Featured Application
The review aims to provide guidance on the definitions and regulation of products containing botanical ingredients, which are at present quite complex.
Abstract
In recent decades, the interest in products containing botanicals and claiming “functional” properties has increased exponentially. Functional foods, novel foods and food supplements have a special impact on the consumers, who show significant expectation for their well-being. Food supplements with botanical ingredients are the food area that has witnessed the greatest development, in terms of the number of available products, budget, and consumer acceptability. This review refers to and discusses some open points, such as: (1) the definitions and regulation of products containing botanicals; (2) the difficulty in obtaining nutritional and functional claims (botanical ingredients obtaining claims in the EU are listed and summarized); (3) the safety aspects of these products; and (4) the poor harmonization between international legislations. The availability of these “new” products can positively influence the well-being of the population, but it is essential to provide the consumers with the necessary recommendations to guide them in their purchase and use.
1. Introduction
The sentence “Let thy food be thy medicine and medicine be thy food”, commonly attributed to Hippocrates (400 BC), indicates the importance that nutrition has always had in the concept of health and prevention of disease. This relationship has obviously undergone changes and historical recurrences with an evolution parallel to that of human civilization with its variable habits in social behavior and diet. More than 2500 years after Hippocrates’ claim, the concept of diet has evolved to include aspects based on research in the field of nutrition and on knowledge relating to active molecules contained in foods with potential benefit to human health.
The vegetables entered massively in the diet of humans with the Agricultural Revolution that occurred about 12,000 years ago. Cereals, only rarely consumed in the Paleolithic era, have become the basis of human nutrition with milk derivatives [1]. However, considering the use of plants for their healing properties, archaeological excavations place their use up to 60,000 years ago. These were plants not commonly consumed as food: poppy (opium), ephedra and cannabis.
The possible placement of the plants among food and products with therapeutic properties is therefore ancient history and reappears, with its contradictions, in the current legislation, as described below. The aim of this review was the clarification and relative discussion of the most critical aspects regarding products containing botanicals, in order to promote the correct use of plant food supplements among consumers.
2. Definitions and Regulation
There is no internationally shared definition of the term “botanical”. However, it is possible to cite what was defined by European Food Safety Authority (EFSA) in its guidelines published in 2009 [2]. The term botanical includes whole, fragmented or cut plants, plant parts, fungi and lichens. The term botanical preparations include all preparations obtained from botanicals by various processes, such as pressing, squeezing, extraction, fractionation, distillation, concentration, drying up and fermentation. A similar definition is reported by a group of food and beverage producers: “botanicals are fresh or dried plants, plant parts or plants’ isolated or collective chemical components, extracted in water, ethanol, or other organic solvents, plus essential oils, oleoresins, and other extractives used for flavoring, fragrance, functional health benefits, medicine, or other uses” [3].
Based on the definitions listed above, it is clear that botanicals include numerous possible ingredients with extremely variable composition and characteristics. First of all, the presence and concentration of active molecules can vary greatly, with possible effects on the beneficial effect expected on the consumers’ health.
Hundreds of plants are ingredients commonly used in the food industry as such or derivatives, or food additives (flavorings, colorings, preservatives, etc.), and botanicals can be included in different types of products regulated by the food law as illustrated in Figure 1.

Figure 1.
Categories of food containing botanicals.
2.1. Fruits and Vegetables
Fruits and vegetables have been included in the diet of humans since ancient times; they can be consumed as such or as household or industrial derivatives (jams, juice, purée, etc.). Fruits and vegetables are included in the EC Regulation N. 178/2002, which defines any unprocessed or processed substances or product designed for human consumption [4]. Generally speaking, consumers prefer products from the geographical area of belonging, but with the increasing globalization it is possible to expand the choice to imported products.
2.2. Enriched or Functional Food
Although there is no international agreement, according to the consensus document on “Scientific Concepts of Functional Foods in Europe” of the European Commission Concerted Action on Functional Food Science in Europe (FUFOSE), functional food is usually considered a product to which one or more ingredients have been added (or more rarely subtracted) with a positive consequence on the functionality of human organs or systems [5]. It is, therefore, a food that has not only the function of providing calories and nutrients but intends to carry out a favorable action on the consumer’s health. This effect must be reached with the quantity of food normally consumed, it must be in “traditional” form (and not in pharmaceutical form) and must guarantee the safety of the subjects taking the product, even if they present common pathologies. As an example, the prebiotic ingredients are frequently added to food; they are soluble fibers capable to promote the growth of the positive intestinal microbiota; among others, the most important fibers in this field are inulin and fructo-oligosaccharides (from chicory or Jerusalem artichoke). Further details will be reported in Section 4.1.2.
2.3. Novel Food and Traditional Food from Third Countries
At the European level the regulation of new food, or novel food, was amended in 2015 (EU Regulation 2015/2283). Compared to the previous regulations, the definition of this products was maintained: “novel food means any food that was not used for human consumption to a significant degree within the Union before 15 May 1997”. Some of the categories listed in novel food include botanicals: (1) food consisting of, isolated from or produced from microorganisms, fungi or algae; (2) food consisting of, isolated from or produced from plants or their parts. Novel food category also includes products obtained with new technologies. Before marketing them, appropriate documentation must be provided in order to demonstrate their safety. Examples of novel food include: new sources of vitamin K (menaquinone) or some extracts from existing food (Antarctic krill oil rich in phospholipids from Euphausia superba) [6].
The aforementioned regulation has introduced a new authorization procedure for “traditional foods deriving from Third Countries”, where a food with proven tradition of safe use in a third country for at least 25 years is included. Compared to the novel food procedure, this new category must present less documentation for their approval. Examples of foods belonging to this category are the dried aerial parts from Hoodia parviflora N.E.Br. (hoodia) and seeds from Salvia hispanica L. (chia) [6].
2.4. Food/Dietary Supplements
Botanicals are widely used in the formulation of food (or dietary in US) supplements, that can be defined as “products intended to supplement the common diet and which constitute a concentrated source of nutrients, such as vitamins and minerals, or other substances having a nutritional or physiological effect, in particular, but not exclusively, amino acids, essential fatty acids, fibers and extracts of vegetable origin, both single- and multi-compound, in pre-dosed forms” [7]. The role of food supplements is limited to correcting nutritional deficiencies, maintaining the recommended intake of some essential nutrients and supporting specific nutritional needs such as pregnancy, breastfeeding and menopause. They must not show therapeutic activity or significantly modify the physiological functions [8]. Among the most frequently botanical ingredients used in food supplements, there are Ginkgo biloba L. (ginkgo), Oenothera biennis L. (evening primrose), Cynara scolymus L. (artichoke) and Panax ginseng C.A. Meyer (ginseng) [9].
2.5. Other Categories
Botanicals can be regulated by drug law. In fact, their use can enter into conventional medicine, or, as in some European countries (e.g., Germany), in “Traditional Herbal Medicinal Products (THMPs)”. THMPs must guarantee their safety in the expected conditions of use and their biological activity/efficacy must be plausible on the basis of a chronic use and of available experience [10].
Botanicals can also be ingredients of products belonging to the category of medical devices; among others: (1) ophthalmic solutions containing chamomile (Matricaria recutita L.), calendula (Calendula officinalis L.), cornflower (Centaurea cyanus L.), etc.; (2) some liquids used in contact lens care, which include arabinogalactans and plant extracts; (3) some solutions used for oral hygiene containing mainly chamomile, aloe (Aloe vera (L.) Burm.f.) and marshmallow (Althaea officinalis L.). Since this chapter is about food, only examples of the use of botanicals in functional foods and food supplements will be described.
3. Claims
One of the most interesting aspects associated with functional food and food supplements is the possibility to boast on the label their health properties, the so-called “claims”.
The European Commission allows health claims when they can be supported by the scientific literature and if they can be understood by consumers. In the EU, the European Food Safety Authority (EFSA) is in charge of evaluating the scientific evidence [11] and provides the guidelines, which describes in detail the studies required for the approval of nutritional/physiological claims [2,12].
There are two types of health sentences, those related to art. 13, and those relating to art. 14 of the European Regulation 1924/2006. Article 13 claims refer to: (1) growth, development and functions of the body; (2) psychological and behavioral functions; (3) slimming or weight control. Article 14 claims are associated with: (1) a reduction of risk factors in the development of diseases; (2) support of normal children’s development and health. Table 1 lists the most important health claims allowed for vegetable/botanical ingredients in the EU [13].

Table 1.
List of main claims allowed in the EU for plant (botanical) ingredients contained in, or added to, functional food or food supplements [13].
4. Botanicals in Functional Foods
Some botanical derivatives have been successfully included in functional food for their healthy properties. Among others, soluble and insoluble fibers, beta glucans and phytosterols/phytostanols deserve a more in-depth description.
4.1. Soluble and Insoluble Fibers
The beneficial properties of dietary fiber intake have been known for a long time and have been publicized since the 1920s. Over the years, the importance of fiber for human health has been fluctuating to be re-evaluated in the second half of the 20th century [14]. With the progress of knowledge, dietary fiber has been classified in various ways; the first subdivision concerns the difference between fiber, which are soluble or insoluble in aqueous medium. In addition to the obvious difference in “chemical” behavior, the solubility (or less) of the fiber in water greatly conditions the activity on humans’ health. Insoluble fiber acts mainly on the intestinal transit, while the soluble fibers mediate beneficial properties based on different biological mechanisms.
4.1.1. Insoluble Fiber
Insoluble fiber includes mainly cellulose, hemicellulose and lignin, and it is characterized by its “inertia” at the level of human organism. In other words, it does not undergo any metabolic modification during the transit in the gastro-intestinal tract. Products containing insoluble fiber from different plant sources can use the health claim “the fiber contributes: (1) to normal bowel function; or (2) to increase faecal bulk; or (3) to an acceleration of intestinal transit” (see barley, oat, rye, sugar beet, and wheat fiber in Table 1). Insoluble fiber increases fecal bulk, that leads to a speeding of intestinal transit with the consequent beneficial effects (elimination of waste containing toxic molecules, less contact of fecal waste with the intestinal mucosa, etc.). These effects have been recognized as protective factors versus some cancers including the colon cancer.
4.1.2. Prebiotic Fibers
The prebiotic fibers fall into the category of soluble fibers. The definition of the term “prebiotic” has undergone a certain number of changes since the 1990s, a period in which they were identified as beneficial ingredients of the human diet. In agreement with the International Scientific Association for Prebiotics and Probiotics, a prebiotic fiber is “a substrate, that is selectively used by host microorganisms conferring a health benefit” [15]. Similarly, the Food and Agriculture Organization of the United Nations (FAO) published the following definition in 2008: “a nonviable food component that confers a health benefit on the host associated with modulation of the microbiota” [16].
In recent decades, the awareness of the critical role played by the intestinal microbiota on human health has progressively grown. Studies to support this knowledge have stimulated the formulation of food products capable to contribute to the modulation of microbiota towards positive bacterial strains (mainly lactic bacteria) at the expense of pathogenic ones. These foods, mostly milk-based, contain particular strains of live bacteria (probiotics) or soluble fibers capable of promoting their growth. In functional foods, the most commonly used prebiotic fibers are: inulin, fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS); the latter are mostly included in products for infants. Inulin is a linear polymer of 2-60 fructose molecules linked by β-(2-1)-bonds.
The nature of chemical bonds prevents inulin from the usual digestion of caloric sugars. FOS are similar to inulin for chemical bonds, but the number of units ranges between 2 and 10 [17]. Inulin and FOS are storage carbohydrates contained in several plants; the most important sources are chicory, sugar beet, leeks, asparagus, and Jerusalem artichokes. Inulin and FOS reach the colon undigested where they are fermented by the “positive” microbiota. A health claim for “native chicory inulin” is allowed in EU; it states that “chicory inulin contributes to normal bowel function by increasing stool frequency”. This claim must be associated with those products, providing at least a daily intake of 12 g of inulin, as a non-fractionated mixture of monosaccharides (<10%), disaccharide, inulin-type fructans and inulin, with an average of polymerization > 9 (see Table 1).
Although from animal origin, galacto-oligosaccharides (GOS) are considered here to describe the possible healthy effect due to prebiotic fiber. GOS are oligosaccharides containing 2-10 molecules of galactose and one molecule of glucose. The purity and degree of polymerization largely influence the prebiotic properties of these oligosaccharides, as shown in clinical studies [18,19]. GOS are widely used in lactating formulas to improve the microbiota colonization and modulate the intestinal disorders such as colic. The effects of GOS added to infant formulas were evaluated in a randomized, double-blind, controlled, parallel-group clinical trial including healthy term infants [20]. Three groups were considered: breastfed, formula-fed, and GOS-supplemented formula-fed infants. The effects of supplemented formula were assessed considering four bacterial targets: Bifidobacterium, Lactobacillus, Clostridium, and Escherichia coli. The effect of the prebiotic-supplemented formula was very close to that of breast-feeding in stimulating Bifidobacterium and Lactobacillus growth and in inhibiting Clostridium growth. The whole result was a significantly lower incidence of colic [20].
4.1.3. Beta-Glucans
Beta-glucans are soluble fibers contained in cereals, such as barley, oat, rye, wheat and some mushrooms [21]. They are linear polymers of glucose linked by β-(1-4) and β-(1-3) bounds. Two claims for beta-glucans received positive opinion by EFSA and were included in the list of wordings allowed in the EU (Table 1) [22].
The first claim is “consumption of beta-glucans from oats or barley as part of a meal contributes to the reduction of the blood glucose rise after that meal” (Table 1). In fact, beta-glucans modulate the post-prandial glycemic response by increasing the meal bolus viscosity, with a consequent delayed absorption of nutrients (including glucose) in the small intestine [23].
On the basis of intervention studies performed in healthy subjects, EFSA concluded that quantities of about 4 g of beta-glucans/30 g of available carbohydrates are capable to decrease the post-prandial glycemic response without modifying the insulin release [22]. The claim must be associated with the recommendation to use beta glucans during meals.
The EFSA received from the European Commission a second request for an opinion regarding the claim associating beta-glucans with the positive modulation of normal blood cholesterol levels [24]. On the basis of a certain number of randomized-controlled trials in adults with normal or mildly increased cholesterolemia, the EFSA Panel formulated the following wording “regular consumption of beta-glucans contributes to the maintenance of normal blood cholesterol levels”. The beneficial effect is linked to a daily consumption of 3 g of beta-glucans in one or more servings. The proposed mechanism of action is based on the fact that dietary beta-glucan forms, in the small intestine, a viscous mixture that reduces the intestinal absorption of dietary cholesterol and the re-uptake of bile acids. As a consequence, new bile acids are synthetized from circulating cholesterol [25].
4.2. Phytosterols/Phytostanols
Phytosterols and phytostanols are compounds of plant origin, which are commonly part of the human diet. Phytosterols can be transformed in phytostanols by hydrogenation and both class of molecules can be esterified with fatty acids from vegetable origin to the corresponding esters [26,27]. Phytosterols and phytostanols are structurally similar to cholesterol, apart from their side chain. The most common phytosterols and phytostanols are sitosterol, sitostanol, campesterol, campestanol, stigmasterol and brassicasterol [26].
In the EU, the use of phytosterols, phytostanols and their esters in foods refers to the Regulation (EC) No. 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods/novel food ingredients.
These molecules reduce the intestinal absorption of cholesterol and, as a consequence, cause a decrease in blood cholesterol levels. For this property, phytosterols and phytostanols, in free or esterified form, can be added to foods for their properties to maintain normal (claim under Art. 13) or reduce blood cholesterol levels (claim under Art. 14). The first wording is allowed for food providing 0.8 g of plant sterols/stanols with the daily dose and the second for daily doses ranging from 1.5 to 3 g. Doses of 1.5–2.4 g/day are associated with a cholesterol level reduction of 7–10%, while 2.4–3 g/day can produce a decrease of 10–12.5% [28].
5. Botanicals in Food Supplements
The category of food/dietary supplements is that in which the presence of ingredients from plant origin is more frequent (botanicals). Food supplements containing plants or derivatives have found an increasing diffusion and acceptance by the consumers, which consider the word “natural” synonymous with “non-chemical” and for association with safe. This belief explains the success of these products and therefore their consistent market presence.
The plants contained in food supplements are many and most of them derive from the tradition of use, that is the preparation of infusions or decoctions. The same plants have been then used by the industries to prepare extracts, in order to enrich the products in active molecules and enhance the expected positive properties. Supplements may contain a single plant ingredient or a mixture of them. It is clear that the greater the number of botanicals present in the finished product, the greater the problems in the correct use by the consumer and in the quality control especially when the product is already on sale.
Another critical aspect derives from the fact that there is no harmonization at the international level (even in Europe) as regards the lists of plants allowed in food supplements. Many countries of the European Union, but also of other continents, have published positive and/or negative lists of botanical ingredients, which are allowed or prohibited in food/dietary supplements. Unfortunately, there is few correlations between them.
The inconsistent situation of the European market of products with botanical ingredients (presence both in products regulated by food or drug laws, see Section 2) leads to several consequences, including the difficulty in discriminating “healthy” or “therapeutic” information for the same plant used in different product classes. As the researchers well know, the dose makes the difference, but discriminating the dose with physiological effects from the one that is suitable for clinical applications is a complex objective even for plants of more ancient tradition. These difficulties were reflected in the very small number of claims approved by EFSA in the field of botanicals.
To boast a nutritional or health claim as a guide for the consumer, it is necessary to refer to the European Regulation n. 1924/2006, according to which: "Scientific substantiation should be the main aspect to be taken into account for the use of nutrition and health claims and the food business operators using claims should justify them”. To obtain authorization to associate a nutrition/health claim with a botanical in EU, the manufacturer must submit a dossier to EFSA, which evaluates the scientific evidence of the studies provided and publishes the relative opinion. The guidelines provided by EFSA [2,8] describe in detail the studies required for the approval of nutritional/physiological claims.
The guidelines published by EFSA, although extremely well-articulated, have nevertheless led to immediate difficulties in the creation of a dossier that could meet all the listed requirements. Among the most critical and important aspects:
(a) speaking of dietary supplements, food industries cannot claim any therapeutic effect, but only physiological ones; as a consequence, apart from few exceptions, it is very difficult to prove a healthy activity that aims to maintain homeostasis or reducing disease risk factors in the long term;
(b) to obtain statistical significance, it is necessary to recruit a very large “healthy” population that is willing to take a certain product for very long periods. All this is economically unsustainable, and the results could still be affected by the dietary habits (and not only) of the subjects considered;
(c) it seems unreasonable that the tradition of use has been accepted for traditional medicine drugs and not for food supplements.
Considering the list of claims listed in Table 1, only one case can be clearly associated with food supplements: Monascus purpureus (red yeast rice); in the remaining cases, claims are related to ingredients generally used in all foods.
Red Yeast Rice (RYR) is a rice fermented by the red yeast Monascus purpureus, which contain a certain number of active polyketides named monacolins, that are secondary metabolites deriving from the fermentation process [29]. The most abundant active molecule is monacolin K.
RYR obtained the approval of the claim: “Monacolin K from red yeast rice contributes to the maintenance of normal blood cholesterol levels” [30]. This wording was supported by two randomized controlled studies, where RYR preparations providing 10 mg of monacolin K (with the daily dose) were responsible of a reduction of blood Low-Density Lipoprotein- cholesterol (LDL-cholesterol) level in subjects suffering from hypercholesterolemia. EFSA considered also the fact that the effect of pure monacolin K (chemically identical to the drug lovastatin) on blood LDL-cholesterol concentration was well known and based on the inhibition of HMG-CoA (3-Hydroxy-3-Methyl-Glutaryl-Coenzyme A) reductase [31].
6. Safety Aspects
This chapter deals with food, the safety of which should be able to be taken for granted. Actually, when botanicals are food supplement ingredients, some distinction has to be made. Compared to common or functional food, food supplements containing botanicals have no or little role from the nutritional point of view, as these do not contribute significantly to the intake of calories and nutrients. In fact, normally these products do not contain caloric nutrients (sugars, fats and proteins) and the presence of vitamins and minerals (not mandatory) covers, with a few exceptions, very limited percentages of the recommended daily dose. On the other hands, food supplements with botanicals are frequently promoted for their possible healthy properties, leading the consumer to prolonged use over time. Furthermore, as mentioned above, the growing popularity among consumers of “natural products” has led to an increasing number of producers and thousands of food supplements on the international market, without certainties relating their composition, activity and safety. While biological activity may be at least partly based on the “tradition of use”, the risk/benefit assessment still remains orphaned of sufficiently reliable scientific data.
Botanicals, especially if in their extract form, can have biological effects which, in relation to the dose and quality of the ingredient, can be functional, therapeutic and also toxic [32]. Different factors could be involved in the incidence of adverse effects such as the quality of the raw botanical material, the presence of environmental contaminants, the specific technological process applied (extraction and concentration), misidentifications of the plant ingredients, adulterations, counterfeits. The phytochemical fingerprint of a botanical can vary significantly, when the biological variability or the different processes applied are considered. Different factors (extractant solvent, the temperature and time, the time from harvesting, etc.) can modulate the quality of botanical ingredients, with consequent changes in the relative abundance of active molecules present in the final product. Consumer’s age and gender, unsuitable use of botanical food supplements, genetic factors and specific pathological or physiological conditions are further possible sources of unexpected adverse reactions.
Confirming the particularity of the supplements with respect to usual food, there are cases in which limits on the content of molecules naturally present in botanicals are regulated by national and international directives. The established values must also be indicated on the label, such as in the case of bitter orange (Citrus aurantium L.) for which it is necessary to declare the content of synephrine and other active amines with the daily dose (Figure 2). Limits are established for molecules that can lead to unwanted side effects at high levels and therefore for which safe daily doses for consumers need to be “standardized”.
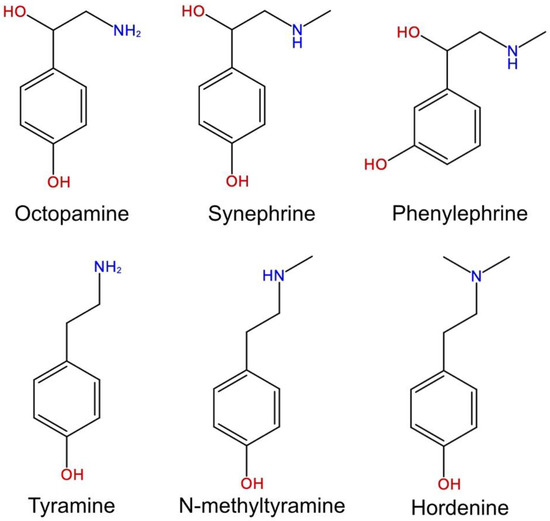
Figure 2.
Active amines of Citrus aurantium L. regulated for their content in food supplements.
6.1. Adverse Events
Even in the case of adverse events, supplements containing botanicals show specific characteristics. Excluding the accidental presence of toxic xenobiotics, the adverse events associated with common foods are mostly due to allergies and intolerances, which concern restricted groups of at-risk populations. On the contrary, in the case of supplements with botanicals, the consumer must be informed that adverse events can occur. Moreover, pregnant, lactating women and children should avoid food supplements or strictly follow the doctor’s/pediatrician’s indications.
Since botanicals are “natural”, the average consumer considers them safe and rarely communicates their use to doctors in the case of short- or long-term therapy. This point is probably the most important factor leading to unexpected adverse effects. Botanicals could modify the efficacy of other molecules (pharmaceutical drugs or other dietetic compounds) reducing or increasing their plasmatic concentration. To give an example of the possible adverse events to supplements containing botanicals, the case of RYR is described here.
6.2. The Case of RYR
As described previously (see Section 5), in 2011 RYR received a positive opinion for the claim supporting the reduction of LDL-cholesterol plasma level, if the daily intake of monacolin K was 10 mg. In 2018, the EFSA was asked a second time in relation to RYR, with the request being to evaluate the safety of the monacolins contained in it and to provide advice on the daily dose of monacolins that does not give rise to concerns for harmful effects [33]. Since the lactonic form of monacolin K has the same chemical structure of lovastatin (a pharmaceutical drug), the EFSA, after consulting the scientific material provided, concluded that the intake of 10 mg of monacolin K with a food supplement would lead to the intake of a therapeutic dose. Moreover, the evaluation of the data presented showed that several adverse events were reported following the consumption of RYR. These adverse events involved musculoskeletal system (the most severe clinical event associated was rhabdomyolysis) and liver. The clinical symptoms appeared after consumption of RYR, with an intake of 3–10 mg/day of monacolin K.
Two problems have therefore been highlighted for RYR:
- the dose of monacolin K required to boast the claim about the LDL-cholesterol reduction corresponded to the lower therapeutic dose of the drug lovastatin, chemically identical to the lactonic form of monacolin K. This fact is not acceptable due to the legal definition of a dietary supplement, which must not show pharmacological activity.
- EFSA “was unable to identify a dietary intake of monacolins from RYR that does not give rise to concerns about harmful effects to health, for the general population, and as appropriate, for vulnerable subgroups of the population” [33]. This fact makes uncertain the marketing of RYR as food supplement, products that do not require medical supervision.
7. Conclusions
Since the last century, a progressive lengthening of the average life of the population has been observed; this social phenomenon is in itself a positive fact, but inevitably entails an increase in the occurrence of chronic-generative diseases more frequent in elderly: cardiovascular diseases, tumors, dementias, etc. At the same time, new data on the role of nutrition in human health has stimulated important international researches and industrial activities:
- the tables of nutritional requirements (RDA) have been prepared which, with some differences, indicate to consumers all over the world the quantity of calories and nutrients necessary to maintain health;
- the possible protective role of some components present in foods (antioxidants are the most popular) against the chronic-degenerative diseases described above has been highlighted.
These premises explain the great interest of the food industry for products that present, in addition to nutritional ingredients (proteins, carbohydrates and fats), new “functional” properties, which have special impact on the consumer. In this area, particular interest has been paid to ingredients of vegetable origin, the botanicals. Most products containing botanicals are regulated by food law: fruits and vegetables, functional foods, novel foods, traditional foods from third countries, and food supplements. Some botanical ingredients have received positive opinion by EFSA to support their health claims; fibers, beta glucans and phytosterols/phytostanols are among the most known functional ingredients. Food supplements with botanical ingredients are the sector that has witnessed the greatest development, in terms of both the number of products on the market and consumer acceptability. The availability of these “new” functional products can positively influence the well-being of the population, but it is essential to provide the consumer with the necessary information to guide him in the purchase and use of food supplements.
The difficulties in demonstrating scientifically the supposed physiological/healthy properties of botanicals are the reasons of the limited number of claims approved by EFSA. In addition, safety aspects should be considered in relation to the high number of products available on the market and their growing popularity among consumers: among other things, the quality of raw materials, possible adulterations, presence of active molecules regulated by national/international directives and factors associated with consumers (age, gender, concomitant diseases/drugs). These factors can contribute to adverse effects occurring, underlying the need for tools and intervention to promote consumers safety.
In conclusion, this review provides an overview on the actual knowledge about products containing botanicals aimed to promote consumers’ wellbeing. The main purpose is to provide useful information to consumers in order to guide their choices. In fact, although much progress has been made in the field of botanicals, there is still a lack of data, information, and guidelines to ensure the suitable use of these products.
Author Contributions
Investigation, F.C. and S.B.; Writing-Original Draft Preparation, P.R.; Data curation, S.B. and F.C.; Writing-Review & Editing, C.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This paper has been prepared in the framework of the MIUR Progetto di Eccellenza.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cordain, L.; Miller, J.B.; Eaton, S.B.; Mann, N.; Holt, S.H.A.; Speth, J.D. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am. J. Clin. Nutr. 2000, 71, 682–692. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Guidance on Safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements. EFSA J. 2009, 7, 1249. [Google Scholar]
- Food Processing Website. Available online: https://www.foodprocessing.com/articles/2012/defining-botanicals/ (accessed on 2 March 2020).
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Union L 2002, 31, 1–24.
- Diplock, A.; Aggett, P.J.; Ashwell, M.; Bornet, F.; Fern, E.B.; Roberfroid, M.B. The buckling of a cylindrical shell. Br. J. Nutr. 1999, 81, S1–S27. [Google Scholar] [CrossRef]
- European Council Regulations No. 2015/2283 Regulation (Eu) 2015/2283 on novel foods. Off. J. Eur. Union 2015, 327, 1–22.
- Italian Ministry of Health. Available online: http://www.salute.gov.it/portale/temi/p2_5.jsp?lingua=italiano&area=Alimentiparticolarieintegratori&menu=integratori (accessed on 2 March 2020).
- EFSA Website. Available online: https://www.efsa.europa.eu/en/topics/topic/food-supplements (accessed on 2 March 2020).
- Trovato, M.; Ballabio, C. Botanical Products: General Aspects. In Food Supplements Containing Botanicals: Benefits, Side Effects and Regulatory Aspects; Restani, P., Ed.; Springer: Cham, Switzerland, 2018; pp. 3–26. [Google Scholar]
- Coppens, P.; Pettman, S. The regulatory situation in Europe and other continents. In Food Supplements Containing Botanicals: Benefits, Side Effects and Regulatory Aspects; Restani, P., Ed.; Springer: Cham, Switzerland, 2018; pp. 27–59. [Google Scholar]
- EU Website. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/health_claims_en (accessed on 2 March 2020).
- EFSA. General scientific guidance for stakeholders on health claim applications. EFSA J. 2016, 14, 4367. [Google Scholar]
- EU Website. Available online: http://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=search (accessed on 2 March 2020).
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- International Scientific Association for Probiotics and Prebiotics (ISAPP). Available online: https://isappscience.org/for-scientists/resources/prebiotics/ (accessed on 2 March 2020).
- Pineiro, M.; Asp, N.G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, J.O.; Tuohy, K. FAO Technical Meeting on Prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef]
- Niness, K.R. Inulin and oligofructose: What are they? J. Nutr. 1999, 129, 1402S–1406S. [Google Scholar] [CrossRef]
- Torres, D.P.M.; Gonçalves, M.D.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, properties, applications, and significance as prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Verduci, E.; Gregori, D.; Ballali, S.; Soldi, S.; Ghisleni, D.; Riva, E.; PLAGOS Trial Study Group. Prebiotic Effect of an Infant Formula Supplemented with Galacto-Oligosaccharides: Randomized Multicenter Trial. J. Am. Coll. Nutr. 2014, 33, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Helsper, J.P.F.G.; Wei, S.; Baars, J.J.P.; van Griensven, L.J.L.D.; Sonnenberg, A.S.M.; Mensink, R.P.; Plat, J. Effects of mushroom-derived β-glucan-rich polysaccharide extracts on nitric oxide production by bone marrow-derived macrophages and nuclear factor-κb transactivation in Caco-2 reporter cells: Can effects be explained by structure? Mol. Nutr. Food Res. 2010, 54, 268–276. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID1236, 1299); increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycemic responses (ID 821 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2207. [Google Scholar]
- Battilana, P.; Ornstein, K.; Minehira, K.; Schwarz, J.M.; Acheson, K.; Schneiter, P.; Burri, J.; Jéquier, E.; Tappy, L. Mechanisms of action of β-glucan in postprandial glucose metabolism in healthy men. Eur. J. Clin. Nutr. 2001, 55, 327–333. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the substantiation of health claims related to beta-glucans and maitenence of normal blood cholesterol concentrations (ID 754, 755, 757, 801, 1465, 2934) and maintenance or achievement of a normal body weight (ID 820, 823) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1254. [Google Scholar]
- Wang, Y.; Harding, S.V.; Thandapilly, S.J.; Tosh, S.M.; Jones, P.J.H.; Ames, N.P. Barley β-glucan reduces blood cholesterol levels via interrupting bile acid metabolism. Br. J. Nutr. 2017, 118, 822–829. [Google Scholar] [CrossRef]
- Cantrill, R. Phytosterols, Phytostanols and Their Esters. Chemical and Technical Assessment. Available online: http://www.fao.org/fileadmin/templates/agns/pdf/jecfa/cta/69/Phytosterols.pdf (accessed on 2 March 2020).
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- EFSA. Plant Sterols and Blood Cholesterol-Scientific substantiation of a health claim related to plant sterols and lower/reduced blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006-Scientific. EFSA J. 2008, 781, 1–12. [Google Scholar]
- Liu, J.; Zhang, J.; Shi, Y.; Grimsgaard, S.; Alraek, T.; Fønnebø, V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: A meta-analysis of randomized controlled trials. Chin. Med. 2006, 1, 4. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL cholesterol concentrations (ID 1648, 1700) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2304. [Google Scholar]
- Alberts, A.W. Lovastatin and simvastatin–inhibitors of hmg coa reductase and cholesterol biosynthesis. Cardiology 1990, 77, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Ceschi, A.; Kupferschmidt, H.; Lüde, S.; De Souza Nascimento, E.; Dos Santos, A.; Colombo, F.; Frigerio, G.; Nørby, K.; Plumb, J.; et al. Adverse effects of plant food supplements and botanical preparations: A systematic review with critical evaluation of causality. Br. J. Clin. Pharmacol. 2015, 79, 578–592. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 2018, 16, 5368. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).