 15 mm, three 12 mm, and seven 10 mm implants were inserted. All of them were 3.8 mm of diameter. During implant insertion, 3 patients exhibited exposed implant threads or facial bone defect; therefore, an additional guided bone regeneration procedure was performed with HA/β-TCP in combination with a collagen membrane. The remaining patients did not show any peri-implant bone defect. After four months of healing, the second-stage implant surgery was performed, and subsequently a prosthesis was delivered to all patients.
None of the implants had mobility or any biological or mechanical complications during this study.
3.1. Histological Results
All 13 biopsies were available for histological evaluation. However, three specimens were not evaluated because the biopsies were damaged during the removal from the trephine burs. A total of 10 biopsies were examined.
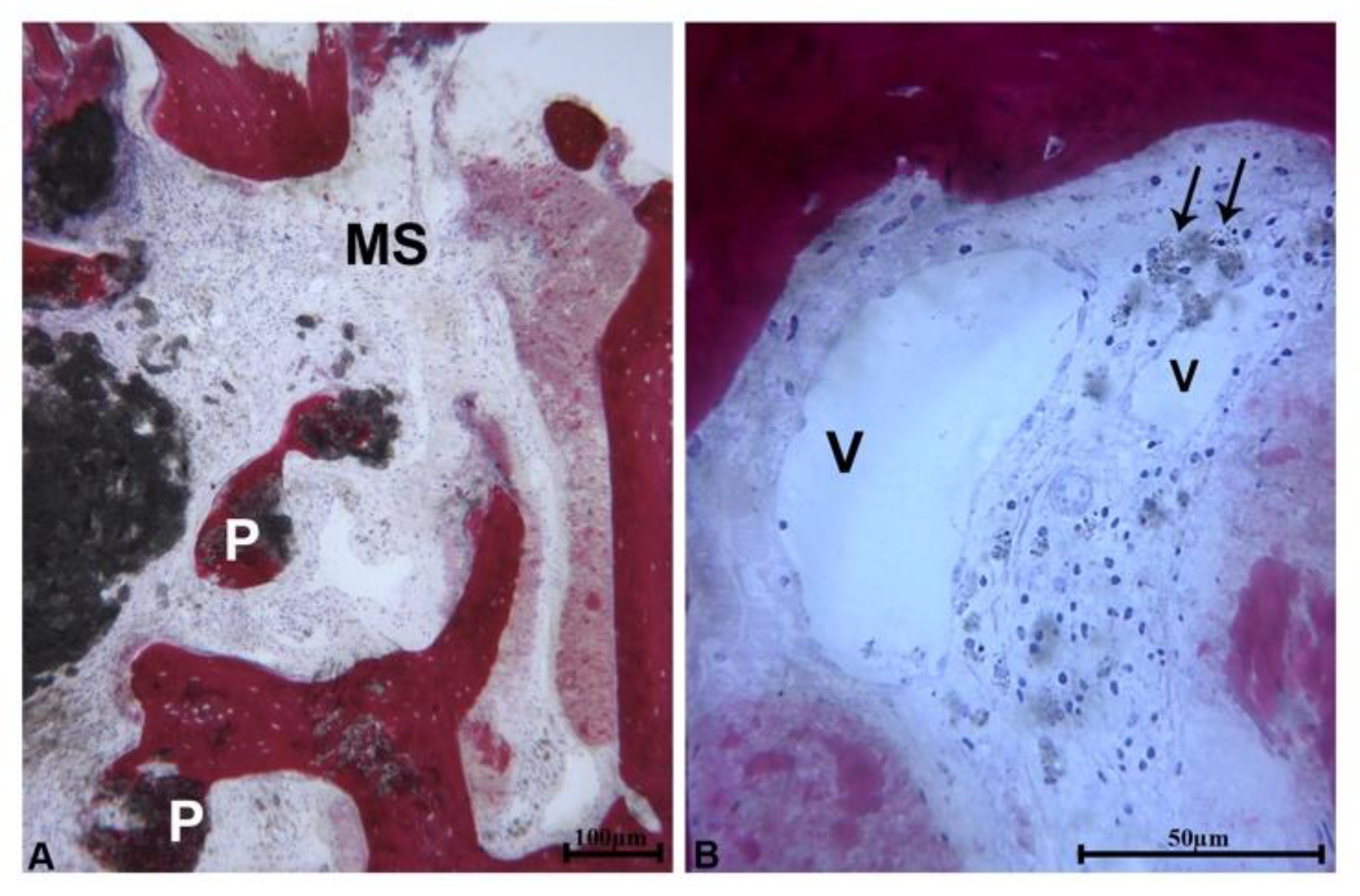
At low magnification, 3 samples showed a portion of bone wall consisting of pre-existing bone which lined the area of bone regeneration. Close to the pre-existing bone and in the apical portion of the samples, newly formed bone with small marrow spaces and residual biomaterial particles were present (
Figure 2a). In these portions of the samples, the residual biomaterial particles were embedded in the newly formed bone. These particles had an irregular shape and showed signs of resorption (
Figure 2b).
In the coronal portion of the biopsies (occlusal region), the regenerated area consisted of newly formed trabecular bone, soft tissues, and residual biomaterial particles which were partially surrounded by new bone (
Figure 3a). The connective tissue adjacent to the newly formed bone was well vascularized; thus, it could be hypothesized that this tissue plays a crucial role in the vascularization of the newly augmented bone. The surface of residual biomaterial particles showed signs of degradation. Some lymphocytes and many blood vessels were present close to them (
Figure 3b).
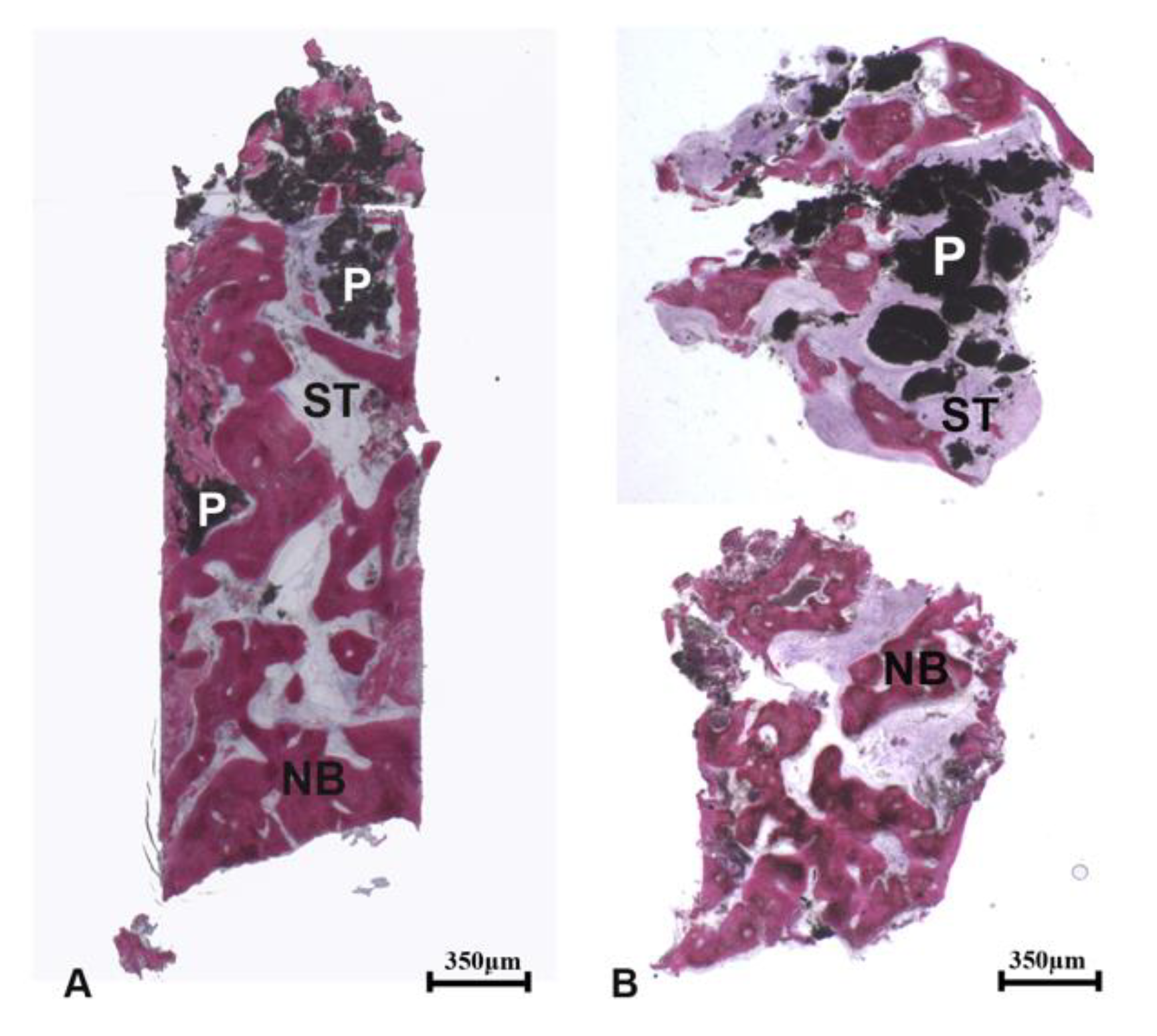
The remaining seven samples were obtained approximately from the center of the socket; they were almost completely composed of newly formed bone.
At low magnification, the samples presented newly formed trabecular bone, soft tissues, and residual biomaterial particles (
Figure 4a,b).
At high magnification, large osteocytes embedded in new bone were observed- a typical structure of newly formed bone (
Figure 5a). The residual biomaterial particles were generally positioned in the center of the samples, and the residual amount was greater than in the three specimens previously described (
Figure 5b).
The osteoblastic rim was close to the biomaterial surface, which was partially covered by osteoid matrix and newly formed bone (
Figure 6a). In some fields, the biomaterial particles were undergoing resorption and being replaced by newly formed bone (
Figure 6b). Close to the residual biomaterial particles, some lymphocytes and many blood vessels were present.
After a 6 months healing period, the main component—upon histomorphometric analysis—was soft tissue with a percentage of 48.9 ± 11.9%; newly formed bone and residual graft particles had a mean percentage of 29.0 ± 9.3% and 22.0± 9.7%, respectively (
Table 2). The best result was obtained by “Patient 1” with considerable new bone formation (43.4%), a moderate amount of connective tissue (35.1%), and few biomaterial residual particles (21.5%).
3.2. Clinical Results
Measurements from clinical analyses, performed at ridge preservation surgery and after six months, exhibited a mean value of alveolar horizontal bone loss (ΔAHB) of 0.5±1.0mm, which was statistically significant (p-value < 0.05).
Alveolar vertical bone loss (ΔAVB), at the mid-crestal point on the buccal side of the socket, recorded from a landmark on a custom-made acrylic template to the top of the bone ridge, was 0.9±1.3mm at follow-up after six months, revealing a statistically significant loss (
p-value < 0.05) (
Table 3).
4. Discussion
This 6 month histological and histomorphometric prospective cohort study evaluated the quality and quantity of bone after a ridge preservation procedure using a hardening biphasic calcium phosphate (β-TCP/HA) and a bioresorbable matrix barrier.
The histological analysis of the biopsies showed newly formed trabecular bone, a moderately well-vascularized inflamed connective tissue, and residual graft particles after six months after graft procedure. This indicated an appropriate healing, with particles embedded into newly formed bone, showing slight signs of resorption and high biocompatibility of the material without significant inflammatory response. Moreover, other authors in histological studies on humans did not find evidence of inflammatory infiltrate, necrosis, foreign body reaction, or other signs of adverse reaction in case of use of the B-TCP/HA for the treatment of bone defects [
23].
The histological human results obtained in the present study were in agreement with the histological observations described in the study conducted by Kakar et al. [
21]. Indeed, after 5 months of healing, the authors reported that the Beta-TCP/HA granules were still present, and they were in contact with active osteoblasts forming osteoid matrix and newly formed bone; this could demonstrate an osteogenic property. Similarly, in the present histological study, the presence of mineralized bone and rims of osteoblasts in contact with the residual particles were observed, therefore, showing that bone formation activity was still in progress.
Surface topography can be a crucial role in the osteoconductive properties of the biomaterials, as it was demonstrated in in vitro studies where the topography and chemistry properties of a substrate had a very important effect on the stem cell characterization or differentiation [
24,
25,
26].
Moreover, in a histological study on rabbits, three hydroxyapatite-based biomaterials with different physical and topographical characteristics showed different quantities of new bone formation when the biomaterials were implanted in bone regenerated sites [
27].
A long-term histological study, performed on experimental animals, has shown that β-TCP biomaterial was osteoconductive until degradation, acting as a scaffold to support newly formed bone [
18].
Calcium phosphates, such as β-TCP or hydroxyapatites (HA) or a mixture of both, have demonstrated in previous studies to be a highly performing bone substitute with all the characteristics of an ideal biomaterial: good biocompatibility and osteoconduction proprieties, easy to shape and to manipulate, safe, and bioresorbable [
19]. Their structure and chemical composition are very similar to those of natural hydroxy apatite, with appropriate porosity for vascular invasion and a surface chemistry that allows cells to adhere, and it is even characterized by appropriate mechanical properties [
19,
28].
The percentage of residual graft particles observed in this study was in a similar range to that observed for other bone substitutes that have been used for ridge preservation procedures. Some biomaterials like demineralized freeze-dried bone allograft exhibited a low percentage of residual graft particles (8.8%), whereas hydroxyapatite-based material shows rather high levels (40.8%) [
11]. In this study, the residual graft particles constituted the 22.0 ± 9.7% of the whole sample and were slightly higher when compared with some other previous studies that used the same biomaterial. Some authors have reported an amount of residual graft material of 15.99 ± 11.4% or 15.83 ± 8.70% [
19,
29]. This could be explained by the initial application and condensation of the material into the defect and that the buccal bone plate was completely or partially (>5mm) lost in the selected cases.
The percentage of newly formed bone in this study was 29.0 ± 9.3%, after six months. This was slightly higher when compared to the histomorphometrical results from Kakar et al., who in a recent case series on intact sockets used hardening biphasic calcium phosphate revealing an observed 21.3 ± 9.1% of new bone at a 5 month follow up [
21]. That outcome was also in accordance with Kesmas et al., who in a previous prospective cohort study reported a range of 28.00%–36.75% of new bone formation after four months in extraction with buccal defects grafted with biphasic calcium phosphate particles and a collagen membrane [
28]. When compared with results from ridge preservation studies performed with different grafting materials, the percentage of new bone was 24.6% for freeze-dried bone allograft and 47.15% for calcium phosphosilicate [
11]. A previous study, performed by Froum et al., used biphasic calcium phosphate in combination with a hydroxyapatite in sinus augmentation procedures; the authors found that the newly formed bone was 28.35% 6–8 months after surgery. Interestingly, the percentage of newly formed bone increased from 6 to 8 months after grafting procedures. This finding could mean that in a biphasic calcium phosphate augmented site, a longer healing period could be beneficial in terms of higher amounts of newly formed bone [
30].
Clinical parameters of horizontal and vertical bone loss after dental extraction were widely studied, and it was demonstrated that marked alteration occurred during the first eight weeks of healing. In this interval, intense osteoclastic activity results in resorption of crestal bone. During the first phase, the bundle bone, the portion of alveolar process that surrounds the teeth, was resorbed and subsequently replaced with a woven bone. On the vestibular side of the alveolar socket, predominantly composed of bundle bone, a substantial vertical (11%–22%) and horizontal (29%–63%) reduction of the buccal crest could be observed during the spontaneous healing without any grafting biomaterials [
2,
3].
Clinical measurements from this study showed an alveolar horizontal resorption after ridge preservation (ΔAHB) of 0.5 ± 1 mm (7.4%) after 6 months. That was in accordance with Kakar et al., who reported 0.79 ± 0.7 mm of width reduction from CBCT analysis (cone beam computer tomography) [
21]. Mardas et al. showed slightly different results in an RCT using biphasic calcium phosphate or a bovine-derived xenograft in a post-extraction site: after 8 months of healing, the bucco-lingual dimensions of the alveolar ridge decreased by 1.1 ± 1 mm and 2.1 ± 1 mm, respectively [
16]. That difference could probably be explained with the different follow-up analysis and the inclusion of different sites (incisor area) by the authors. Volumetric proprieties of calcium phosphate were recently confirmed in a systematic review that pointed out the high performance of this biomaterial in terms of horizontal ridge preservation, even compared with other biomaterials [
11].
As regards alveolar vertical bone change (ΔAVB), a loss of 0.9±1.3mm (14.8%) at the mid-crestal site of the extraction sockets was observed after six months. That result was higher than the one reported for other biomaterials (0.157 mm), according to a recent systematic review [
1]. On the other hand, the alveolar vertical bone change was much lower than the loss reported (−1.5mm) in another prospective cohort study using BCP and a collagen membrane [
24]. This indicated a wide heterogeneity of data, probably related with different alveolar socket type and length of follow-up.
Randomized clinical trials should be performed to assess the long-term outcomes, for quality and quantity of bone after ridge preservation procedure, using a hardening biphasic calcium phosphate (Beta-TCP/HA) and a bioresorbable matrix barrier.