Foams in Wastewater Treatment Plants: From Causes to Control Methods
Abstract
1. Introduction
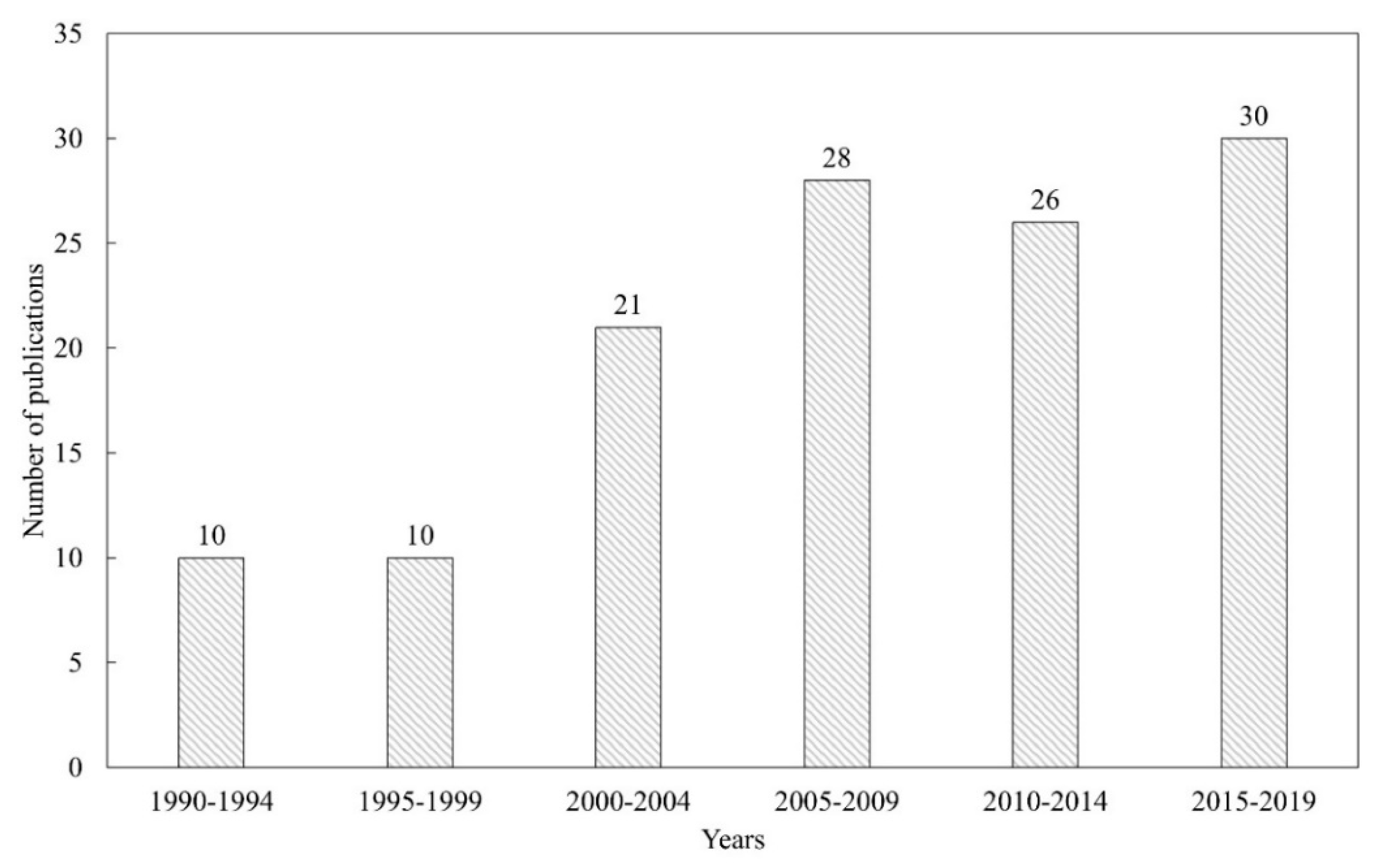
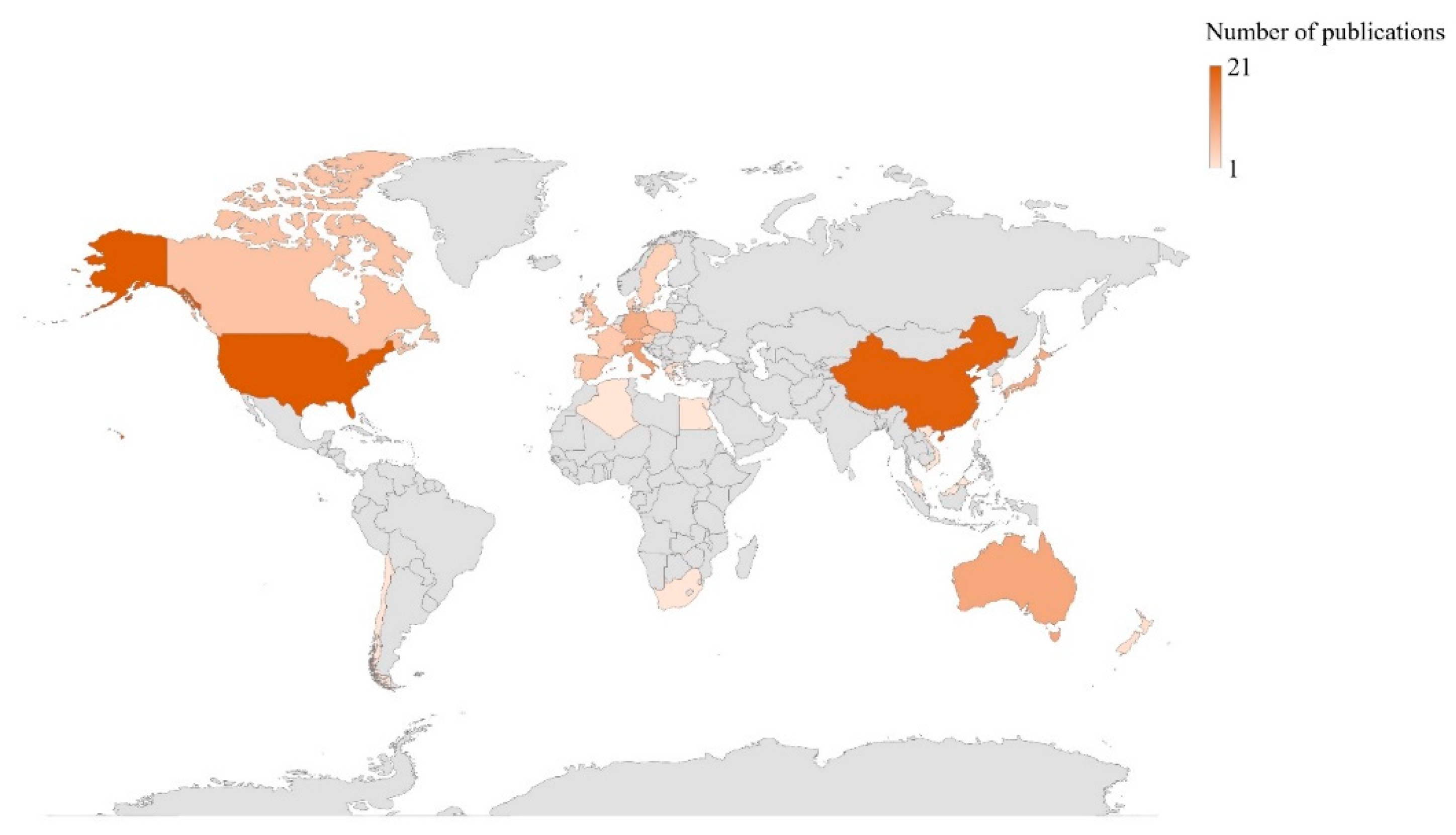
2. Bibliometric Research and Structure
3. Causes
4. Evaluation Method
4.1. Direct Methods
4.1.1. Foam Rating (FR)
4.1.2. Volume
- Shaking: a 100 mL graduated cylinder containing 15 mL of sample for 10 s was shaken vigorously. Foaming was recorded as the volume of foams produced immediately after shaking.
- Air bubbling: an apparatus consisting of a 50 cm high quartz column with a diameter of 3.5 cm and a 0.25 cm capillary on the bottom, was used to generate bubbles. Fifty milliliters of sample were poured into the column using a long funnel that reached the bottom in order to ensure that the cylinder walls remained dry (in this way the matrix was only on the bottom and not on the walls). The airflow was constant at a flow rate of 158.2 mL min−1. The foam volume produced after 2 min was recorded.
4.1.3. Foam Power (FP)
4.1.4. Foam Stability (FS)
4.1.5. Scum Index (SI)
4.1.6. Foaming Scum Index (FSI)
4.1.7. Methods Applicable on Site
Foam Surface Covered (FSC)
Foam Volume (FV)
4.2. Indirect Methods
4.2.1. Surface Tension
4.2.2. EPS Concentration
Heating Method
Steaming Extraction Method
India Ink Reverse Stain Method
4.2.3. Hydrophobicity
4.2.4. Viscosity
4.2.5. Filamentous Bacteria
5. Foam Control Methods
5.1. Physical Methods
5.2. Chemical Methods
6. Discussion and Future Outlooks
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| AD | Anaerobic digester |
| CAS | Conventional activated sludge |
| CSH | Cell surface hydrophobicity |
| EPS | Extracellular polymeric substances |
| EPSb | Bound extracellular polymeric substances |
| EPSs | Soluble extracellular polymeric substances |
| EPSp | Protein fraction of bound extracellular polymeric substances |
| EPSc | Carbohydrate fraction of bound extracellular polymeric substances |
| FP | Foaming power index calculated as consumed sample per liter of supplied air |
| FP2 | Foaming power index calculates as foam volume produced per liter of supplied air |
| FR | Foam rating |
| FS | Foam stability |
| FSI | Foaming scum index |
| FV | Foam volume |
| FSC | Foam surface covered |
| MBR | Membrane bioreactor |
| NIN | Normalized intersections number |
| SDS | Sodium dodecyl sulphate |
| SEM | Structural equation modeling |
| SI | Scum index |
| SMP | Soluble microbial product |
| SMPp | Protein fraction of soluble microbial product |
| SMPc | Carbohydrate fraction of soluble microbial product |
| SS | Suspended solids |
| THM | Trihalomethane |
| VSS | Volatile suspended solids |
| WWTP | Wastewater treatment plant |
References
- Kiselev, A.V.; Magaril, E.R.; Rada, E.C. Energy and sustainability assessment of municipal wastewater treatment under circular economy paradigm. WIT Trans. Ecol. Environ. 2019, 237, 109–120. [Google Scholar]
- Capodici, M.; Di Bella, G.; Nicosia, S.; Torregrossa, M. Effect of chemical and biological surfactants on activated sludge of MBR system: Microscopic analysis and foam test. Bioresour. Technol. 2014, 177, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wang, Z.P.; Yu, K.; Zhang, T. Detailed investigation of the microbial community in foaming activated sludge reveals novel foam formers. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R.J.; Pickering, R.L.; Goodhead, A.K.; Curtis, T.P. A universal threshold concept for hydrophobic mycolata in activated sludge foaming. Water Res. 2008, 42, 3446–3454. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Richard, M.G.; Daigger, G.T. Manual on the Causes and Control of Activated Sludge Bulking, Foaming, and Other Solids Separation Problems, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2003; ISBN 1-849-046-9. [Google Scholar]
- Pitt, P.; Jenkins, D. Causes and control of Nocardia in activated sludge. Res. J. Water Pollut. Control Fed. 1990, 62, 143–150. [Google Scholar]
- Seviour, E.M.; Williams, C.J.; Seviour, R.J.; Soddell, J.A.; Lindrea, K.C. A survey of filamentous bacterial populations from foaming activated sludge plants in Eastern States of Australia. Water Res. 1990, 24, 493–498. [Google Scholar] [CrossRef]
- Seviour, E.M.; Williams, C.; DeGrey, B.; Soddell, J.A.; Seviour, R.J.; Lindrea, K.C. Studies on filamentous bacteria from australian activated sludge plants. Water Res. 1994, 28, 2335–2342. [Google Scholar] [CrossRef]
- Soddell, J.A.; Seviour, R.J. Microbiology of foaming in activated sludge plants. J. Appl. Bacteriol. 1990, 69, 145–176. [Google Scholar] [CrossRef]
- Pujol, R.; Duchene, P.; Schetrite, S.; Canler, J.P. Biological foams in activated sludge plants: Characterization and situation. Water Res. 1991, 25, 1399–1404. [Google Scholar] [CrossRef]
- Wanner, J.; Ruzicková, I.; Jetmarová, P.; Krhutková, O.; Paraniaková, J. A national survey of activated sludge separation problems in the Czech Republic: Filaments, floc characteristics and activated sludge metabolic properties. Water Sci. Technol. 1998, 37, 271–279. [Google Scholar] [CrossRef]
- Heard, J.; Harvey, E.; Johnson, B.B.; Wells, J.D.; Angove, M.J. The effect of filamentous bacteria on foam production and stability. Colloids Surfaces B Biointerfaces 2008, 63, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Blackall, L.L.; Harbers, A.E.; Hayward, A.C.; Greenfield, P.F. Activated sludge foams: Effects of environmental variables on organism growth and foam formation. Environ. Technol. (United Kingdom) 1991, 12, 241–248. [Google Scholar] [CrossRef]
- Schilling, K.; Zessner, M. Foam in the aquatic environment. Water Res. 2011, 45, 4355–4366. [Google Scholar] [CrossRef] [PubMed]
- Moeller, L.; Zehnsdorf, A.; Pokorná, D.; Zábranská, J. Foam Formation in Anaerobic Digesters. Adv. Bioenergy 2018, 3, 1–42. [Google Scholar]
- Hug, T. Characterization and Controlling of Foam and Scum in Activated Sludge Systems. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2006. [Google Scholar]
- Joss, A.; Salzgeber, D.; Eugster, J.; König, R.; Rottermann, K.; Burger, S.; Fabijan, P.; Leumann, S.; Mohn, J.; Siegrist, H.R. Full-scale nitrogen removal from digester liquid with partial nitritation and anammox in one SBR. Environ. Sci. Technol. 2009, 43, 5301–5306. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.Q.; Sun, N.; Yang, H.; Zhang, J.; Ngo, H.H. Distribution of extracellular polymeric substances in anammox granules and their important roles during anammox granulation. Biochem. Eng. J. 2015, 101, 126–133. [Google Scholar] [CrossRef]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C.M. Full-scale partial nitritation/anammox experiences—An application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Fryer, M.; Eoghan, O.F.; Gray, N.F. Evaluating the Measurement of Activated Sludge Foam Potential. Water 2011, 3, 424–444. [Google Scholar] [CrossRef]
- Di Bella, G.; Torregrossa, M. Foaming in membrane bioreactors: Identification of the causes. J. Environ. Manag. 2013, 128, 453–461. [Google Scholar] [CrossRef]
- Nakajima, J.; Mishima, I. Measurement of foam quality of activated sludge in MBR process. Acta Hydrochim. Hydrobiol. 2005, 33, 232–239. [Google Scholar] [CrossRef]
- Di Bella, G.; Torregrossa, M.; Viviani, G. The role of EPS concentration in MBR foaming: Analysis of a submerged pilot plant. Bioresour. Technol. 2011, 102, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Durante, F.; Torregrossa, M.; Viviani, G. Start-up with or without inoculum? Analysis of an SMBR pilot plant. Desalination 2010, 260, 79–90. [Google Scholar] [CrossRef]
- Mannina, G.; Di Bella, G. Comparing two start-up strategies for MBRs: Experimental study and mathematical modelling. Biochem. Eng. J. 2012, 68, 91–103. [Google Scholar] [CrossRef]
- Subramanian, B.; Pagilla, K.R. Mechanisms of foam formation in anaerobic digesters. Colloids Surf. B Biointerfaces 2015, 126, 621–630. [Google Scholar] [CrossRef]
- Jiang, C.; Qi, R.; Hao, L.; McIlroy, S.J.; Nielsen, P.H. Monitoring foaming potential in anaerobic digesters. Waste Manag. 2018, 75, 280–288. [Google Scholar] [CrossRef]
- Moeller, L.; Eismann, F.; Wißmann, D.; Nägele, H.J.; Zielonka, S.; Müller, R.A.; Zehnsdorf, A. Innovative test method for the estimation of the foaming tendency of substrates for biogas plants. Waste Manag. 2015, 41, 39–49. [Google Scholar] [CrossRef]
- Westlund, Å.D.; Hagland, E.; Rothman, M. Operational aspects on foaming in digesters caused by microthrix parvicella. Water Sci. Technol. 1998, 38, 29–34. [Google Scholar] [CrossRef]
- Ganidi, N.; Tyrrel, S.; Cartmell, E. Anaerobic digestion foaming causes—A review. Bioresour. Technol. 2009, 100, 5546–5554. [Google Scholar] [CrossRef]
- Sorlini, S.; Collivignarelli, M.C.; Castagnola, F.; Crotti, B.M.; Raboni, M. Methodological approach for the optimization of drinking water treatment plants’ operation: A case study. Water Sci. Technol. 2015, 71, 597–604. [Google Scholar] [CrossRef]
- Sorlini, S.; Collivignarelli, M.C.; Carnevale Miino, M. Technologies for the control of emerging contaminants in drinking water treatment plants. Environ. Eng. Manag. J. 2019, 18, 2203–2216. [Google Scholar]
- Collivignarelli, M.C.; Abbà, A.; Bestetti, M.; Crotti, B.M.; Carnevale Miino, M. Electrolytic Recovery of Nickel and Copper from Acid Pickling Solutions Used to Treat Metal Surfaces. Water. Air. Soil Pollut. 2019, 230, 101. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Arab, H.; Bestetti, M.; Franz, S. Decolorization and biodegradability of a real pharmaceutical wastewater treated by H2O2-assisted photoelectrocatalysis on TiO2 meshes. J. Hazard. Mater. 2019, 387, 121668. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Carnevale Miino, M.; Baldi, M.; Manzi, S.; Abbà, A.; Bertanza, G. Removal of non-ionic and anionic surfactants from real laundry wastewater by means of a full-scale treatment system. Process Saf. Environ. Prot. 2019, 132, 105–115. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Canato, M.; Abbà, A.; Carnevale Miino, M. Biosolids: What are the different types of reuse? J. Clean. Prod. 2019, 238. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Miino, M.C.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legislation for the reuse of biosolids on agricultural land in Europe: Overview. Sustain. 2019, 11, 6015. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Castagnola, F.; Sordi, M.; Bertanza, G. Sewage sludge treatment in a thermophilic membrane reactor (TMR): Factors affecting foam formation. Environ. Sci. Pollut. Res. 2017, 24, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Aloui, F.; Kchaou, S.; Sayadi, S. Physicochemical treatments of anionic surfactants wastewater: Effect on aerobic biodegradability. J. Hazard. Mater. 2009, 164, 353–359. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, M.I.; Sánchez-Polo, M.; Méndez-Díaz, J.D. Removal of surfactant dodecylbenzenesulfonate by consecutive use of ozonation and biodegradation. Eng. Life Sci. 2012, 12, 113–116. [Google Scholar] [CrossRef]
- Jardak, K.; Drogui, P.; Daghrir, R. Surfactants in aquatic and terrestrial environment: Occurrence, behavior, and treatment processes. Environ. Sci. Pollut. Res. 2016, 23, 3195–3216. [Google Scholar] [CrossRef]
- Malysa, K.; Krasowska, M.; Krzan, M. Influence of surface active substances on bubble motion and collision with various interfaces. Adv. Colloid Interface Sci. 2005, 114–115, 205–225. [Google Scholar] [CrossRef]
- Davenport, R.J.; Curtis, T.P.; Goodfellow, M.; Stainsby, F.M.; Bingley, M. Quantitative use of fluorescent in situ hybridization to examine relationships between mycolic acid-containing actinomycetes and foaming in activated sludge plants. Appl. Environ. Microbiol. 2000, 66, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- De Los Reyes, F.L.; Raskin, L. Role of filamentous microorganisms in activated sludge foaming: Relationship of mycolata levels to foaming initiation and stability. Water Res. 2002, 36, 445–459. [Google Scholar] [CrossRef]
- Madoni, P.; Davoli, D.; Gibin, G. Survey of filamentous microorganisms from bulking and foaming activated- sludge plants in Italy. Water Res. 2000, 34, 1767–1772. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, K.; Taki, H.; Miyata, N.; Fujita, M. Analysis of Nocardia amarae profiles in actual foaming activated sludge plant with viable cell count measurement. J. Ferment. Bioeng. 1997, 84, 98–102. [Google Scholar] [CrossRef]
- Petrovski, S.; Dyson, Z.A.; Quill, E.S.; McIlroy, S.J.; Tillett, D.; Seviour, R.J. An examination of the mechanisms for stable foam formation in activated sludge systems. Water Res. 2011, 45, 2146–2154. [Google Scholar] [CrossRef]
- Menniti, A.; Morgenroth, E. The influence of aeration intensity on predation and EPS production in membrane bioreactors. Water Res. 2010, 44, 2541–2553. [Google Scholar] [CrossRef]
- Laspidou, C.S.; Rittmann, B.E. A unified theory for extracellular polymeric substances, soluble microbial products, and active and inert biomass. Water Res. 2002, 36, 2711–2720. [Google Scholar] [CrossRef]
- Dvořák, L.; Gómez, M.; Dvořáková, M.; Růžičková, I.; Wanner, J. The impact of different operating conditions on membrane fouling and EPS production. Bioresour. Technol. 2011, 102, 6870–6875. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, W.Y.; Lee, C.H. Comparison of the filtration characteristics between attached and suspended growth microorganisms in submerged membrane bioreactor. Water Res. 2001, 35, 2435–2445. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, H.; Yang, F.; Li, Y.; Xiao, J.; Zhang, X. Effect of filamentous bacteria on membrane fouling in submerged membrane bioreactor. J. Memb. Sci. 2006, 272, 161–168. [Google Scholar] [CrossRef]
- You, S.J.; Sue, W.M. Filamentous bacteria in a foaming membrane bioreactor. J. Memb. Sci. 2009, 342, 42–49. [Google Scholar] [CrossRef]
- Oerther, D.B.; De Los Reyes, F.L.; De Los Reyes, M.F.; Raskin, L. Quantifying filamentous microorganisms in activated sludge before, during, and after an incident of foaming by oligonucleotide probe hybridizations and antibody staining. Water Res. 2001, 35, 3325–3336. [Google Scholar] [CrossRef]
- Xie, B.; Dai, X.C.; Xu, Y.T. Cause and pre-alarm control of bulking and foaming by Microthrix parvicella—A case study in triple oxidation ditch at a wastewater treatment plant. J. Hazard. Mater. 2007, 143, 184–191. [Google Scholar] [CrossRef]
- Kragelund, C.; Remesova, Z.; Nielsen, J.L.; Thomsen, T.R.; Eales, K.; Seviour, R.; Wanner, J.; Nielsen, P.H. Ecophysiology of mycolic acid-containing Actinobacteria (Mycolata) in activated sludge foams. FEMS Microbiol. Ecol. 2007, 61, 174–184. [Google Scholar] [CrossRef]
- Blackall, L.L.; Stratton, H.; Bradford, D.; Del Dot, T.; Sjörup, C.; Seviour, E.M.; Seviour, R.J. “Candidatus microthrix parvicella,” a filamentous bacterium from activated sludge sewage treatment plants. Int. J. Syst. Bacteriol. 1996, 46, 344–346. [Google Scholar] [CrossRef]
- Chun, J.; Blackall, L.L.; Kang, S.A.O.; Hah, Y.C.; Goodfellow, M. A proposal to reclassify Nocardia pinensis Blackall et al. as Skermania piniformis gen. nov., comb. nov. Int. J. Syst. Bacteriol. 1997, 47, 127–131. [Google Scholar] [CrossRef]
- Soddell, J.A.; Seviour, R.J. Numerical taxonomy of Skermania piniformis and related isolates from activated sludge. J. Appl. Microbiol. 1998, 84, 272–284. [Google Scholar] [CrossRef]
- Frigon, D.; Michael Guthrie, R.; Timothy Bachman, G.; Royer, J.; Bailey, B.; Raskin, L. Long-term analysis of a full-scale activated sludge wastewater treatment system exhibiting seasonal biological foaming. Water Res. 2006, 40, 990–1008. [Google Scholar] [CrossRef]
- Fryer, M.; Gray, N.F. Foaming Scum Index (FSI)—A new tool for the assessment and characterisation of biological mediated activated sludge foams. J. Environ. Manage. 2012, 110, 8–19. [Google Scholar] [CrossRef]
- Fan, N.; Wang, R.; Qi, R.; Gao, Y.; Rossetti, S.; Tandoi, V.; Yang, M. Control strategy for filamentous sludge bulking: Bench-scale test and full-scale application. Chemosphere 2018, 210, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.H. Fouling characteristics in pure oxygen MBR process according to MLSS concentrations and COD loadings. J. Memb. Sci. 2013, 428, 323–330. [Google Scholar] [CrossRef]
- Yamato, N.; Kimura, K.; Miyoshi, T.; Watanabe, Y. Difference in membrane fouling in membrane bioreactors (MBRs) caused by membrane polymer materials. J. Memb. Sci. 2006, 280, 911–919. [Google Scholar] [CrossRef]
- Wu, B.; Yi, S.; Fane, A.G. Microbial behaviors involved in cake fouling in membrane bioreactors under different solids retention times. Bioresour. Technol. 2011, 102, 2511–2516. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.J.; Judd, C. The MBR Book: Principles and Applications of Membrane Bioreactors. In Water and Wastewater Treatment, 2nd ed.; Elsevier: London, UK, 2010. [Google Scholar]
- Guitián, J.; Joseph, D. Foaminess Measurements Using A Shaker Bottle; University of Minnesota: Minneapolis, MN, USA, 1996. [Google Scholar]
- Bikerman, J.J. Unit of foaminess. Trans. Faraday Soc. 1938, 34, 634–638. [Google Scholar] [CrossRef]
- Blackall, L.L.; Marshall, K.C. The mechanism of stabilization of actinomycete foams and the prevention of foaming under laboratory conditions. J. Ind. Microbiol. 1989. [Google Scholar] [CrossRef]
- Stratton, H.M.; Brooks, P.R.; Griffiths, P.C.; Seviour, R.J. Cell surface hydrophobicity and mycolic acid composition of Rhodococcus strains isolated from activated sludge foam. J. Ind. Microbiol. Biotechnol. 2002, 28, 264–267. [Google Scholar] [CrossRef]
- Patist, A.; Huibers, P.D.T.; Deneka, B.; Shah, D.O. Effect of tetraalkylammonium chlorides on foaming properties of sodium dodecyl sulfate solutions. Langmuir 1998, 14, 4471–4474. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, R.; Xu, Z. Experimental study of foaming agent screening and performance evaluation of nitrogen foam flooding in daqing oilfield. Adv. Mater. Res. 2012, 550–553, 2873–2877. [Google Scholar] [CrossRef]
- Kato, A.; Takahashi, A.; Matsudomi, N.; Kobayashi, K. Determination of Foaming Properties of Proteins by Conductivity Measurements. J. Food Sci. 1983, 48, 62–65. [Google Scholar] [CrossRef]
- Constant, M. A practical method for characterizing poured beer foam quality. J. Am. Soc. Brew. Chem. 1992, 50, 37–47. [Google Scholar] [CrossRef]
- Baniel, A.; Fains, A.; Popineau, Y. Foaming properties of egg albumen with a bubbling apparatus compared with whipping. J. Food Sci. 1997, 62, 377–381. [Google Scholar] [CrossRef]
- Pretorius, W.A.; Laubscher, C.J.P. Control of biological scum in activated sludge plants by means of selective flotation. Water Sci. Technol. 1987, 19, 1003–1011. [Google Scholar] [CrossRef]
- Torregrossa, M.; Viviani, G.; Vinci, V. Foaming estimation tests in activated sludge systems. Acta Hydrochim. Hydrobiol. 2005, 33, 240–246. [Google Scholar] [CrossRef]
- Kocianova, E.; Foot, R.J.; Forster, C.F. Physicochemical Aspects of Activated Sludge in Relation to Stable Foam Formation. Water Environ. J. 1992, 6, 342–350. [Google Scholar] [CrossRef]
- Vardar-Sukan, F. Foaming: Consequences, prevention and destruction. Biotechnol. Adv. 1998, 16, 913–948. [Google Scholar] [CrossRef]
- Verma, S.; Bhargava, R.; Pruthi, V. Oily sludge degradation by bacteria from Ankleshwar, India. Int. Biodeterior. Biodegrad. 2006, 57, 207–213. [Google Scholar] [CrossRef]
- Nitschke, M.; Pastore, G.M. Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresour. Technol. 2006, 97, 336–341. [Google Scholar] [CrossRef]
- Boe, K.; Kougias, P.G.; Pacheco, F.; O-Thong, S.; Angelidaki, I. Effect of substrates and intermediate compounds on foaming in manure digestion systems. Water Sci. Technol. 2012, 66, 2146–2154. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J.; Dewil, R.; De Heyder, B. Advanced sludge treatment affects extracellular polymeric substances to improve activated sludge dewatering. J. Hazard. Mater. 2004, 106, 83–92. [Google Scholar] [CrossRef]
- Mannina, G.; Torregrossa, M.; Viviani, G. BioMac 2013. I Bioreattori a Membrana per la Depurazione Delle Acque Reflue, 1st ed.; Edizioni Caracol: Palermo, PA, USA, 2013. [Google Scholar]
- Rosenberger, S.; Kraume, M. Filterability of activated sludge in membrane bioreactors. Desalination 2003, 151, 195–200. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Memb. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Zhang, X.; Bishop, P.L.; Kinkle, B.K. Comparison of extraction methods for quantifying extracellular polymers in biofilms. Water Sci. Technol. 1999, 39, 211–218. [Google Scholar] [CrossRef]
- Morgan, J.W.; Forster, C.F.; Evison, L. A comparative study of the nature of biopolymers extracted from anaerobic and activated sludges. Water Res. 1990, 24, 743–750. [Google Scholar] [CrossRef]
- Cosenza, A.; Di Bella, G.; Mannina, G.; Torregrossa, M. The role of EPS in fouling and foaming phenomena for a membrane bioreactor. Bioresour. Technol. 2013, 147, 184–192. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Brown, M.J.; Lester, J.N. Comparison of bacterial extracellular polymer extraction methods. Appl. Environ. Microbiol. 1980, 40, 179–185. [Google Scholar] [CrossRef]
- Hladikova, K.; Ruzickova, I.; Klucova, P.; Wanner, J. An investigation into studying of the activated sludge foaming potential by using physicochemical parameters. Water Sci. Technol. 2002, 46, 525–528. [Google Scholar] [CrossRef]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Pollice, A.; Giordano, C.; Laera, G.; Saturno, D.; Mininni, G. Rheology of sludge in a complete retention membrane bioreactor. Environ. Technol. 2006, 27, 723–732. [Google Scholar] [CrossRef]
- Pollice, A.; Giordano, C.; Laera, G.; Saturno, D.; Mininni, G. Physical characteristics of the sludge in a complete retention membrane bioreactor. Water Res. 2007, 41, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Khairnar, K.; Paunikar, W. Causes and remedies for filamentous foaming in activated sludge treatment plant. Glob. Nest J. 2014, 16, 762–772. [Google Scholar]
- Rossetti, S.; Tomei, M.C.; Nielsen, P.H.; Tandoi, V. “microthrix parvicella”, a filamentous bacterium causing bulking and foaming in activated sludge systems: A review of current knowledge. FEMS Microbiol. Rev. 2005, 29, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Caravelli, A.; Contreras, E.M.; Giannuzzi, L.; Zaritzky, N. Modeling of chlorine effect on floc forming and filamentous micro-organisms of activated sludges. Water Res. 2003, 37, 2097–2105. [Google Scholar] [CrossRef]
- Chudoba, J.; Grau, P.; Ottová, V. Control of activated-sludge filamentous bulking-II. Selection of microorganisms by means of a selector. Water Res. 1973, 7, 1389–1406. [Google Scholar] [CrossRef]
- Noutsopoulos, C.; Mamais, D.; Andreadakis, A. Effect of solids retention time on Microthrix parvicella growth. Water SA 2006, 32, 315–321. [Google Scholar] [CrossRef]
- Mamais, D.; Kalaitzi, E.; Andreadakis, A. Foaming control in activated sludge treatment plants by coagulants addition. Glob. Nest J. 2011, 13, 237–245. [Google Scholar]
- Tsang, Y.F.; Sin, S.N.; Chua, H. Nocardia foaming control in activated sludge process treating domestic wastewater. Bioresour. Technol. 2008, 99, 3381–3388. [Google Scholar] [CrossRef]
- Liu, Y. Chemically reduced excess sludge production in the activated sludge process. Chemosphere 2003, 50, 1–7. [Google Scholar] [CrossRef]
- Saby, S.; Djafer, M.; Chen, G.H. Feasibility of using a chlorination step to reduce excess sludge in activated sludge process. Water Res. 2002, 36, 656–666. [Google Scholar] [CrossRef]
- Petrovski, S.; Seviour, R.J.; Tillett, D. Characterization of the genome of the polyvalent lytic bacteriophage GTE2, which has potential for biocontrol of Gordonia-, Rhodococcus-, and Nocardia-stabilized foams in activated sludge plants. Appl. Environ. Microbiol. 2011, 77, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, S.; Seviour, R.J.; Tillett, D. Prevention of Gordonia and Nocardia stabilized foam formation by using bacteriophage GTE7. Appl. Environ. Microbiol. 2011, 77, 7864–7867. [Google Scholar] [CrossRef]
- Petrovski, S.; Seviour, R.J.; Tillett, D. Genome sequence and characterization of the Tsukamurella bacteriophage TPA2. Appl. Environ. Microbiol. 2011, 77, 1389–1398. [Google Scholar] [CrossRef] [PubMed]

| Direct Methods | Equipment | Operative Conditions | References |
|---|---|---|---|
| Foam Rating (FR) | Glass tube (50 cm high, 4 cm diameter), air diffuser (porosity 40–90 μm) on the bottom | Sample volume: 50 mL, Airflow: 200 mL min−1 | [2,13,23] |
| Shaking | 100 mL graduated cylinder | Sample volume: 14 mL, Stirring time: 10 s | [72] |
| Air bubbling | Quartz column (50 cm high, 3.5 cm diameter), 0.25 cm capillary on the bottom | Sample volume: 50 mL, Airflow: 158 mL min−1 for 2 min | [72] |
| Waring Blender | High speed mixer | Sample volume: 100 mL, Mixing speed >1000 r min−1 for 3 min | [73] |
| Foam Power (FP) | Vertical acrylic cylinder (100 cm height, 3 cm diameter, 7 cm2 of cross section) | Sample volume: 100 mL, Airflow: 5 L min−1 for 20 s or 30 s | [22] |
| Foam Power 2 (FP2) | Glass tube (1 L, 6 cm diameter), borosilicate sintered disc (porosity 160–250 μm, 100–160 μm, 40–100 μm) on the bottom | Sample volume: 150 mL, Airflow: 0.5 L min−1 for 1 min | [20,62] |
| Foam Stability (FS) | Graduated cylinder, sintered glass diffuser on the bottom | Sample volume: 150 mL, Airflow: 0.5 L min−1 for 1 min | [22,62] |
| Scum Index (SI) | Flotation cell (50 cm height, 8 cm internal diameter) | Sample volume: 2L, Airflow: 10 L (L h)−1 for 15 min | [2,21,78] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collivignarelli, M.C.; Baldi, M.; Abbà, A.; Caccamo, F.M.; Carnevale Miino, M.; Rada, E.C.; Torretta, V. Foams in Wastewater Treatment Plants: From Causes to Control Methods. Appl. Sci. 2020, 10, 2716. https://doi.org/10.3390/app10082716
Collivignarelli MC, Baldi M, Abbà A, Caccamo FM, Carnevale Miino M, Rada EC, Torretta V. Foams in Wastewater Treatment Plants: From Causes to Control Methods. Applied Sciences. 2020; 10(8):2716. https://doi.org/10.3390/app10082716
Chicago/Turabian StyleCollivignarelli, Maria Cristina, Marco Baldi, Alessandro Abbà, Francesca Maria Caccamo, Marco Carnevale Miino, Elena Cristina Rada, and Vincenzo Torretta. 2020. "Foams in Wastewater Treatment Plants: From Causes to Control Methods" Applied Sciences 10, no. 8: 2716. https://doi.org/10.3390/app10082716
APA StyleCollivignarelli, M. C., Baldi, M., Abbà, A., Caccamo, F. M., Carnevale Miino, M., Rada, E. C., & Torretta, V. (2020). Foams in Wastewater Treatment Plants: From Causes to Control Methods. Applied Sciences, 10(8), 2716. https://doi.org/10.3390/app10082716










