Quinazolin-4(3H)-ones: A Tangible Synthesis Protocol via an Oxidative Olefin Bond Cleavage Using Metal-Catalyst Free Conditions
Abstract
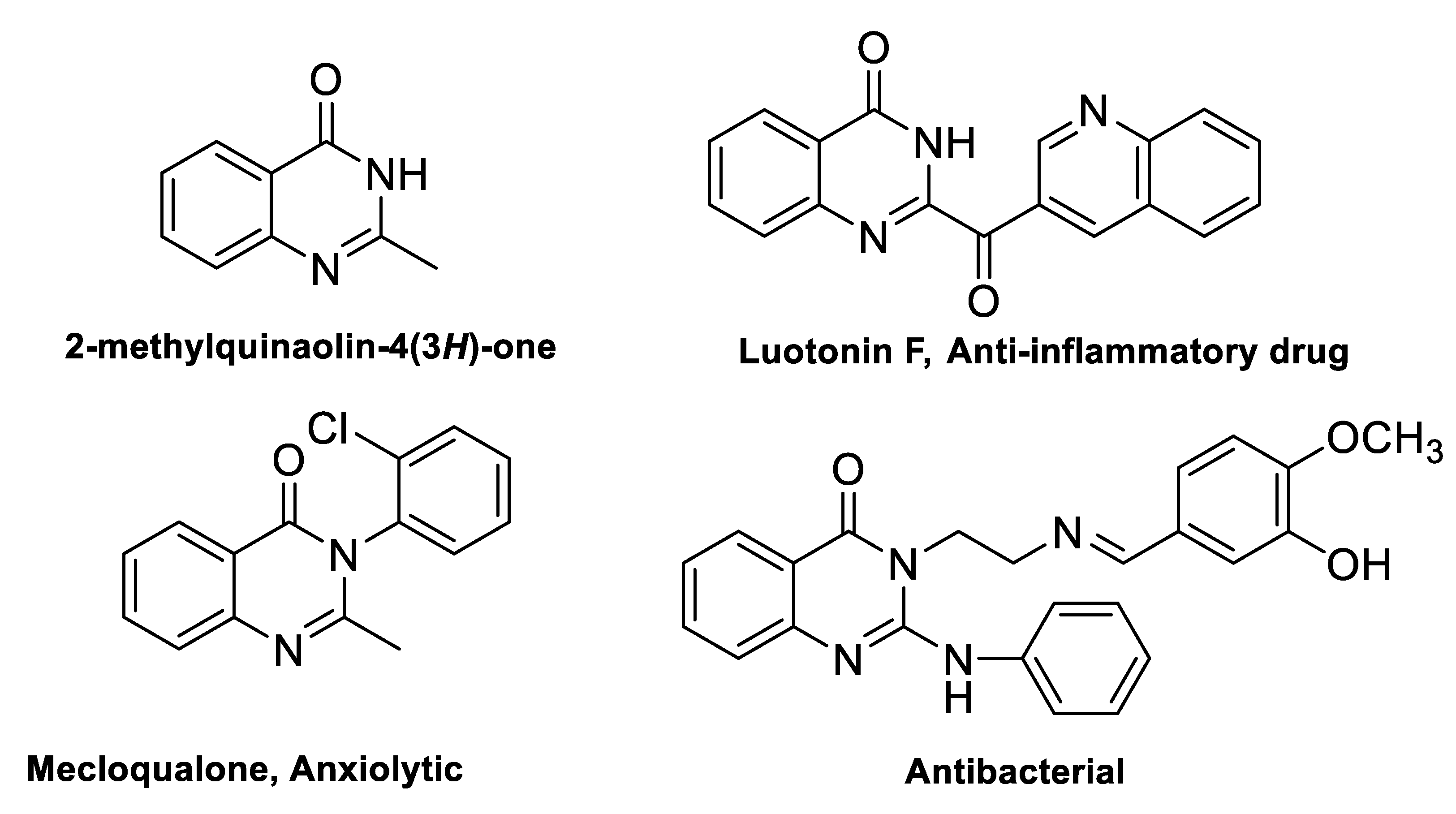
1. Introduction
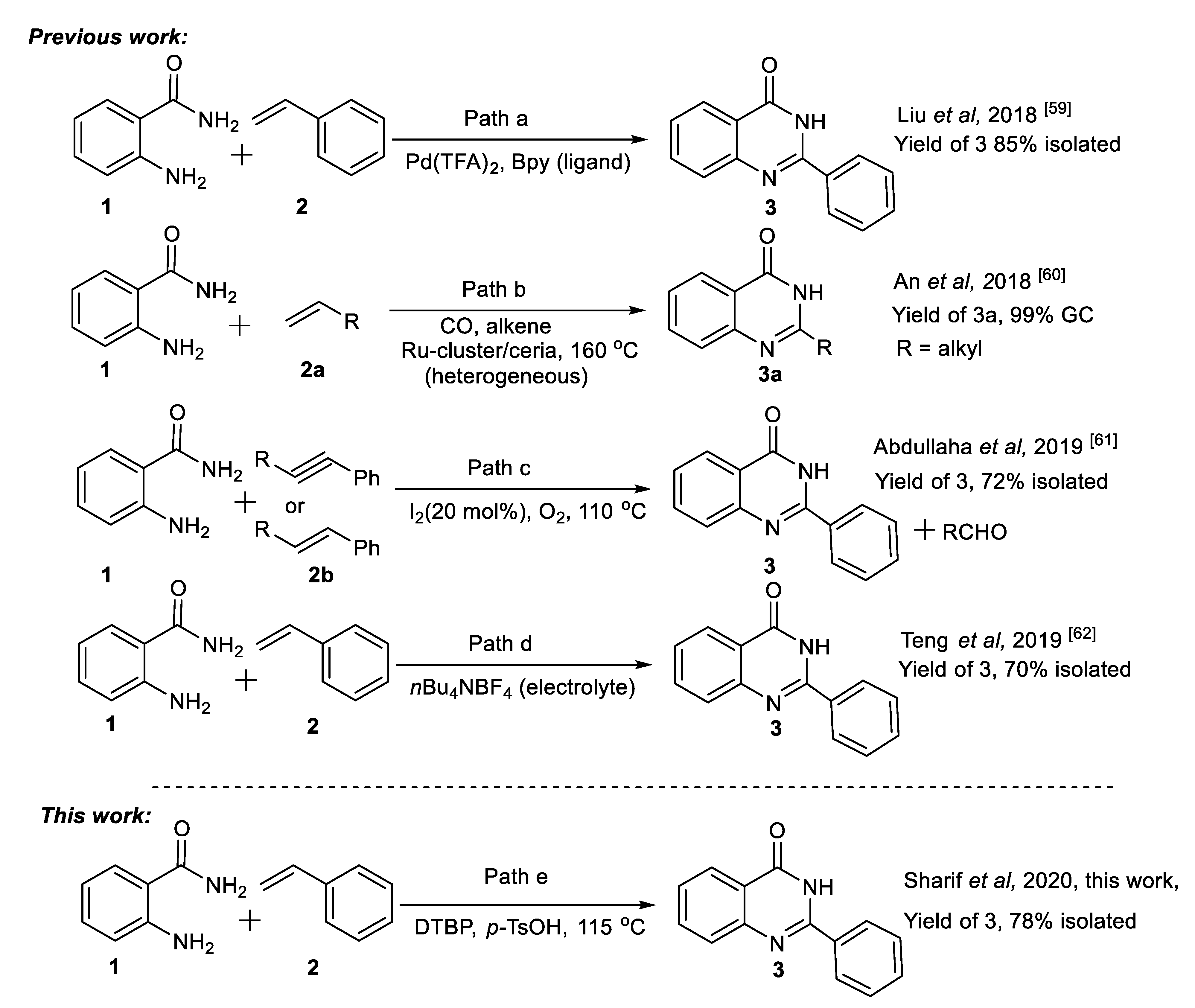
2. Results and Discussion
2.1. Model Reaction for Synthesis of Quinazolin-4(3H)-one
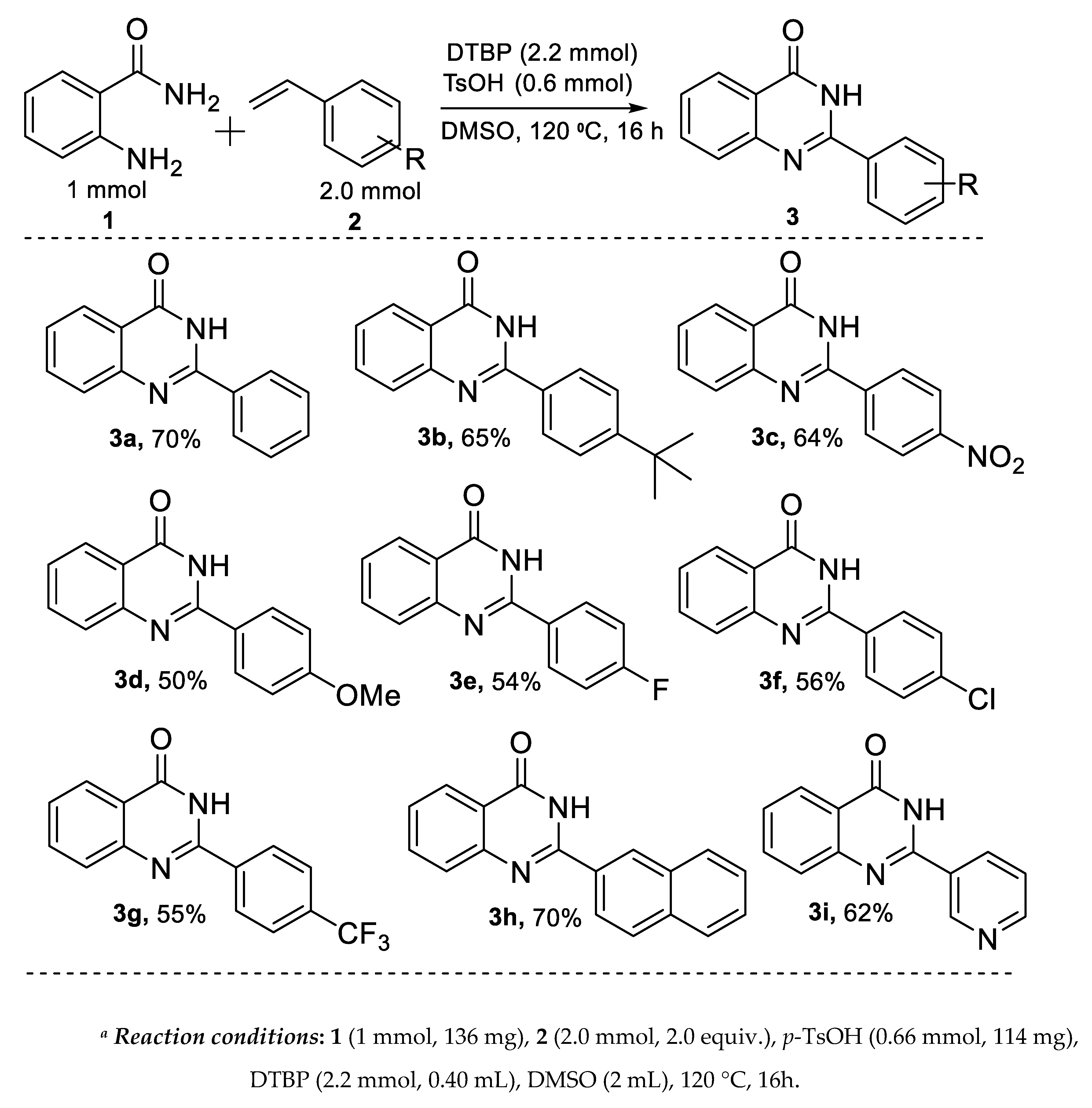
2.2. Synthesis of Quinazolin-4(3H)-one: Substrate Scope
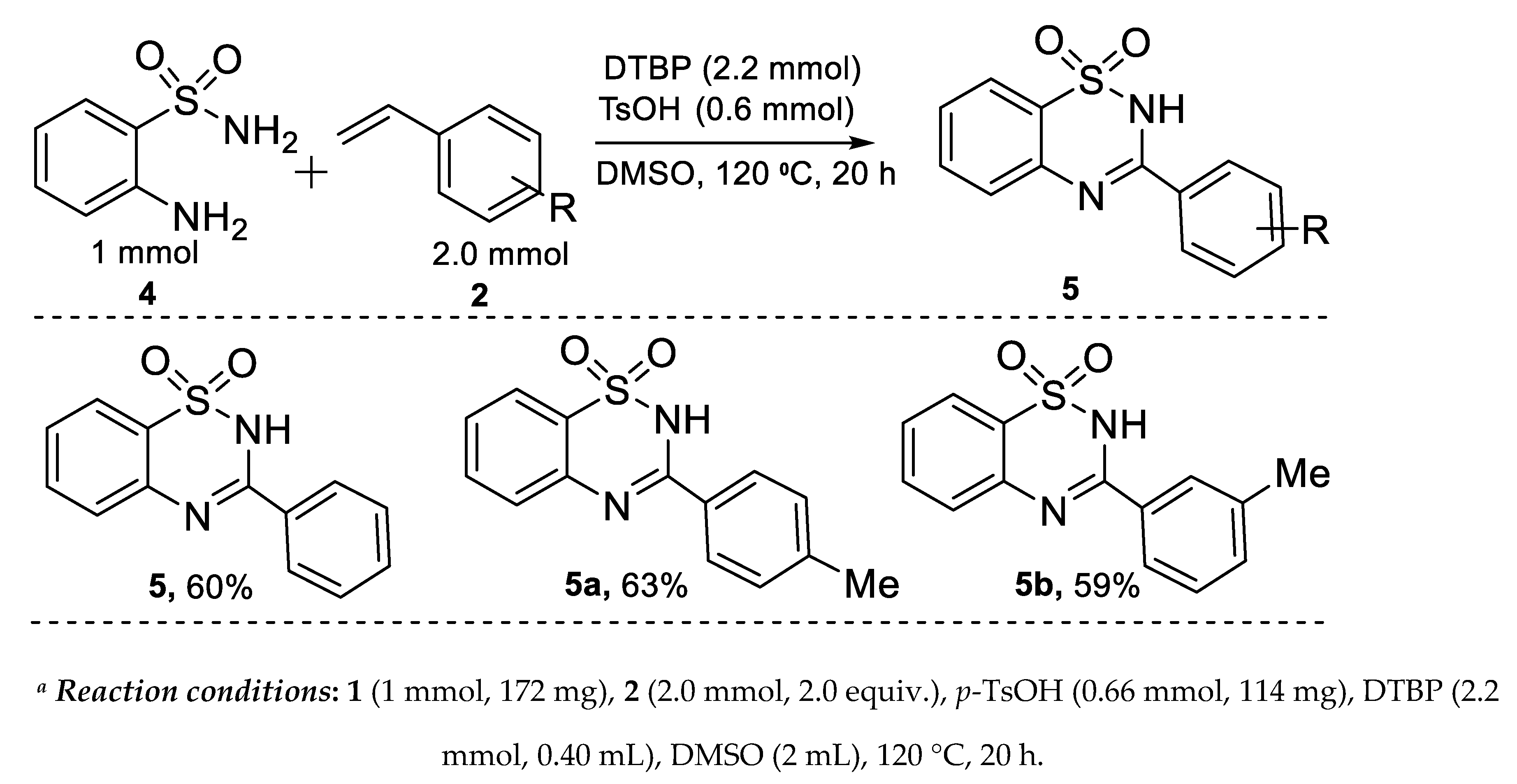
2.3. Synthesis of Benzothiadiazine-1,1-dioxides: Substrate Scopes
2.4. Understanding of the Reaction Mechanism
3. Materials and Methods
3.1. Typical Experimental Procedure for Synthesis of Quinazolin-4(3H)-ones
2-phenylquinazolin-4(3H)-one (3)
3.2. Typical Procedure for Synthesis of Benzothiadiazine-1,1-dioxides
3-phenyl-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide (5)
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, G.; Teo, P.; Wickens, Z.K.; Grubbs, R.H. Primary alcohols from terminal olefins: Formal anti-markovnikov hydration via triple relay catalysis. Science 2011, 333, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Namboodiri, V.V.; Varma, R.S.; Demessie, E.S.; Pillai, U.R. Selective oxidation of styrene to acetophenone in the presence of ionic liquids. Green Chem. 2002, 4, 170–173. [Google Scholar] [CrossRef]
- Olah, G.A.; Reddy, V.P.; Prakash, G.K.S. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley and Sons: New York, NY, USA, 1994; Volume 11, pp. 1042–1081. [Google Scholar]
- Spannring, P.; Bruijnincx, P.C.A.; Weckhuysen, B.M.; Klein Gebbink, R.J.M. Transition metal-catalyzed oxidative double bond cleavage of simple and bio-derived alkenes and unsaturated fatty acids. Catal. Sci. Technol. 2014, 4, 2182–2209. [Google Scholar] [CrossRef]
- Kulciţki, V.; Bourdelais, A.; Schuster, T.; Baden, D. Synthesis of a functionalized furan via ozonolysis—further confirmation of the Criegee mechanism. Tetrahedron Lett. 2010, 51, 4079–4084. [Google Scholar] [CrossRef]
- Wong, C.T.; Lam, H.Y.; Li, X. Effective synthesis of kynurenine-containing peptides via on-resin ozonolysis of tryptophan residues: Synthesis of cyclomontanin B. Org. Biomol. Chem. 2013, 11, 7616–7620. [Google Scholar] [CrossRef]
- Cochran, B. One-pot oxidative cleavage of olefins to synthesize carboxylic acids by a telescoped ozonolysis-oxidation process. Synlett 2015, 27, 245–248. [Google Scholar] [CrossRef]
- Schiaffo, C.E.; Dussault, P.H.J. Ozonolysis in solvent/water mixtures: Direct conversion of alkenes to aldehydes and ketones. Org. Chem. 2008, 73, 4688–4690. [Google Scholar] [CrossRef]
- Lemieux, R.U.; Rudloff, E.V. Periodate–permanganate oxidations: I. Oxidation of oelfins. Can. J. Chem. 1955, 33, 1701–1709. [Google Scholar] [CrossRef]
- Lee, D.G.; Chang, V.S. Oxidation of hydrocarbons. 8. Use of dimethyl polyethylene glycol as a phase transfer agent for the oxidation of alkenes by potassium permanganate. J. Org. Chem. 1978, 43, 1532–1536. [Google Scholar] [CrossRef]
- Travis, B.; Narayan, R.S.; Borhan, B. Osmium tetroxide-promoted catalytic oxidative cleavage of olefins: An organometallic ozonolysis. J. Am. Chem. Soc. 2002, 124, 3824–3825. [Google Scholar] [CrossRef]
- Whitehead, D.C.; Travis, B.R.; Borhan, B. The OsO4-mediated oxidative cleavage of olefins catalyzed by alternative osmium sources. Tetrahedron Lett. 2006, 47, 3797–3800. [Google Scholar] [CrossRef]
- Hiroshi, O.; Kazuhiro, O.; Shinji, B. Use of the composite material RuO2/BaTi4O9 as an environmentally benign solid catalyst for the oxidative cleavage of olefins. Synlett 2007, 20, 3201–3205. [Google Scholar]
- Hart, S.R.; Whitehead, D.C.; Travis, B.R.; Borhan, B. Catalytic oxidative cleavage of olefins promoted by osmium tetroxide and hydrogen peroxide. Org. Biomol. Chem. 2011, 9, 4741–4744. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, S.; Ramesh, R.; Semeril, D. Synthesis and characterisation of cycloruthenated benzhydrazone complexes: Catalytic application to selective oxidative cleavage of olefins to aldehydes. RSC Adv. 2016, 6, 97107–97115. [Google Scholar] [CrossRef]
- Kim, S.; Chung, J.; Kim, B.M. Recycling of osmium catalyst in oxidative olefin cleavage: A chemoentrapment approach. Tetrahedron Lett. 2011, 52, 1363–1367. [Google Scholar] [CrossRef]
- Singh, F.V.; Milagre, H.M.S.; Eberlin, M.N.; Stefani, H.A. Synthesis of benzophenones from geminal biaryl ethenes using m-chloroperbenzoic acid. Tetrahedron Lett. 2009, 50, 2312–2316. [Google Scholar] [CrossRef]
- Goulart, M.O.F.; Cioletti, A.G.; de Souza Filho, J.D.; de Simone, C.A.; Castellano, E.E.; Emery, F.S.; de Moura, K.C.G.; Pintod, M.C.F.R.; Pinto, A.V. Unexpected oxidation of a substituted benzo[a]phenazine: Oxidative cleavage of a double bond and formation of a macrolactone. Tetrahedron Lett. 2003, 44, 3581–3585. [Google Scholar] [CrossRef]
- Wang, A.; Jiang, H. Palladium-catalyzed direct oxidation of alkenes with molecular oxygen: General and practical methods for the preparation of 1,2-diols, aldehydes, and ketones. J. Org. Chem. 2010, 75, 2321–8282. [Google Scholar] [CrossRef]
- Wu, X.-F.; Schranck, J.; Neumann, H.; Beller, M. Convenient and mild synthesis of nitroarenes by metal-free nitration of arylboronic acids. Chem. Commun. 2011, 47, 12462–12463. [Google Scholar] [CrossRef]
- Wu, X.-F.; Sharif, M.; Feng, J.-B.; Neumann, H.; Pews-Davtyan, A.; Langer, P.; Beller, M. A general and practical oxidation of alcohols to primary amides under metal-free conditions. Green Chem. 2013, 15, 1956–1961. [Google Scholar] [CrossRef]
- Wu, X.-F.; Oschatz, S.; Block, A.; Spannenberg, A.; Langer, P. Base mediated synthesis of 2-aryl-2,3-dihydroquinazolin-4(1H)-ones from 2-aminobenzonitriles and aromatic aldehydes in water. Org. Biomol. Chem. 2014, 12, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- EI-Hashash, M.; Salem, M.S.; Al-Mabrook, S. Synthesis and anticancer activity of novel quinazolinone and benzamide derivatives. Res. Chem. Intermed. 2018, 44, 2545–2559. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Rees, C.W. The Structure, Reaction, Synthesis and Uses of Heterocyclic Compounds, Comprehensive Heterocyclic Chemistry; Pergamon Press: New York, NY, USA, 1984. [Google Scholar]
- Pelletier, S.W. Alkaloids: Chemical and Biological Prospective; John Wiley & Sons Ltd.: New York, NY, USA, 1983. [Google Scholar]
- Ramesh, V.; Rao, B.; Sharma, P.; Swarna, B.; Thummuri, D.; Srinivas, K.; Naidu, V.; Rao, V. Synthesis and biological evaluation of new rhodanine analogues bearing 2-chloroquinoline and benzo [h] quinoline scaffolds as anticancer agents. Eur. J. Med. Chem. 2014, 83, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Noolvi, M.N.; Patel, H.M.; Bhardwaj, V.; Chauhan, A. Synthesis and in vitro antitumor activity of substituted quinazoline and quinoxaline derivatives: Search for anticancer agent. Eur. J. Med. Chem. 2011, 46, 2327–2346. [Google Scholar] [CrossRef]
- Samira, I.; Hasmin, M.; Patel, S. Biological profile of quinazoline. Int. J. Pharm. Chem. Sci. 2012, 1, 1863–1872. [Google Scholar]
- Singh, V.K.; Singh, S.K.; Gangwar, L. Synthesis and antimicrobial activity of novel fused 4-(3H) quinazolinone derivatives. Int. J. Sci. Res. 2013, 2, 425–428. [Google Scholar]
- Ghorab, M.M.; Ismail, Z.; Radwan, A.A.; Abdalla, M. Synthesis and pharmacophore modeling of novel quinazolines bearing a biologically active sulfonamide moiety. Acta Pharm. 2013, 63, 1–18. [Google Scholar] [CrossRef]
- Ighachane, H.; Sedra, M.H.; Lazrek, H. Synthesis and evaluation of antifungal activities of (3H)-quinazolin-4-one derivatives against tree plant fungi. J. Mater. Environ. Sci. 2017, 8, 134–143. [Google Scholar]
- Vijayakumar, K.; Ahamed, A.J.; Thiruneelakandan, G. Synthesis, antimicrobial, and anti-HIV1 activity of quinazoline-4(3H)-one derivatives. J. Appl. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Deep, A.; Narasimhan, B.; Ramasamy, K.; Mani, V.; Mishra, R.K.; Majeed, A.B. Synthesis, antimicrobial, anticancer evaluation and QSAR studies of thiazolidin-4-ones clubbed with quinazolinone. Curr. Top. Med. Chem. 2013, 13, 2034–2046. [Google Scholar] [CrossRef][Green Version]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Shamel, M.; Satar, M.; Khalid, Y.; Mohamad, A.B. Antioxidant and antimicrobial activities of novel quinazolinones. Med. Chem. Res. 2014, 23, 236–242. [Google Scholar] [CrossRef]
- Jafari, E.; Khajouei, M.R.; Hassanzadeh, F.; Hakimelahi, G.H.; Khodarahmi, G.A. Quinazolinone and quinazoline derivatives: Recent structures with potent antimicrobial and cytotoxic activities. Res. Pharm. Sci. 2016, 11, 1–14. [Google Scholar] [PubMed]
- Jiang, S.; Zeng, Q.; Gettayacamin, M.; Tungtaeng, A.; Wannaying, S.; Lim, A. Antimalaria activities and therapeutic properties of febrifugine analogs. Antimicrob. Agents Chemoter. 2005, 49, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Deetz, M.J.; Malerich, J.P.; Beatty, A.M.; Smith, B.D. One-step synthesis of 4(3H) quinazolinones. Tetrahedron Lett. 2001, 42, 1851–1854. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Ganguly, S.; Veeramy, R.; Jan, B. Synthesis, antiviral and cytotoxic investigation of 2-phenyl-3-substituted quinazolin-4(3H)-ones. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 673–681. [Google Scholar] [PubMed]
- He, L.; Li, H.; Chen, J.; Wu, X.-F. Recent advances in 4(3H)-quinazolinone syntheses. RSC Adv. 2014, 4, 12065–12077. [Google Scholar] [CrossRef]
- Yousefi, B.; Azimi, A.; Majidinia, M.; Shafiei-Irannejad, V.; Badalzadeh, R.; Baradaran, B. Balaglitazone reverses P-glycoproteinmediated multidrug resistance via upregulation of PTEN in a PPARγdependent manner in leukemia cells. Tumor Biol. 2017, 39, 1–11. [Google Scholar] [CrossRef]
- Potewar, T.M.; Nadaf, R.N.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. A novel one-pot synthesis of 2-aryl-4(3H)-quinazolinones using room temperature ionic liquid as reaction medium as well as promoter. Synth. Commun. 2005, 35, 231–241. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Fei, Z.; Liu, M.-C.; Jia, F.-C.; Wu, A.-X. Direct one-pot synthesis of Luotonin F and analogues via rational logical design. Org. Lett. 2013, 15, 378–381. [Google Scholar] [CrossRef]
- Watson, A.J.A.; Maxwell, A.C.; Williams, J.M. Ruthenium-catalysed oxidative synthesis of heterocycles from alcohols. J. Org. Biomol. Chem. 2012, 12, 240–243. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, J. One-pot synthesis of quinazolinones via iridium-catalyzed hydrogen transfers. J. Org. Chem. 2011, 76, 7730–7736. [Google Scholar] [CrossRef] [PubMed]
- Hikawa, H.; Ino, Y.; Suzuki, H.; Yokoyama, Y. Pd-catalyzed benzylic C–H amidation with benzyl alcohols in water: A strategy to construct quinazolinones. J. Org. Chem. 2012, 77, 7046–7051. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Zhu, X.; Wei, Y. Iodine-catalyzed oxidative system for cyclization of primary alcohols with o-aminobenzamides to quinazolinones using DMSO as the oxidant in dimethyl carbonate. RSC Adv. 2013, 3, 10817–10822. [Google Scholar] [CrossRef]
- Mohammed, S.; Vishwakarma, R.A.; Bharate, S.B. Iodine catalyzed oxidative synthesis of quinazolin-4(3H)-ones and pyrazolo[4,3-d]pyrimidin-7(6H)-ones via amination of sp3 C-H bond. J. Org. Chem. 2015, 80, 6915–6921. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Dong, J.Y.; Chen, X.L.; Li, Q.; Zhou, Y.B.; Yin, S.-F. Metal- and oxidant-Free synthesis of quinazolinones from β-Ketoesters with o-aminobenzamides via phosphorous acid-catalyzed cyclocondensation and selective C–C bond cleavage. J. Org. Chem. 2015, 80, 9392–9400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, T.; Li, J.-X. Metal-free oxidative synthesis of quinazolinones via dual amination of sp3 C-H bonds. Chem. Commun. 2014, 50, 6471–6474. [Google Scholar] [CrossRef]
- Majumdar, B.; Mandani, S.; Bhattacharya, T.; Sarma, D.; Sarma, T.K. Probing carbocatalytic activity of carbon nanodots for the synthesis of biologically active dihydro/spiro/glyco quinazolinones and aza-michael adducts. J. Org. Chem. 2017, 82, 2097–2106. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Y.; Peng, J.L.; Zhu, Q. Synthesis of quinazolin-4(3H)-ones via Pd(II)-catalyzed intramolecular C(sp2)–H carboxamidation of N-arylamidines. J. Org. Chem. 2011, 76, 6362–6366. [Google Scholar] [CrossRef]
- Sadig, J.E.R.; Foster, R.; Wakenhut, F.; Willis, M.C. Palladium-catalyzed synthesis of benzimidazoles and quinazolinones from common precursors. J. Org. Chem. 2012, 77, 9473–9486. [Google Scholar] [CrossRef]
- Jia, F.-C.; Zhou, Z.-W.; Xu, C.; Wu, Y.-D.; Wu, A.-X. Divergent synthesis of quinazolin-4(3H)-ones and tryptanthrins enabled by a tert-butyl hydroperoxide/K3PO4-promoted oxidative cyclization of isatins at room temperature. Org. Lett. 2016, 18, 2942–2945. [Google Scholar] [CrossRef]
- Zhou, R.; Qi, X.; Wu, X.-F. Selenium-catalyzed carbonylative synthesis of 3,4-dihydroquinazolin-2(1H)-one derivatives with TFBen as the CO source. ACS Comb. Sci. 2019, 21, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-B.; Chen, B.; Qi, X.; Ying, J.; Wu, X.-F. Palladium-catalyzed synthesis of quinolin-2(1H)-ones: The unexpected reactivity of azodicarboxylate. Org. Biomol. Chem. 2018, 16, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-B.; Geng, H.-Q.; Wang, W.; Qi, X.; Ying, J.; Wu, X.-F. Palladium-catalyzed four-component carbonylative synthesis of 2,3-disubstituted quinazolin-4(3H)-ones: Convenient methaqualone preparation. J. Catal. 2018, 365, 10–13. [Google Scholar] [CrossRef]
- Wu, X.-F.; Oschatz, S.; Sharif, M.; Beller, M.; Langer, P. Palladium-catalyzed carbonylative synthesis of N-(2-cyanoaryl)benzamides and sequential synthesis of quinazolinones. Tetrahedron 2014, 70, 23–29. [Google Scholar] [CrossRef]
- He, L.; Sharif, M.; Neumann, H.; Beller, M.; Wu, X.-F. A convenient palladium-catalyzed carbonylative synthesis of 4(3H)-quinazolinones from 2-bromoformanilides and organo nitros with Mo(CO)6 as a multiple promoter. Green Chem. 2014, 16, 3763–3767. [Google Scholar] [CrossRef]
- Liu, W.; Wu, G.; Gao, W.X.; Ding, J.C.; Huang, X.B.; Liu, M.C.; Wu, H.Y. Palladium-catalyzed oxidative C=C bond cleavage with molecular oxygen: One-pot synthesis of quinazolinones from 2-amino benzamides and alkenes. Org. Chem. Front. 2018, 5, 2734–2738. [Google Scholar] [CrossRef]
- An, J.H.; Wang, Y.H.; Zhang, Z.X.; Zhao, Z.T.; Zhang, J.; Wang, F. The Synthesis of Quinazolinones from Olefins, CO, and Amines over aHeterogeneous Ru-clusters/Ceria Catalyst. Angew. Chem. Int. Ed. 2018, 57, 12308–12312. [Google Scholar] [CrossRef]
- Abdullaha, M.; Shabber, M.; Ali, A.; Kumar, A.; Vishwakarma, R.A.; Bharate, S.B. Discovery of quinazolin-4(3h)-ones as nlrp3 inflammasome inhibitors: Computational design, metal-free synthesis, and in vitro biological evaluation. J. Org. Chem. 2019, 84, 5129–5140. [Google Scholar] [CrossRef]
- Teng, Q.-H.; Sun, Y.; Yao, Y.; Tang, H.-T.; Li, J.-R.; Pan, Y.-M. Metal- and catalyst-free electrochemical synthesis of quinazolinones from alkenes and 2-aminobenzamides. ChemElectroChem 2019, 6, 3120–3124. [Google Scholar] [CrossRef]
- Sharif, M.; Opalach, J.; Jackstell, R.; Beller, M. Synthesis of pharmacologically relevant new derivatives of maleimides via ligand-free Pd-catalyzed suzuki–miyaura cross-coupling reactions. Arab. J. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Sharif, M.; Wu, X.-F. Palladium-catalyzed synthesis of primary benzamides from aryl bromides via a cyanation and hydration sequence. RSC Adv. 2015, 5, 21001–21004. [Google Scholar] [CrossRef]
- Sharif, M.; Feng, J.-B.; langer, P.; Beller, M.; Wu, X.-F. A novel oxidative procedure for the synthesis of benzamides from styrenes and amines under metal-free conditions. Chem. Commun. 2014, 50, 4747–4750. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.; Opalach, J.; Langer, P.; Beller, M.; Wu, X.-F. Oxidative synthesis of quinazolinones and benzothiadiazine-1,1-dioxides from 2-aminobenzamide and 2-aminobenzenesulfonamide with benzyl alcohols and aldehydes. RSC Adv. 2014, 4, 8–17. [Google Scholar] [CrossRef]
- Close, W.J.; Swett, R.L.; Bready, L.E.; Short, J.H.; Vernsten, M. Synthesis of potential diuretic agents. I. Derivatives of 7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide. J. Am. Chem. Soc. 1960, 82, 1132–1135. [Google Scholar] [CrossRef]
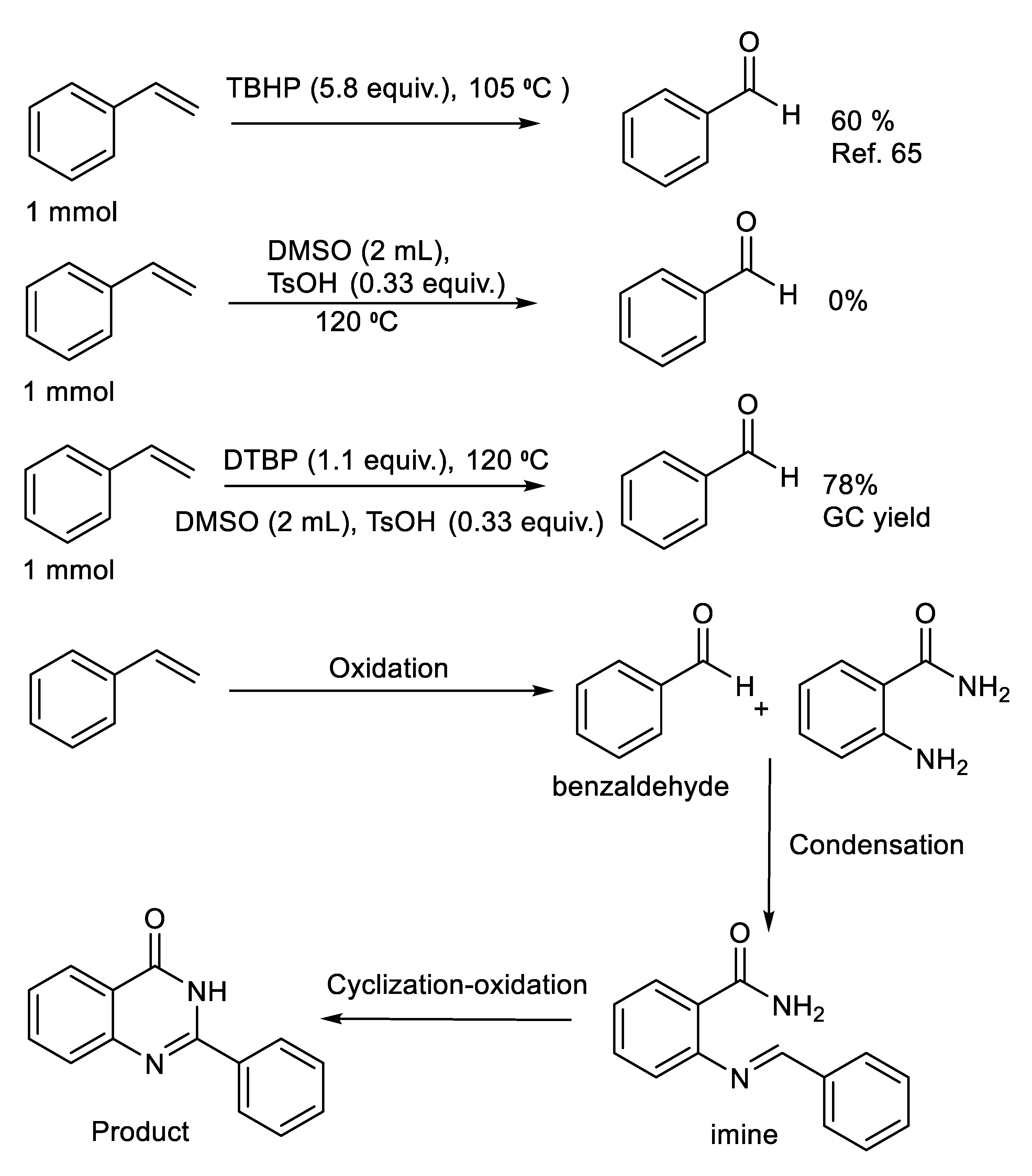
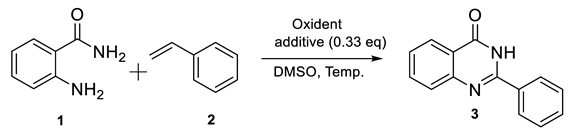
| Entry | Oxidant (Equiv) e | Additive | Temp., Time | Solvent | Yield % b |
|---|---|---|---|---|---|
| 1 | TBHP (5.8) | - | 105 °C | - | 56 |
| 2 | TBHP (5.8) | - | 105 °C | H2O | 30 |
| 3 | TBHP (5.8) | - | 105 °C | DMSO | 40 |
| 4 | TBHP (1.5) | - | 80 °C, 24 h | - | 20 |
| 5 | TBHP (1.5) | - | 120 °C, 24 h | - | 30 |
| 6 | DTBP (0.54) | - | 110 °C | DMSO | 35 |
| 7 | DTBP (0.54) | p-TsOH | 110 °C | DMSO | 58 |
| 8 | DTBP (1.09) | p-TsOH | 120 °C | DMSO | 70 |
| 9 | DTBP (0.8) | p-TsOH | 105 °C | DMSO | 45 |
| 10 | DTBP (1.09) | p-TsOH | 105 °C | DMSO | 50 |
| 11 | DTBP (1.4) | p-TsOH | 105 °C | DMSO | 14 |
| 12 | DTBP (1.6) | p-TsOH | 105 °C | DMSO | 23 |
| 13 | DTBP (2.1) | p-TsOH | 105 °C | DMSO | 65 |
| 14 | - | - | 120 °C | DMSO | 0 |
| 15 | - | p-TsOH | 120 °C | DMSO | 20 |
| 16 c | DTBP (1.4) | p-TsOH | 120 °C | CH3CN | 60 |
| 17 d | DTBP (1.09) | p-TsOH | 120 °C | DMSO | 50 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, M. Quinazolin-4(3H)-ones: A Tangible Synthesis Protocol via an Oxidative Olefin Bond Cleavage Using Metal-Catalyst Free Conditions. Appl. Sci. 2020, 10, 2815. https://doi.org/10.3390/app10082815
Sharif M. Quinazolin-4(3H)-ones: A Tangible Synthesis Protocol via an Oxidative Olefin Bond Cleavage Using Metal-Catalyst Free Conditions. Applied Sciences. 2020; 10(8):2815. https://doi.org/10.3390/app10082815
Chicago/Turabian StyleSharif, Muhammad. 2020. "Quinazolin-4(3H)-ones: A Tangible Synthesis Protocol via an Oxidative Olefin Bond Cleavage Using Metal-Catalyst Free Conditions" Applied Sciences 10, no. 8: 2815. https://doi.org/10.3390/app10082815
APA StyleSharif, M. (2020). Quinazolin-4(3H)-ones: A Tangible Synthesis Protocol via an Oxidative Olefin Bond Cleavage Using Metal-Catalyst Free Conditions. Applied Sciences, 10(8), 2815. https://doi.org/10.3390/app10082815





