Antioxidant Properties of a Supercritical Fluid Extract of the Halophyte Mesembryanthemum nodiflorum L. from Sicilian Coasts: Nutraceutical and Cosmeceutical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Plant Material
2.1.1. Extraction with Solvents
2.1.2. Extraction with Supercritical Fluid Extraction (SFE)
2.1.3. Total Phenolic Content
2.1.4. DPPH Assay
2.1.5. Iron-Reducing Power
2.2. Evaluation of Cytotoxicity and Protective Effect in Fibroblast Cell Line HS-68
2.2.1. Hs68 Cell Culture
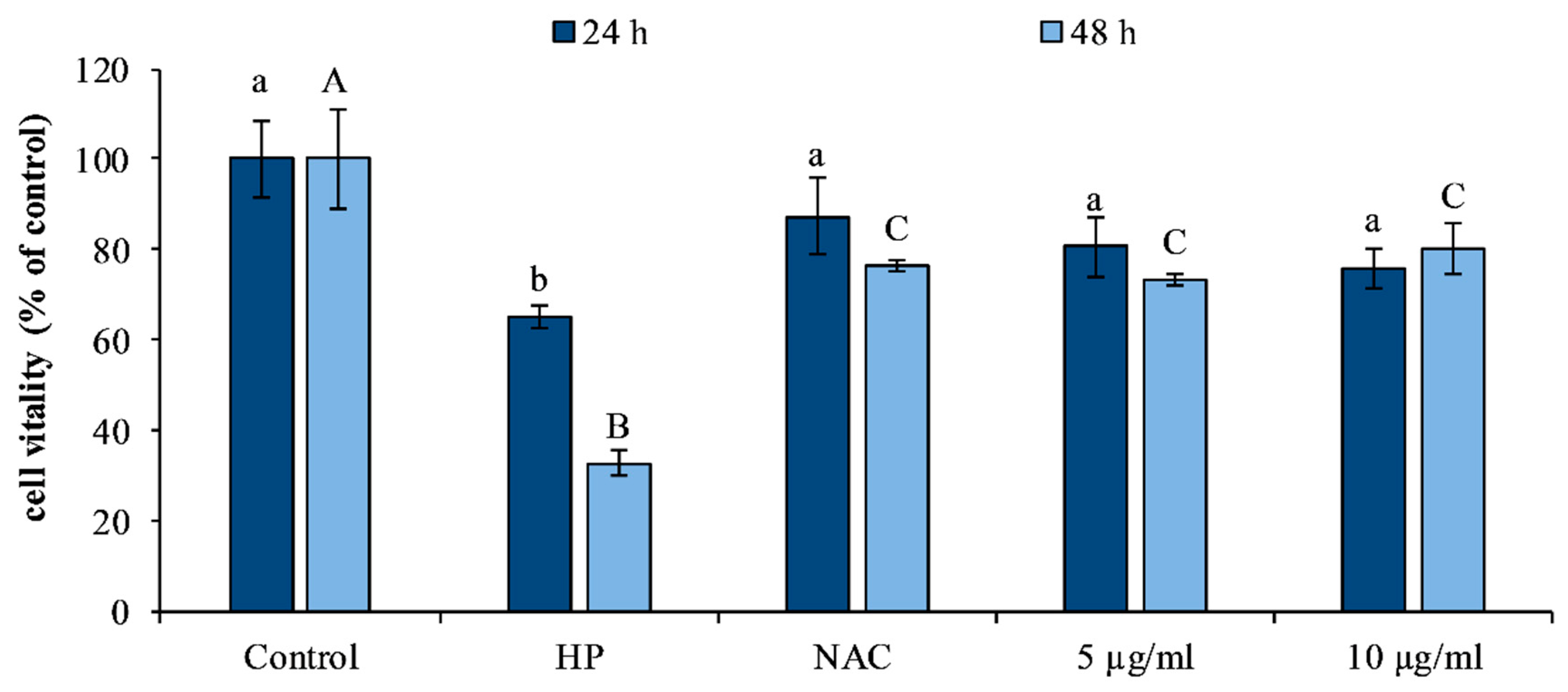
2.2.2. Cytotoxicity Assay and Protective Effect of M. nodiflorum Extract
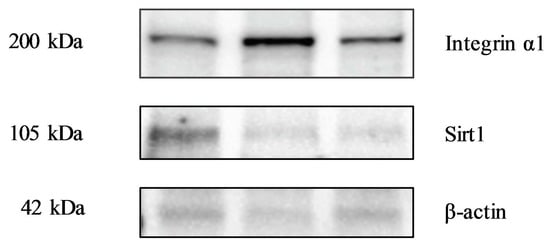
2.2.3. Evaluation of Molecular Markers by Immunoblotting
2.3. Statistical Analysis
3. Results and Discussions
3.1. Extraction and Evaluation of Antioxidant Power
3.2. Total Phenolic Content Analysis
3.3. DPPH Assay Analysis
3.4. Iron-Reducing Power Analysis
3.5. Evaluation of Cytotoxicity and Protective Effect in Fibroblast Cell Line HS-68
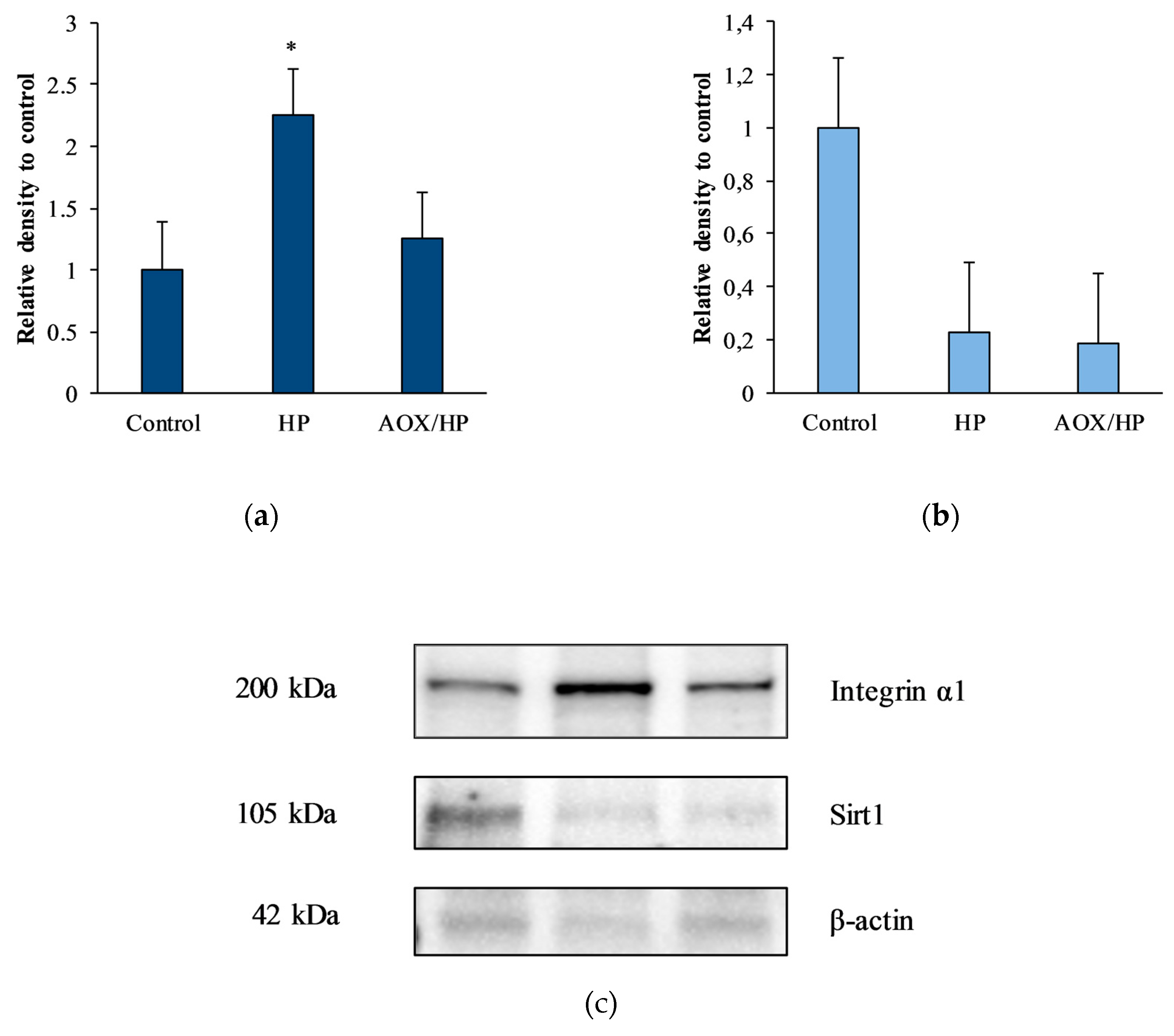
3.6. Evaluation of Biomolecular Markers by Immunoblotting
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sabovljevic, M.; Sabovljevic, A. Contribution to the coastal bryophytes of the Northern Mediterranean: Are there halophytes among bryophytes? Phytol. Balc. 2007, 13, 131–135. [Google Scholar]
- Aslam, R.; Bostan, N.; Nabgha-e-Amen; Maria, M.; Safdar, W. A critical review on halophytes: Salt tolerant plants. J. Med. Plant Res. 2011, 5, 7108–7118. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Rodrigues, M.J.; Pereira, C.; Oliveira, M.; Barreira, L.; Varela, J.; Trampetti, F.; Custódio, L. Natural products from extreme marine environments: Searching for potential industrial uses within extremophile plants. Ind. Crops Prod. 2016, 94, 299–307. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C. R. Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef]
- Boestfleisch, C.; Wagenseil, N.B.; Buhmann, A.K.; Seal, C.E.; Wade, E.M.; Muscolo, A.; Papenbrock, J. Manipulating the antioxidant capacity of halophytes to increase their cultural and economic value through saline cultivation. AoB Plants 2014, 6, 1–16. [Google Scholar] [CrossRef]
- Messina, C.M.; Troia, A.; Arena, R.; Manuguerra, S.; Ioannou, T.; Curcuraci, E.; Renda, G.; Hellio, C.; Santulli, A. Species-Specific Antioxidant Power and Bioactive Properties of the Extracts Obtained from Wild Mediterranean Calendula spp. (Asteraceae). Appl. Sci. 2019, 9, 4627. [Google Scholar] [CrossRef]
- Messina, C.M.; Renda, G.; Laudicella, V.A.; Trepos, R.; Fauchon, M.; Hellio, C.; Santulli, A. From ecology to biotechnology, study of the defense strategies of algae and halophytes (from Trapani Saltworks, NW Sicily) with a focus on antioxidants and antimicrobial properties. Int. J. Mol. Sci. 2019, 20, 881. [Google Scholar] [CrossRef]
- Yang, J.; Yen, H.E. Early salt stress effects on the changes in chemical composition in leaves of ice plant and Arabidopsis. A Fourier transform infrared spectroscopy study. Plant Physiol. 2002, 130, 1032–1042. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Oueslati, S.; Guyot, S.; Magné, C.; Abdelly, C. Interspecific variability of antioxidant activities and phenolic composition in Mesembryanthemum genus. Food Chem. Toxicol. 2009, 47, 2308–2313. [Google Scholar]
- Lee, S.Y.; Choi, H.D.; Yu, S.N.; Kim, S.H.; Park, S.K.; Ahn, S.C. Biological Activities of Mesembryanthemum crystallinum (Ice plant) Extract. J. Life Sci. 2015, 25, 638–645. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Medini, F.; Guyot, S.; Abdelly, C.; Magné, C. Antioxidant activity and phenolic composition of the medicinal and edible halophyte Mesembryanthemum edule L. Ind. Crops Prod. 2011, 34, 1066–1071. [Google Scholar] [CrossRef]
- Soliman, M.I.; Zaghloul, M.S.; Heikal, Y.M. Genetic variation within and among three Egyptian Mesembryanthemum species using different genetic markers. Egypt. J. Basic Appl. Sci. 2014, 1, 127–135. [Google Scholar] [CrossRef][Green Version]
- Mustafa, A.I.; Al-Jassir, M.S.; Nawawy, M.A.; Ahmed, S.E. Studies on samh seeds (Mesembryanthemum forsskalei Hochst) growing in Saudi Arabia: 3. Utilization of samh seeds in bakery products. Plant Foods Hum. Nutr. 1995, 48, 279–286. [Google Scholar] [CrossRef] [PubMed]
- El-Amier, Y.A.; Haroun, S.A.; El-Shehaby, O.A.; Al-Hadithy, O.N. Antioxidant and Antimicrobial Properties of Some Wild Aizoaceae Species Growing in Egyptian Desert. J. Environ. Sci. 2016, 45, 1–10. [Google Scholar]
- Doudach, L.; Meddah, B.; Benbacer, L.; Hammani, K.; El Mzibri, M.; Verité, P.; Elomri, A.; Cherrah, Y. Ethnopharmacological studies of Mesembryanthemum nodiflorum. Phytopharmacology 2013, 4, 246–258. [Google Scholar]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef]
- Boudjadi, S.; Beaulieu, J.F. ITGA1 (integrin, alpha 1). Atlas Genet. Cytogenet. Oncol. Haematol. 2017, 19, 86–89. [Google Scholar] [CrossRef][Green Version]
- Law, I.K.M.; Liu, L.; Xu, A.; Lam, K.S.L.; Vanhoutte, P.M.; Che, C.M.; Leung, P.T.Y.; Wang, Y. Identification and characterization of proteins interacting with SIRT1 and SIRT3: Implications in the antiaging and metabolic effects of sirtuins. Proteomics 2009, 9, 2444–2456. [Google Scholar] [CrossRef]
- Franco, D.; Sineiro, J.; Rubilar, M.; Sánchez, M.; Jerez, M.; Pinelo, M.; Costoya, N.; Núñez, M.J. Polyphenols from plant materials: Extraction and antioxidant power. Electron. J. Environ. Agric. Food Chem. 2008, 7, 3210–3216. [Google Scholar]
- Trabelsi, N.; Megdiche, W.; Ksouri, R.; Falleh, H.; Oueslati, S.; Soumaya, B.; Hajlaoui, H.; Abdelly, C. Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves. LWT Food Sci. Technol. 2010, 43, 632–639. [Google Scholar] [CrossRef]
- Gharbi, S.; Renda, G.; La Barbera, L.; Amri, M.; Messina, C.M.; Santulli, A. Tunisian tomato by-products, as a potential source of natural bioactive compounds. Nat. Prod. Res. 2017, 31, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A Quantitative Assay for Lycopene That Utilizes Reduced Volumes of Organic Solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant Activity of Pink-Flesh Guava (Psidium guajava L.): Effect of Extraction Techniques and Solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Manuguerra, S.; Caccamo, L.; Mancuso, M.; Arena, R.; Rappazzo, A.C.; Genovese, L.; Santulli, A.; Messina, C.M.; Maricchiolo, G. The antioxidant power of horseradish, Armoracia rusticana, underlies antimicrobial and antiradical effects, exerted In Vitro. Nat. Prod. Res. 2018, 1–4. [Google Scholar] [CrossRef]
- Messina, C.M.; Manuguerra, S.; Renda, G.; Santulli, A. Biotechnological Applications for the Sustainable Use of Marine By-products: In Vitro Antioxidant and Pro-apoptotic Effects of Astaxanthin Extracted with Supercritical CO2 from Parapeneus longirostris. Mar. Biotechnol. 2019, 21, 565–576. [Google Scholar] [CrossRef]
- Messina, C.; Renda, G.; Randazzo, M.; Laudicella, A.; Gharbi, S.; Pizzo, F.; Morghese, M.; Santulli, A. Extraction of bioactive compounds from shrimp waste. Bull. Inst. Natl. Sci. Technol. Mer Salammbô 2015, 42, 27–29. [Google Scholar]
- Anaëlle, T.; Serrano Leon, E.; Laurent, V.; Elena, I.; Mendiola, J.A.; Stéphane, C.; Nelly, K.; Stéphane, L.B.; Luc, M.; Valérie, S.P. Green improved processes to extract bioactive phenolic compounds from brown macroalgae using Sargassum muticum as model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Oki, T.; Masuda, M.; Furuta, S.; Nishiba, Y.; Terahara, N.; Suda, I. Involvement of anthocyanins and other phenolic compounds in radical-scavenging activity of purple-fleshed sweet potato cultivars. J. Food Sci. 2002, 67, 1752–1756. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Masteikova, R.; Davalgiene, J.; Peciura, R.; Gauryliene, R.; Bernatoniene, R.; Majiene, D.; Lazauskas, R.; Civinskiene, G.; Velziene, S.; et al. Topical application of Calendula officinalis (L.): Formulation and evaluation of hydrophilic cream with antioxidant activity. J. Med. Plants Res. 2011, 5, 868–877. [Google Scholar]
- Yeddes, N.; Chérif, J.; Guyot, S.; Sotin, H.; Ayadi, M. Comparative Study of Antioxidant Power, Polyphenols, Flavonoids and Betacyanins of the Peel and Pulp of Three Tunisian Opuntia Forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from Glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Messina, C.M.; Pizzo, F.; Santulli, A.; Bušelić, I.; Boban, M.; Orhanović, S.; Mladineo, I. Anisakis pegreffii (Nematoda: Anisakidae) products modulate oxidative stress and apoptosis-related biomarkers in human cell lines. Parasit. Vectors 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Abbes, M.; Baati, H.; Guermazi, S.; Messina, C.; Santulli, A.; Gharsallah, N.; Ammar, E. Biological properties of carotenoids extracted from Halobacterium halobium isolated from a Tunisian solar saltern. BMC Complement. Altern. Med. 2013, 13, 1–8. [Google Scholar] [CrossRef]
- Taherian, A.; Li, X.; Liu, Y.; Haas, T.A. Differences in integrin expression and signaling within human breast cancer cells. BMC Cancer 2011, 11, 1–15. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Manuguerra, S.; Ruiz, C.E.; Santulli, A.; Messina, C.M. Sub-lethal doses of polybrominated diphenyl ethers, In Vitro, promote oxidative stress and modulate molecular markers related to cell cycle, antioxidant balance and cellular energy management. Int. J. Environ. Res. Public Health 2019, 16, 588. [Google Scholar] [CrossRef] [PubMed]
- Kalpna, R.; Mital, K.; Sumitra, C. Vegetable and fruit peels as a novel source of antioxidants. J. Med. Plants Res. 2011, 5, 63–71. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative Properties of Xanthan on the Autoxidation of Soybean Oil in Cyclodextrin Emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Falleh, H.; Oueslati, S.; Guyot, S.; Dali, A.B.; Magné, C.; Abdelly, C.; Ksouri, R. LC/ESI-MS/MS characterisation of procyanidins and propelargonidins responsible for the strong antioxidant activity of the edible halophyte Mesembryanthemum edule L. Food Chem. 2011, 127, 1732–1738. [Google Scholar] [CrossRef]
- Zhao, G.R.; Xiang, Z.J.; Ye, T.X.; Yuan, Y.J.; Guo, Z.X. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng. Food Chem. 2006, 99, 767–774. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT Food Sci. Technol. 2004, 37, 717–721. [Google Scholar] [CrossRef]
- Ghafoor, K.; Park, J.; Choi, Y.H. Optimization of supercritical fluid extraction of bioactive compounds from grape (Vitis labrusca B.) peel by using response surface methodology. Innov. Food Sci. Emerg. Technol. 2010, 11, 485–490. [Google Scholar] [CrossRef]
- Messina, C.M.; Panettieri, V.; Arena, R.; Renda, G.; Espinosa Ruiz, C.; Morghese, M.; Piccolo, G.; Santulli, A.; Bovera, F. The Inclusion of a Supercritical Fluid Extract, Obtained From Honey Bee Pollen, in the Diet of Gilthead Sea Bream (Sparus aurata), Improves Fish Immune Response by Enhancing Anti-oxidant, and Anti-bacterial Activities. Front. Vet. Sci. 2020, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Deters, A.M.; Meyer, U.; Stintzing, F.C. Time-dependent bioactivity of preparations from cactus pear (Opuntia ficus indica) and ice plant (Mesembryanthemum crystallinum) on human skin fibroblasts and keratinocytes. J. Ethnopharmacol. 2012, 142, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, Y.K.; Lin, Y.H. Photoprotective Effects of Ice Plant (Mesembryanthemum crystallinum) Callus Extract on Gene Expression of Human Dermal Fibroblast Against UV Exposure. J. Biobased Mater. Bioenerg. 2019, 13, 570–575. [Google Scholar] [CrossRef]
- Giancotti, F.G. Complexity and specificity of integrin signalling. Nat. Cell Biol. 2000, 2, E13–E14. [Google Scholar] [CrossRef]
- Beauséjour, M.; Noël, D.; Thibodeau, S.; Bouchard, V.; Harnois, C.; Beaulieu, J.F.; Demers, M.J.; Vachon, P.H. Integrin/Fak/Src-mediated regulation of cell survival and anoikis in human intestinal epithelial crypt cells: Selective engagement and roles of PI3-K isoform complexes. Apoptosis 2012, 17, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Langholz, O.; Röckel, D.; Mauch, C.; Kozlowska, E.; Bank, I.; Krieg, T.; Eckes, B. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by α1β1 and α2β1 integrins. J. Cell Biol. 1995, 131, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Jean, C.; Gravelle, P.; Fournie, J.J.; Laurent, G. Influence of stress on extracellular matrix and integrin biology. Oncogene 2011, 30, 2697–2706. [Google Scholar] [CrossRef]
- Defilippi, P.; Van Hinsbergh, V.; Bertolotto, A.; Rossino, P.; Silengo, L.; Tarone, G. Differential distribution and modulation of expression of Alpha1/beta1 integrin on human endothelial cells. J. Cell Biol. 1991, 114, 855–863. [Google Scholar] [CrossRef]
- Lin, Z.; Fang, D. The Roles of SIRT1 in Cancer. Genes Cancer 2013, 4, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Q.; Wang, M.; Liang, M.; Yang, X.; Xu, X.; Zou, H.; Qiu, J. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in Fibroblasts. PLoS ONE 2011, 6, e27081. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gan, Q.; Han, L.; Li, J.; Zhang, H.; Sun, Y.; Zhang, Z.; Tong, T. SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PLoS ONE 2008, 3, e1710. [Google Scholar] [CrossRef]
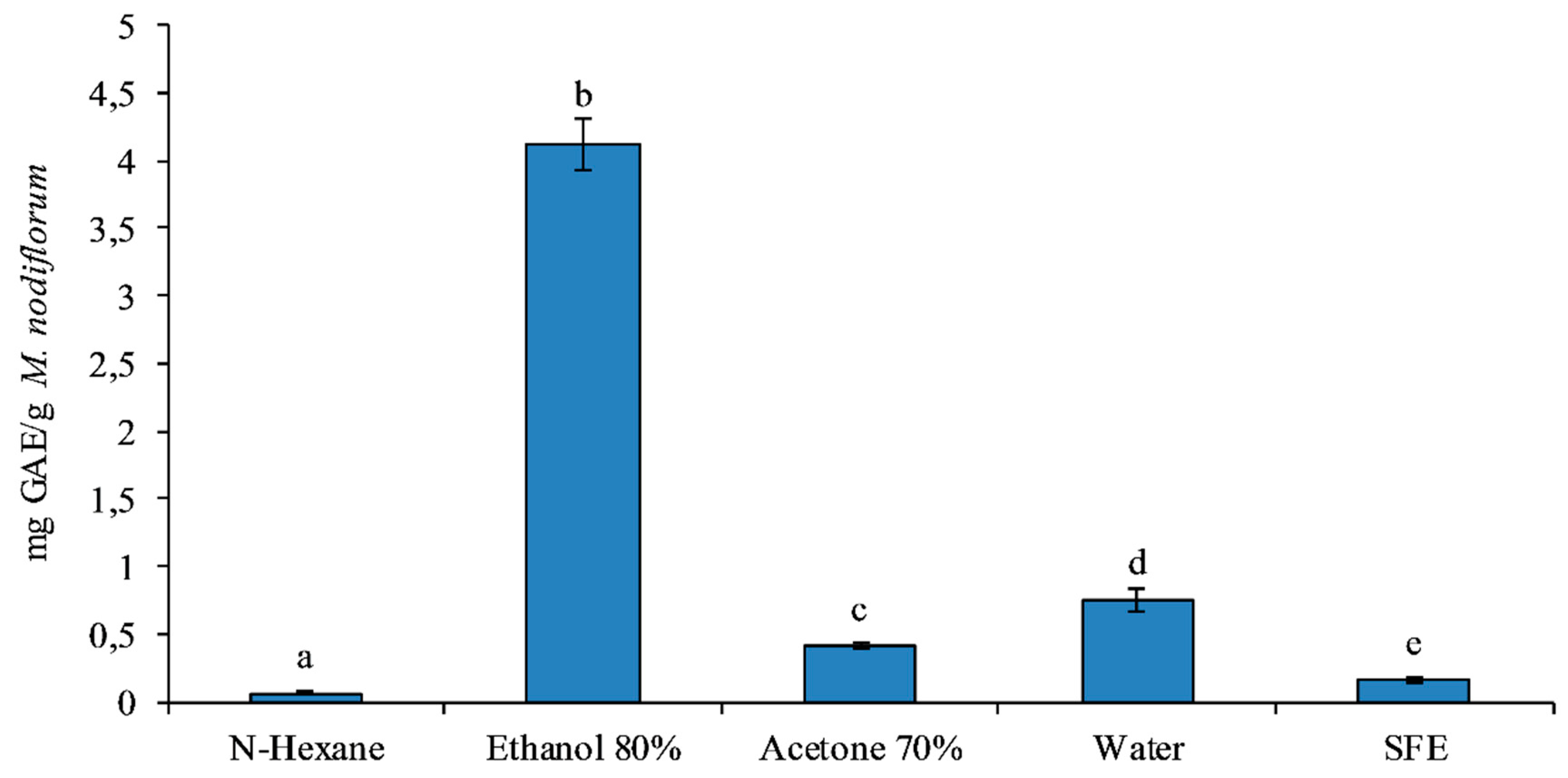

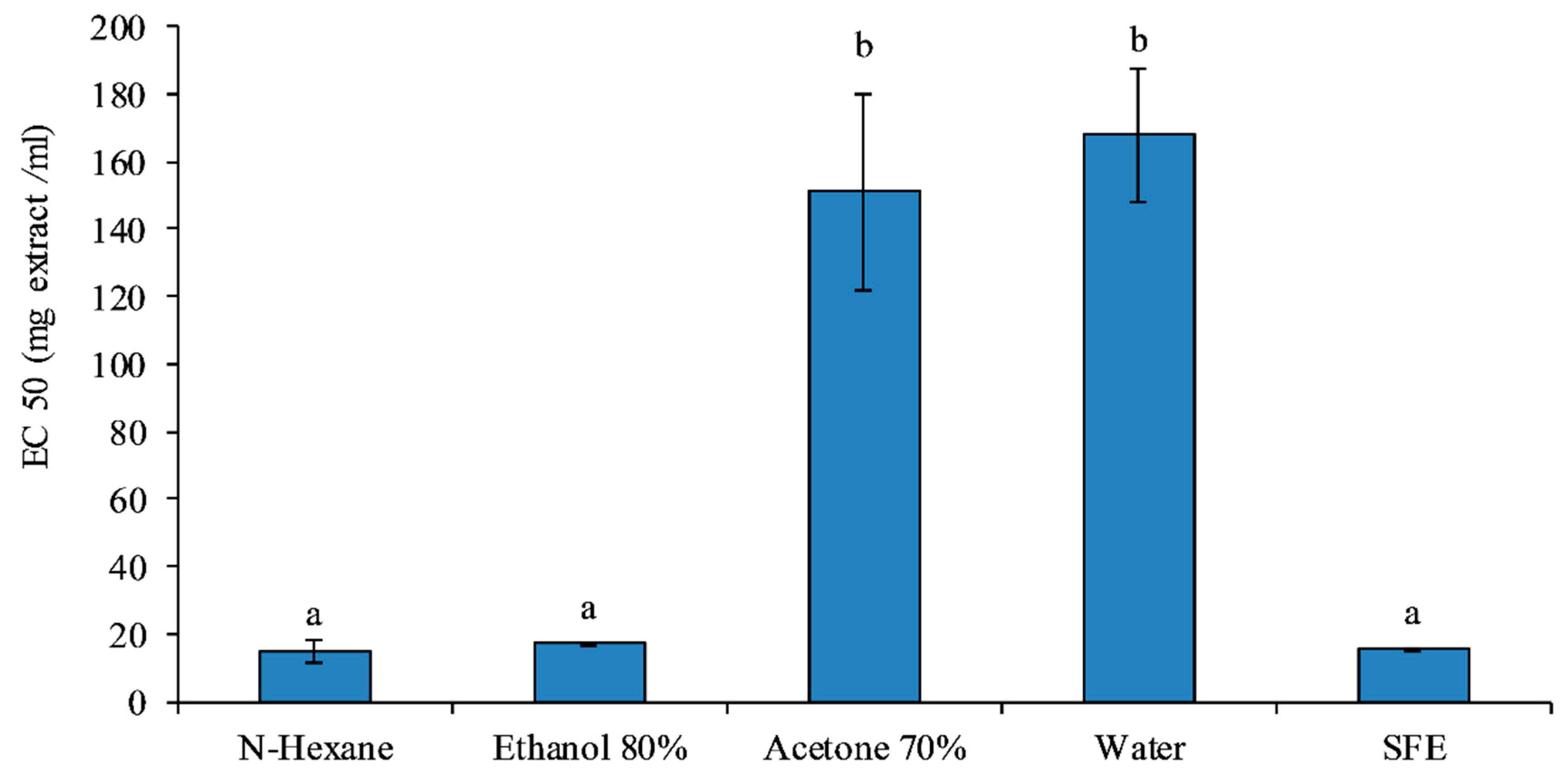
| Solvent | Yield (g/100) |
|---|---|
| N-Hexane | 0.43 ± 0.28 a |
| Ethanol | 18.39 ± 1.69 b |
| Acetone | 12.30 ± 2.45 c |
| Water | 33.32 ± 8.37 d |
| SFE | 0.79 ± 0.27 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arena, R.; Manuguerra, S.; Collins, E.; Mahdhi, A.; Renda, G.; Messina, C.M.; Santulli, A. Antioxidant Properties of a Supercritical Fluid Extract of the Halophyte Mesembryanthemum nodiflorum L. from Sicilian Coasts: Nutraceutical and Cosmeceutical Applications. Appl. Sci. 2020, 10, 2374. https://doi.org/10.3390/app10072374
Arena R, Manuguerra S, Collins E, Mahdhi A, Renda G, Messina CM, Santulli A. Antioxidant Properties of a Supercritical Fluid Extract of the Halophyte Mesembryanthemum nodiflorum L. from Sicilian Coasts: Nutraceutical and Cosmeceutical Applications. Applied Sciences. 2020; 10(7):2374. https://doi.org/10.3390/app10072374
Chicago/Turabian StyleArena, Rosaria, Simona Manuguerra, Edward Collins, Abdelkarim Mahdhi, Giuseppe Renda, Concetta Maria Messina, and Andrea Santulli. 2020. "Antioxidant Properties of a Supercritical Fluid Extract of the Halophyte Mesembryanthemum nodiflorum L. from Sicilian Coasts: Nutraceutical and Cosmeceutical Applications" Applied Sciences 10, no. 7: 2374. https://doi.org/10.3390/app10072374
APA StyleArena, R., Manuguerra, S., Collins, E., Mahdhi, A., Renda, G., Messina, C. M., & Santulli, A. (2020). Antioxidant Properties of a Supercritical Fluid Extract of the Halophyte Mesembryanthemum nodiflorum L. from Sicilian Coasts: Nutraceutical and Cosmeceutical Applications. Applied Sciences, 10(7), 2374. https://doi.org/10.3390/app10072374