Surface Functionalization of Polyethylene by Silicon Nitride Laser Cladding
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Production
2.2. Samples Characterization
2.3. Biological Testing
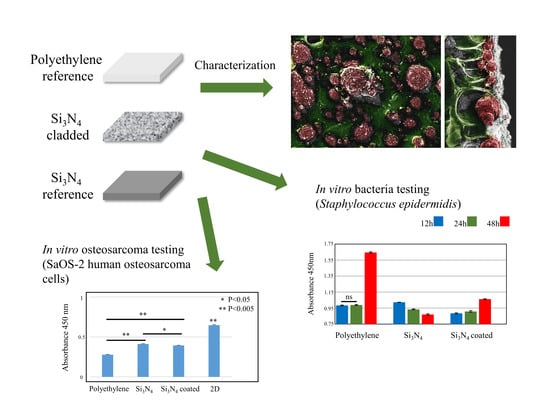
3. Experimental Results
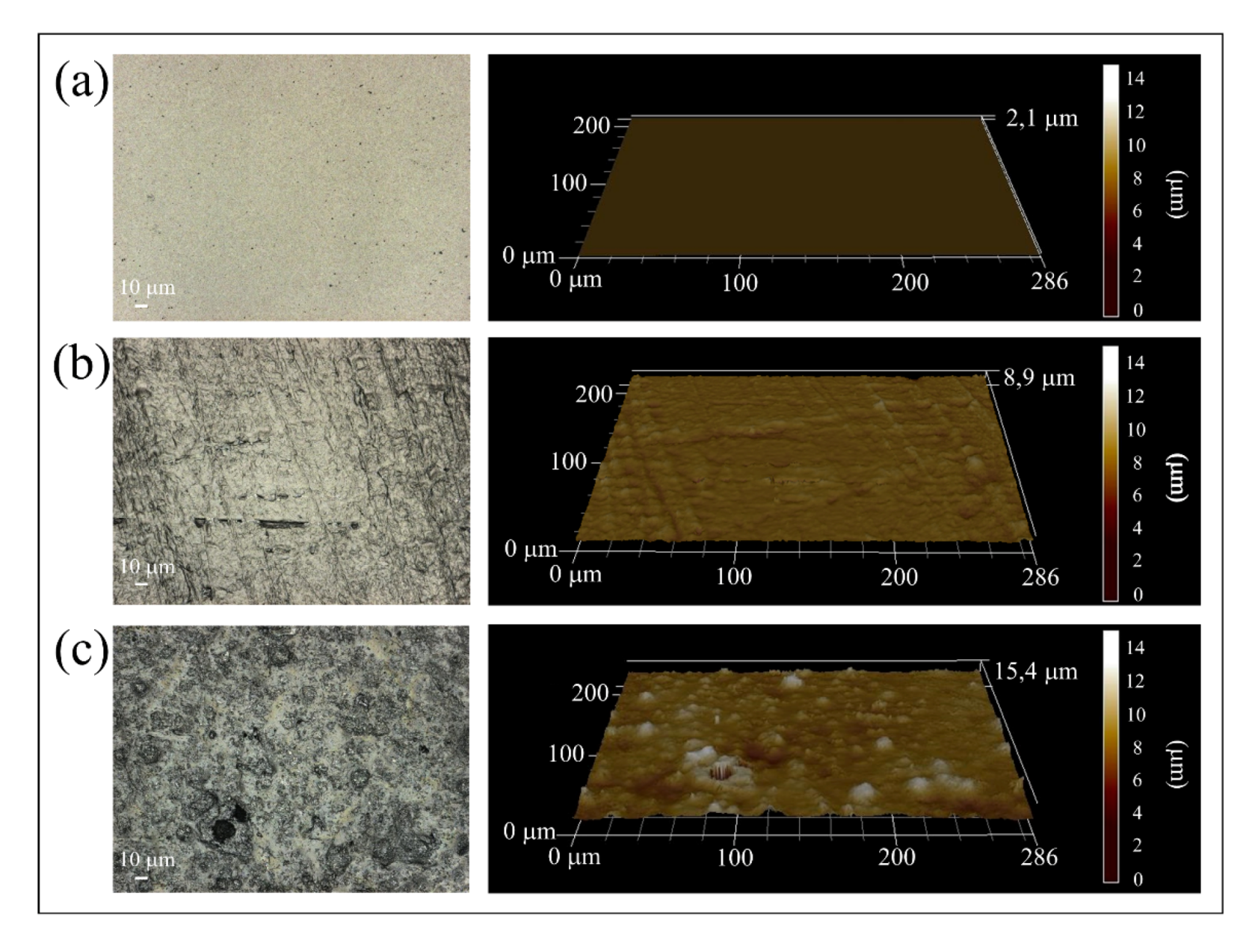
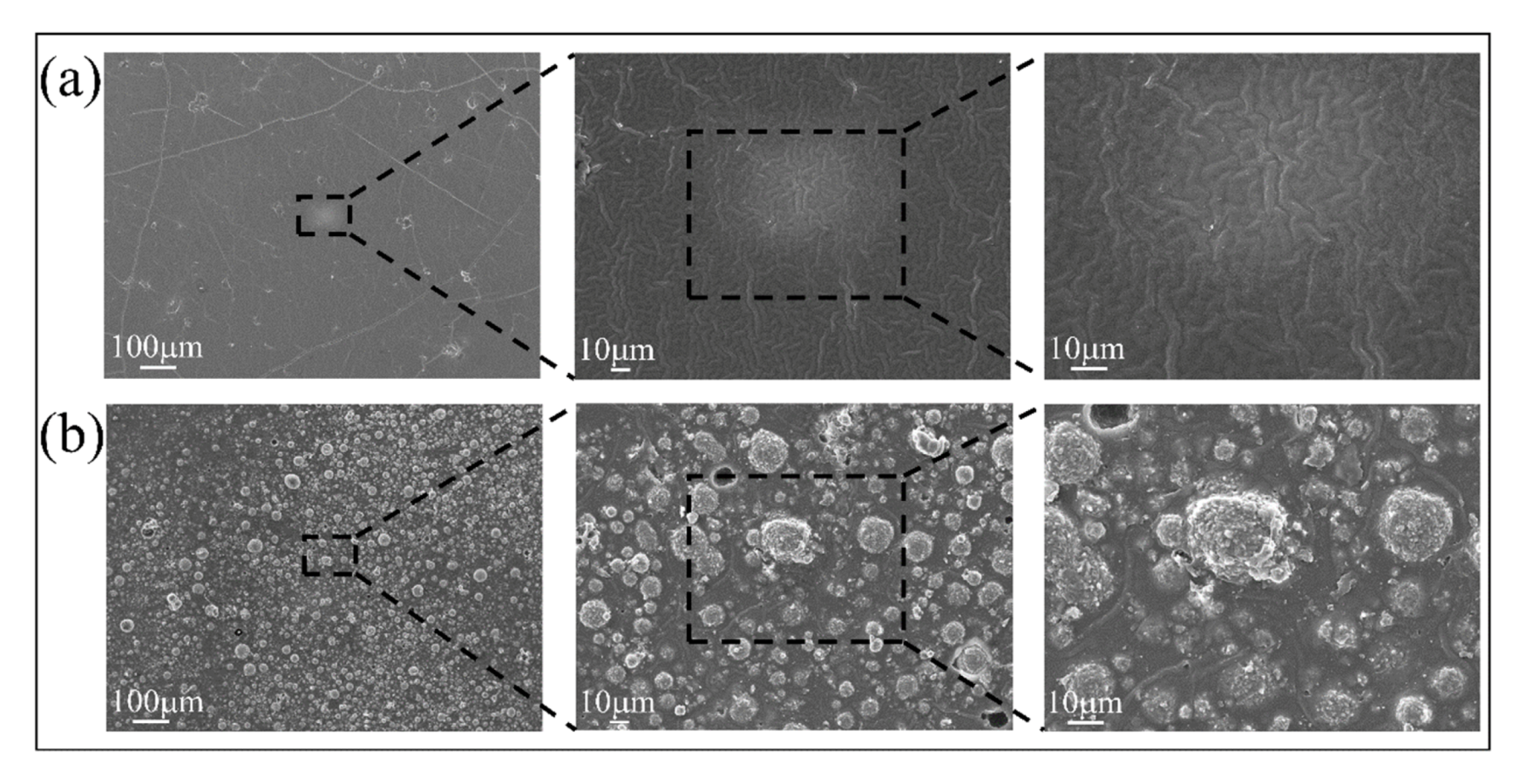
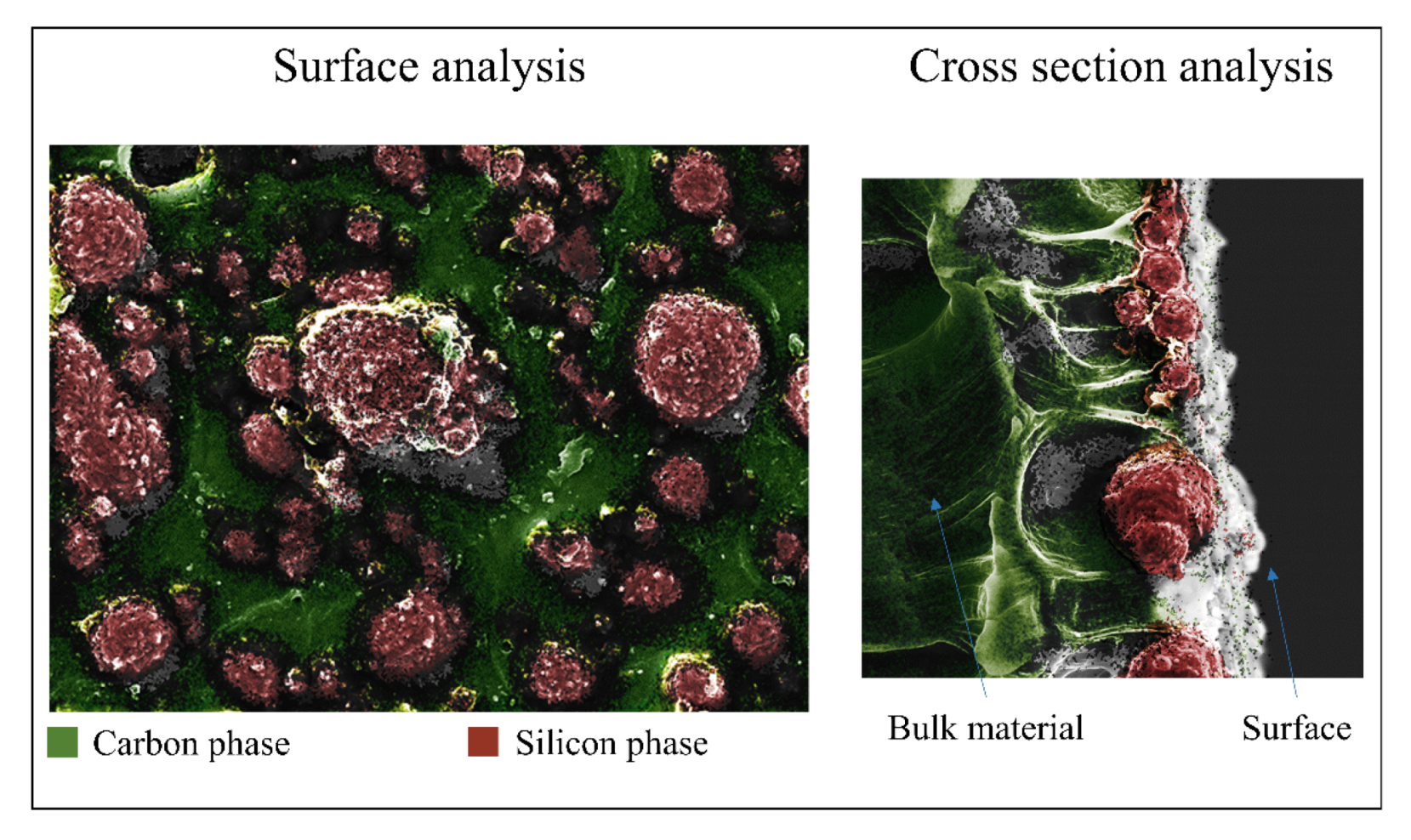
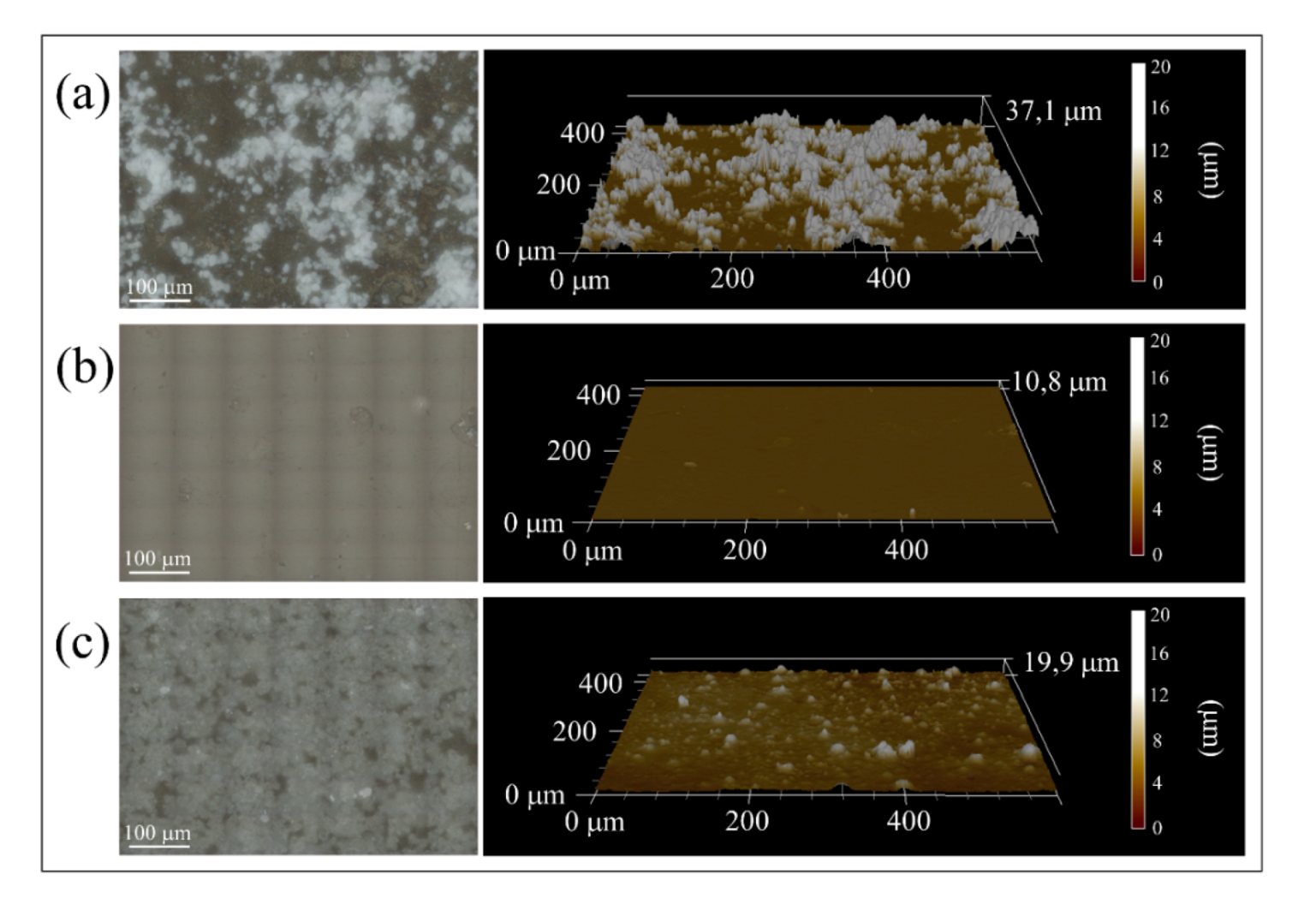
3.1. Surface Characterizations of Pristine and Coated Substrates
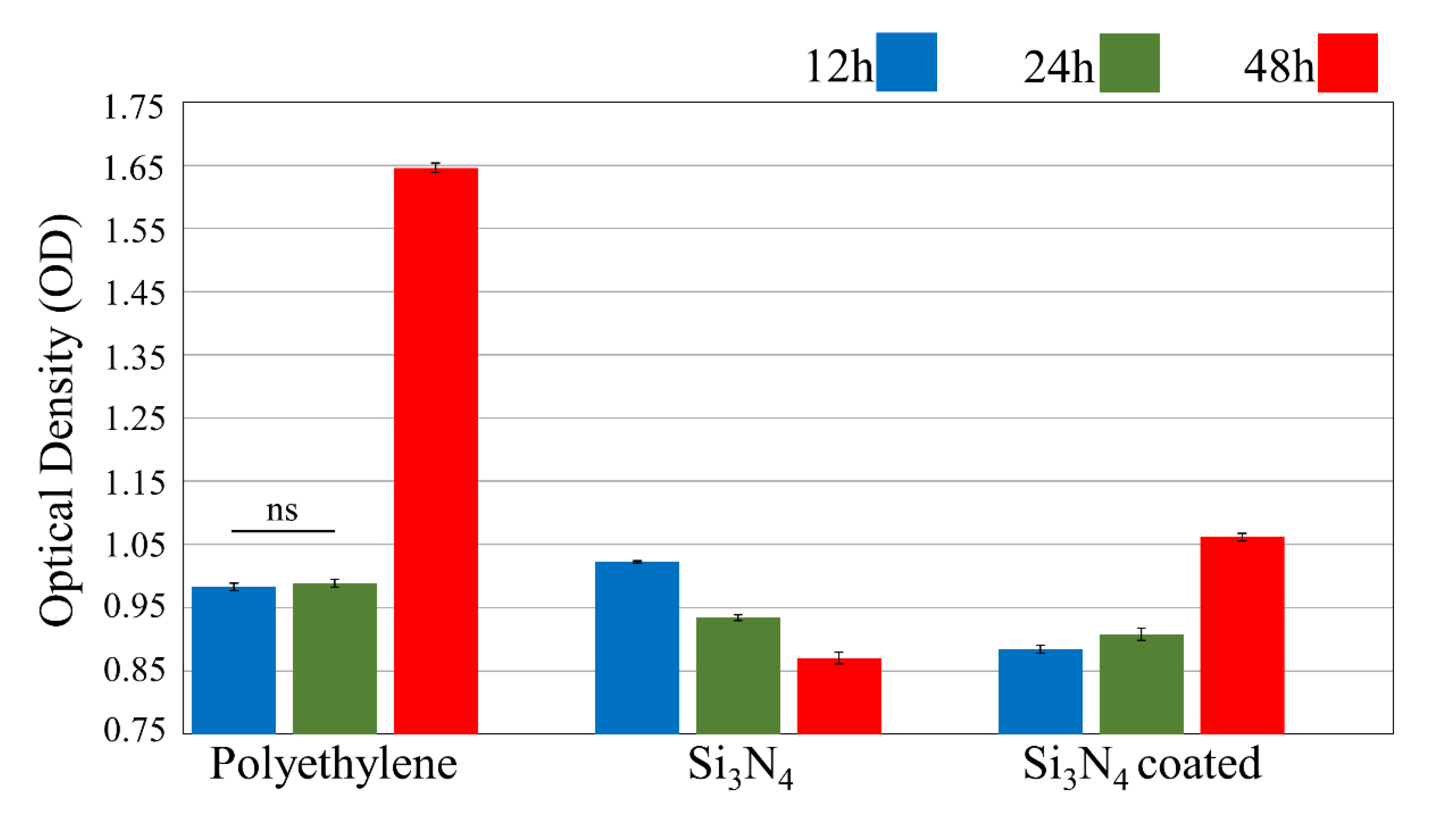
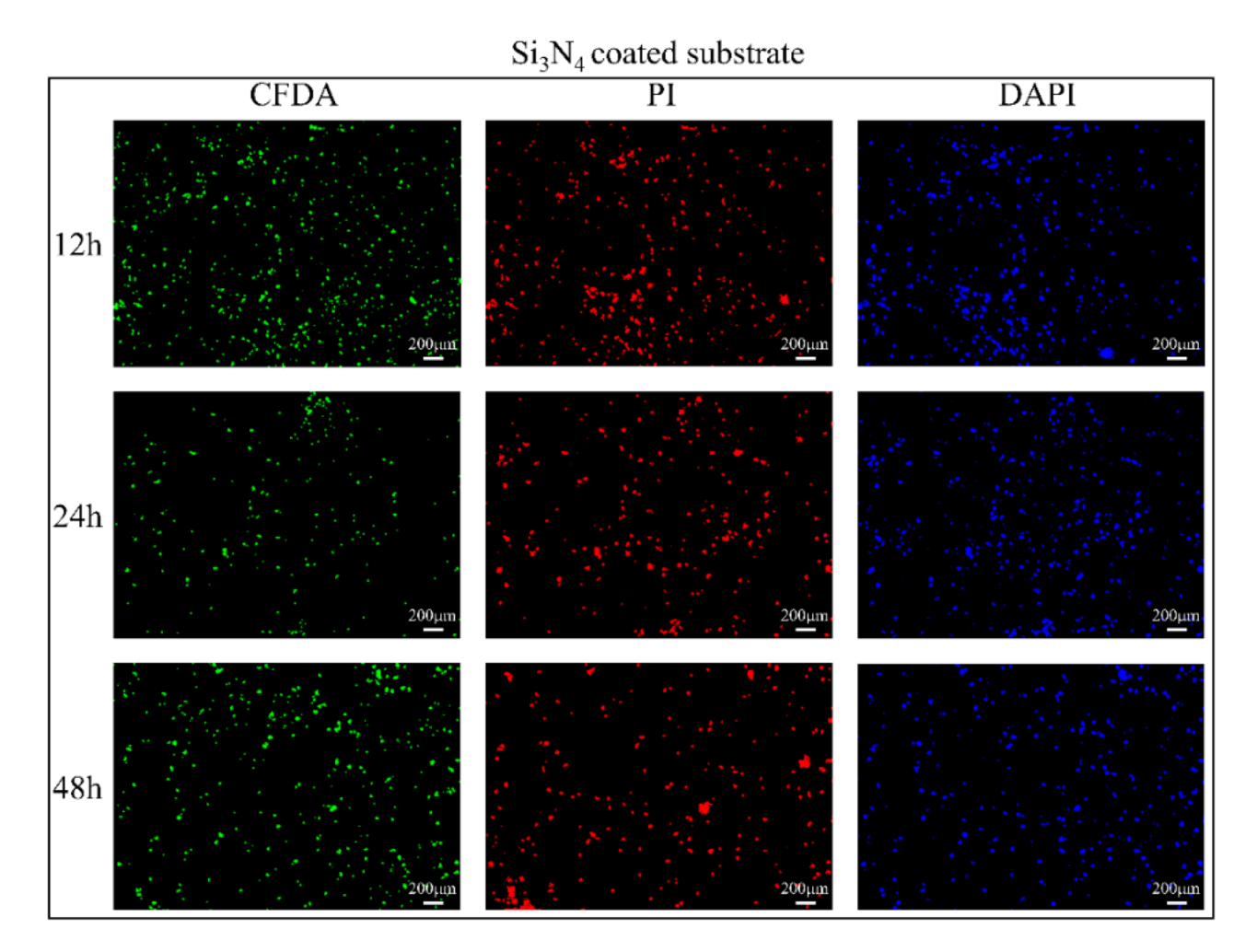
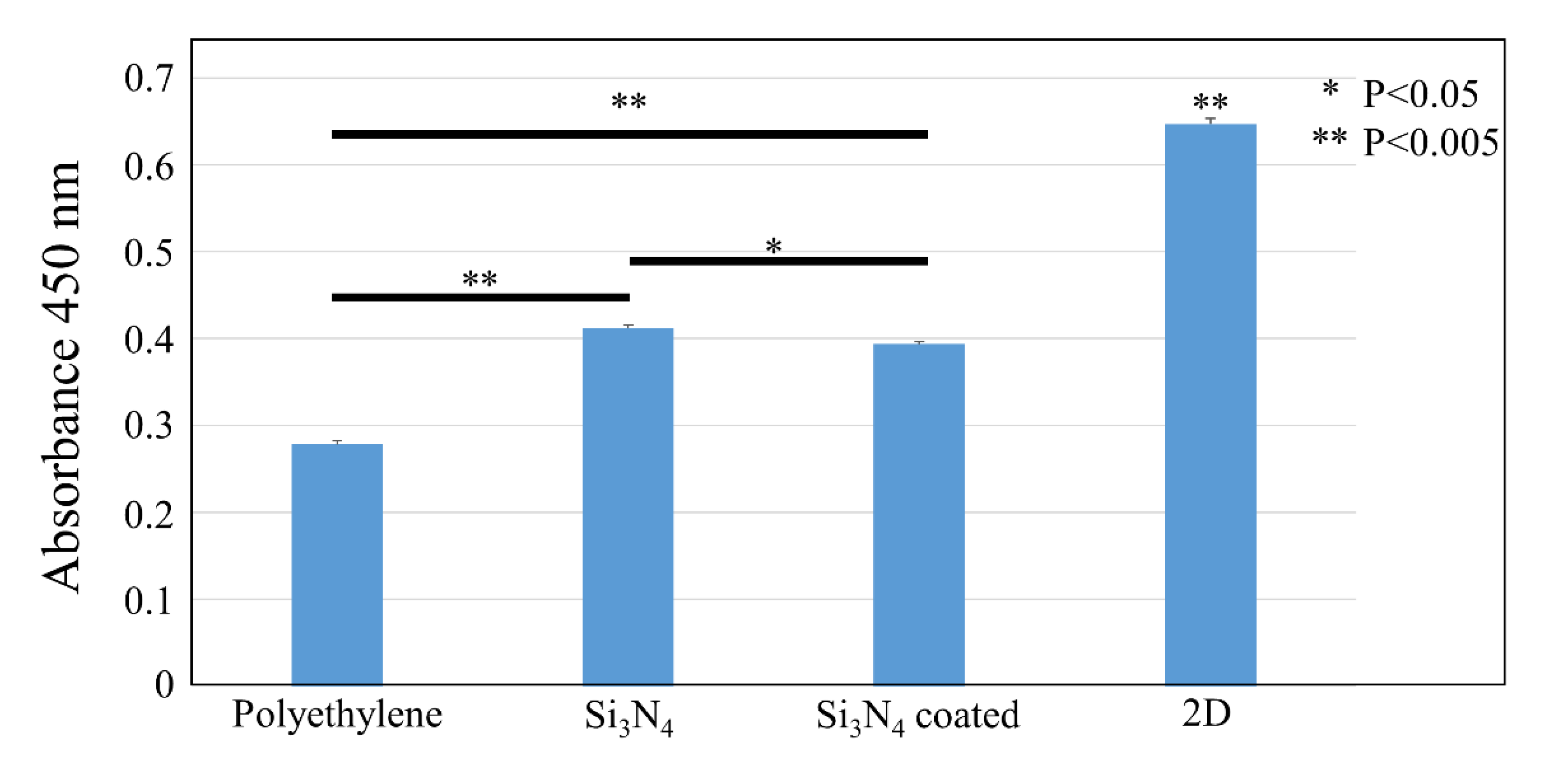
3.2. In Vitro Bacterial Testing
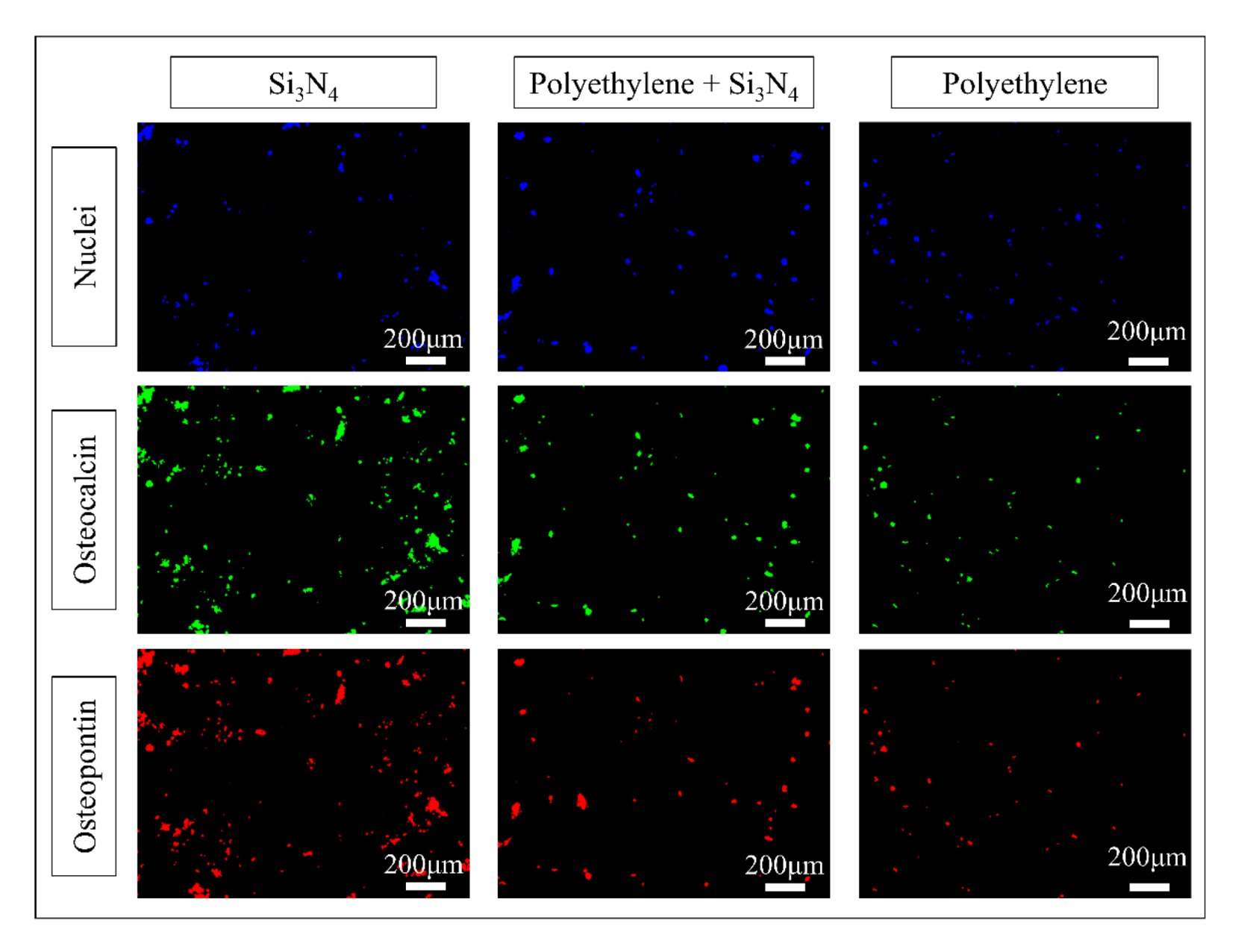
3.3. In Vitro Osteosarcoma Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Teoh, S.H.; Tang, Z.G.; Hastings, G.W. Thermoplastic polymers in biomedical applications: Structures, properties and processing. In Handbook of Biomaterial Properties, 2nd ed.; Springer: Boston, MA, USA, 2016; pp. 261–290. [Google Scholar]
- Fernández-Pradas, J.M.; Naranjo-León, S.; Morenza, J.L.; Serra, P. Surface modification of UHMWPE with infrared femtosecond laser. Appl. Surf. Sci. 2012, 258, 9256–9259. [Google Scholar] [CrossRef]
- Riveiro, A.; Soto, R.; Comesaña, R.; Boutinguiza, M.; del Val, J.; Quintero, F.; Lusquiños, F.; Pou, J. Laser surface modification of PEEK. Appl. Surf. Sci. 2012, 258, 9437–9442. [Google Scholar] [CrossRef]
- Lee, E.H.; Rao, G.R.; Lewis, M.B.; Mansur, L.K. Ion beam application for improved polymer surface properties. Nucl. Inst. Methods Phys. Res. B 1993, 74, 326–330. [Google Scholar] [CrossRef]
- Chan, C.M.; Ko, T.M.; Hiraoka, H. Polymer surface modification by plasmas and photons. Surf. Sci. Rep. 1996, 24, 1–54. [Google Scholar] [CrossRef]
- Riveiro, A.; Soto, R.; del Val, J.; Comesaña, R.; Boutinguiza, M.; Quintero, F.; Lusquiños, F.; Pou, J. Laser surface modification of ultra-high-molecular-weight polyethylene (UHMWPE) for biomedical applications. Appl. Surf. Sci. 2014, 302, 236–242. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Chan, C.M.; Cheng, C.L.; Yuen, M.M.F. Electrical properties of polymer composites prepared by sintering a mixture of carbon black and ultra-high molecular weight polyethylene powder. Polym. Eng. Sci. 1997, 37, 1127–1136. [Google Scholar] [CrossRef]
- Thongruang, W.; Balik, C.M.; Spontak, R.J. Volume-exclusion effects in polyethylene blends filled with carbon black, graphite, or carbon fiber. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1013–1025. [Google Scholar] [CrossRef]
- Hashimoto, M.; Takadama, H.; Mizuno, M.; Yasutomi, Y.; Kokubo, T. Titanium dioxide/ultra high molecular weight polyethylene composite for bone-repairing applications: Preparation and biocompatibility. Key Eng. Mater. 2003, 240–242, 415–418. [Google Scholar] [CrossRef]
- Roy, S.; Pal, S. Characterization of silane coated hollow sphere alumina-reinforced ultra high molecular weight polyethylene composite as a possible bone substitute material. Bull. Mater. Sci. 2002, 25, 609–612. [Google Scholar] [CrossRef]
- Suwanprateeb, J. Binary and ternary particulated composites: UHMWPE/CaCO3/HDPE. J. Appl. Polym. Sci. 2000, 75, 1503–1513. [Google Scholar] [CrossRef]
- Fang, L.; Leng, Y.; Gao, P. Processing of hydroxyapatite reinforced ultrahigh molecular weight polyethylene for biomedical applications. Biomaterials 2005, 26, 3471–3478. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.C.; Bloom, P.D.; Baikerikar, K.G.; Sheares, V.V.; Mallapragada, S.K. Al-Cu-Fe quasicrystal/ultra-high molecular weight polyethylene composites as biomaterials for acetabular cup prosthetics. Biomaterials 2002, 23, 1761–1768. [Google Scholar] [CrossRef]
- Bal, B.S.; Rahaman, M.N. Orthopedic Applications of Silicon Nitride Ceramics. Acta Biomater. 2012, 8, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Bock, R.M.; Jones, E.N.; Ray, D.A.; Sonny Bal, B.; Pezzotti, G.; McEntire, B.J. Bacteriostatic behavior of surface modulated silicon nitride in comparison to polyetheretherketone and titanium. J. Biomed. Mater. Res. Part A 2017, 105, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Patel, A.A.; Rahaman, M.N.; Sonny Bal, B. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater. 2012, 8, 4447–4454. [Google Scholar] [CrossRef]
- Pezzotti, G.; Marin, E.; Adachi, T.; Lerussi, F.; Rondinella, A.; Boschetto, F.; Zhu, W.; Kitajima, T.; Inada, K.; McEntire, B.J.; et al. Incorporating Si 3 N 4 into PEEK to Produce Antibacterial, Osteocondutive, and Radiolucent Spinal Implants. Macromol. Biosci. 2018, 18, e1800033. [Google Scholar] [CrossRef]
- McEntire, B.J.; Lakshminarayanan, R.; Thirugnanasambandam, P.; Seitz-Sampson, J.; Bock, R.; O’Brien, D. Processing and Characterization of Silicon Nitride Bioceramics. Bioceram. Dev. Appl. 2016, 6, 1000093. [Google Scholar] [CrossRef]
- Honda, K.; Yokoyama, S.; Tanaka, S. Assignment of the Raman Active Vibration Modes of ß-Si3N4 using Micro-Raman Scattering. J. Appl. Phys. 1999, 85, 7380–7384. [Google Scholar] [CrossRef]
- Sato, H.; Shimoyama, M.; Kamiya, T.; Amari, T.; Šašic, S.; Ninomiya, T.; Siesler, H.W.; Ozaki, Y. Raman spectra of high-density, low-density, and linear low-density polyethylene pellets and prediction of their physical properties by multivariate data analysis. J. Appl. Polym. Sci. 2002, 86, 443–448. [Google Scholar] [CrossRef]
- Yoda, I.; Koseki, H.; Tomita, M.; Shida, T.; Horiuchi, H.; Sakoda, H.; Osaki, M. Effect of Surface Roughness of Biomaterials on Staphylococcus Epidermidis Adhesion. BMC Microbiol. 2014, 14, 234. [Google Scholar] [CrossRef]
- Pezzotti, G.; Bock, R.M.; Adachi, T.; Rondinella, A.; Boschetto, F.; Zhu, W.; Marin, E.; McEntire, B.J.; Sonny Bal, B.; Mazda, O. Silicon Nitride Surface Chemistry: A Potent Regulator of Mesenchymal Progenitor Cell Activity in Bone Formation. Appl. Mater. Today 2017, 9, 82–95. [Google Scholar] [CrossRef]
- Zanocco, M.; Boschetto, F.; Zhu, W.; Marin, E.; McEntire, B.J.; Sonny Bal, B.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Ohgitani, E.; et al. 3D-Additive Deposition of an Antibacterial and Osteogenic Silicon Nitride Coating on Orthopaedic Titanium Substrate. J. Mech. Behav. Biomed. Mater. 2020, 103, 103557. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Bock, R.M.; McEntire, B.J.; Adachi, T.; Marin, E.; Boschetto, F.; Zhu, W.; Mazda, O.; Sonny Bal, B. In vitro Antibacterial Activity of Oxide and Non-Oxide Bioceramics for Arthroplastic Devices: I. In situ Time-Lapse Raman Spectroscopy. Analyst 2018, 143, 3708–3721. [Google Scholar] [CrossRef] [PubMed]
- Himathongkham, S.; Riemann, H. Destruction of Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes in Chicken Manure by Drying and/or Gassing with Ammonia. FEMS Microbiol. Lett. 1999, 171, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Farrah, S.; Baney, R.H. Membrane Damage of Bacteria by Silanols Treatment. Electron. J. Biotechnol. 2007, 10, 252–259. [Google Scholar] [CrossRef]
- Marin, E.; Rondinella, A.; Boschetto, F.; Zanocco, M.; McEntire, B.J.; Sonny Bal, B.; Pezzotti, G. Understanding Silicon Nitride’s Biological Properties: From Inert to Bioactive Ceramic. Key Eng. Mater. 2018, 782, 289–296. [Google Scholar] [CrossRef]
- Pezzotti, G.; Marin, E.; Adachi, T.; Rondinella, A.; Boschetto, F.; Zhu, W.; Sugano, N.; Bock, R.M.; McEntire, B.J.; Sonny Bal, B. Bioactive Silicon Nitride: A New Therapeutic Material for Osteoarthropathy. Sci. Rep. 2017, 7, 44848. [Google Scholar] [CrossRef]
- Pezzotti, G.; McEntire, B.J.; Bock, R.M.; Boffelli, M.; Zhu, W.; Vitale, E.; Puppulin, L.; Adachi, T.; Yamamoto, T.; Kanamura, N.; et al. Silicon Nitride: A Synthetic Mineral for Vertebrate Biology. Sci. Rep. 2016, 6, 31717. [Google Scholar] [CrossRef]
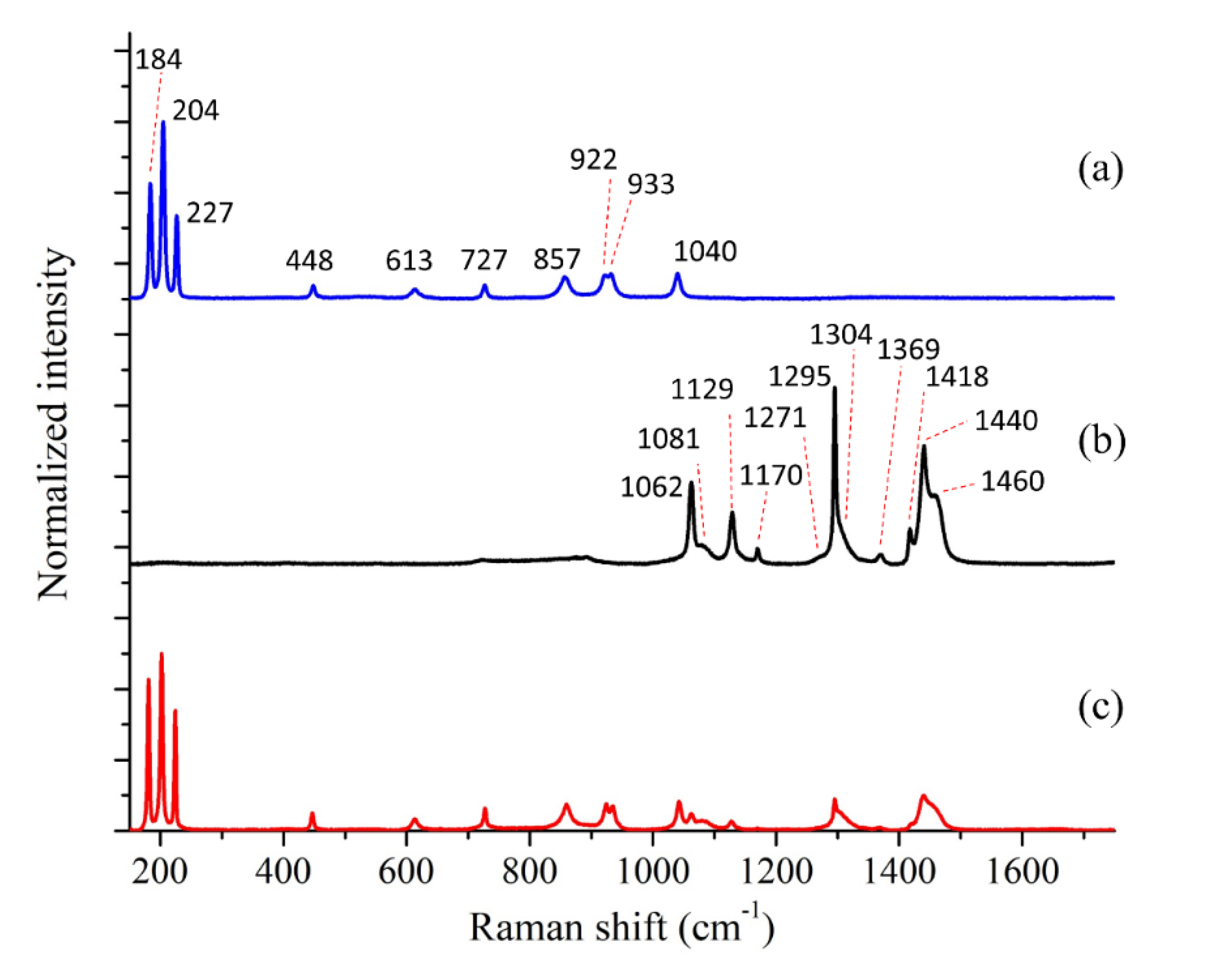

| Bands Position (cm−1) | Assignments | Bands Position (cm−1) | Assignments |
|---|---|---|---|
| 184 | E2g | 1062 | C-C stretching |
| 204 | Ag | 1081 | C-C stretching |
| 227 | E1g | 1129 | C-C stretching |
| 448 | E2g | 1170 | CH2 rocking |
| 613 | E2g | 1271 | CH2 twisting |
| 727 | Ag | 1295 | CH2 twisting |
| 857 | E1g | 1304 | CH2 wagging |
| 922 | E2g | 1369 | CH2 bending |
| 933 | Ag | 1418 | CH2 bending |
| 1040 | E2g | 1440 | CH2 bending |
| 1460 | CH2 rocking |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanocco, M.; Marin, E.; Boschetto, F.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Zhu, W.; McEntire, B.J.; Bal, B.S.; Ashida, R.; et al. Surface Functionalization of Polyethylene by Silicon Nitride Laser Cladding. Appl. Sci. 2020, 10, 2612. https://doi.org/10.3390/app10072612
Zanocco M, Marin E, Boschetto F, Adachi T, Yamamoto T, Kanamura N, Zhu W, McEntire BJ, Bal BS, Ashida R, et al. Surface Functionalization of Polyethylene by Silicon Nitride Laser Cladding. Applied Sciences. 2020; 10(7):2612. https://doi.org/10.3390/app10072612
Chicago/Turabian StyleZanocco, Matteo, Elia Marin, Francesco Boschetto, Tetsuya Adachi, Toshiro Yamamoto, Narisato Kanamura, Wenliang Zhu, Bryan J. McEntire, B. Sonny Bal, Ryutaro Ashida, and et al. 2020. "Surface Functionalization of Polyethylene by Silicon Nitride Laser Cladding" Applied Sciences 10, no. 7: 2612. https://doi.org/10.3390/app10072612
APA StyleZanocco, M., Marin, E., Boschetto, F., Adachi, T., Yamamoto, T., Kanamura, N., Zhu, W., McEntire, B. J., Bal, B. S., Ashida, R., Mazda, O., & Pezzotti, G. (2020). Surface Functionalization of Polyethylene by Silicon Nitride Laser Cladding. Applied Sciences, 10(7), 2612. https://doi.org/10.3390/app10072612