Abstract
The presence of pharmaceuticals in water bodies is associated with the increasing consumption of these substances and limited elimination from wastewater. Pharmaceutical residues and their metabolites may have an unfavorable impact on fish and other aquatic biota. As the purification of wastewater from tramadol is very limited and the knowledge on its effects on non-target organisms is low, we decided to assess the subchronic impact of tramadol hydrochloride on fish—on the mortality, growth and histopathology, together with the impact on selected indices of oxidative stress. The juvenile growth toxicity test was carried out on zebrafish (Danio rerio), in accordance with the Organisation for European Economic Cooperation Guidelines 215 (Fish, Juvenile Growth Test). The fish were exposed to a range of tramadol hydrochloride concentrations (0.2, 2, 20, 200 and 600 µg/L) for 28 days. The outcome of this study suggests that chosen concentrations of tramadol hydrochloride did not affect either mortality or growth (regarding weight, length and specific growth rate). However, the results of this study indicate that 28-day exposure can negatively influence selected indices of oxidative stress, which is a harmful imbalance between free radicals and antioxidants in an organism. A significant increase was observed in glutathione S-transferase activity in the experimental group exposed to 2 µg/L tramadol hydrochloride, compared to the control. Moreover, lipid peroxidation was observed in groups exposed to 20 and 200 µg/L, in comparison to the control.
1. Introduction
In recent years, pharmaceuticals have become one of the most important environmental contaminants. Pharmaceuticals enter the aquatic environment as a consequence of their increasing use in human and veterinary medicine and incomplete removal during wastewater treatment processes [1,2,3,4,5].
Pharmaceutical residues and their metabolites are being reported to be present in surface water, as well as groundwater. In the aquatic environment, pharmaceuticals from many different classes (such as antibiotics, antidepressants, analgesics, antiepileptics, etc.) have been found worldwide, at concentration levels ranging from ng/L to μg/L [6,7,8,9,10,11,12]. Moreover, extremely high concentrations in the order of mg/L have also been reported in India, China, Korea and Israel [13].
Although residues of pharmaceuticals occur in water bodies mostly at minor levels, there is a risk that they may negatively affect a number of non-target organisms (both vertebrates and invertebrates), as these substances are designed to induce a biological effect in organisms at minor concentrations [14,15].
Tramadol hydrochloride is a synthetic opioid analgesic drug used to relieve pain but also to treat anxiety and depression [15,16]. This substance has become the most prescribed opioid worldwide [17]. Tramadol interacts with opioid, adrenergic and serotonin receptors, and with its metabolites, it is mainly excreted through kidneys, with a mean elimination half-life of about 6 h [18,19]. The unchanged excretion of the tramadol parent compound in urine is about 15%–35% [7].
Tramadol hydrochloride belongs to the group of frequently detected substances in aquatic environments, with reported concentrations of tens of ng/L. Higher concentrations, reaching up to hundreds of ng/L, are found in locations with elevated anthropogenic activities or near an effluent of wastewater treatment plants [20,21,22]. Grabicova et al. [11] determined an average tramadol concentration in water samples from Czech stream (Zivny stream—tributary of Blanice river) to be highly affected by wastewater-treatment-plant effluents; the measured values reached up to 1400 ng/L. However, this pharmaceutical was also reported in samples from the control site (upstream of the wastewater treatment plant), with concentrations ranging from 4 to 33 ng/L. Wick et al. [23] reported tramadol at a concentration of 0.24 µg/L in sewage-treatment-plant influent and 0.23 µg/L in effluent (in Germany). Even higher levels were found by Kasprzyk-Hordern et al. [7] in raw sewage in the United Kingdom (average concentration > 30 µg/L). As pointed out by Tanoue et al. [24], most psychoactive drugs are soluble in water, because they need to cross the blood–brain barrier in order to have their desired effects. Unfortunately, their hydrophobicity means that, once they enter aquatic environments, they will be in the form to be taken up by aquatic organisms. Moreover, various drugs might concentrate in invertebrates, resulting in exposing predators (including fish) to levels exceeding surface-water concentrations [25]. Bachour et al. [26] observed hypoactivity in zebrafish larvae after 144 h exposure to a range of various tramadol concentrations, with the lowest observed effect concentration at 320 µg/L. Buřič et al. [14] and Ložek et al. [15] found behavioral changes—in particular, increased shelter dwelling—induced by tramadol exposure at a concentration as low as 1 µg/L in crayfish.
Apart from behavioral effects, tramadol has been proven to have significant effects on development retardation and cause inhibition of growth in Cyprinus carpio early life stages after 32-days-long exposure to tramadol concentrations starting at 10 µg/L. Moreover, histopathological examination revealed gill hyperemia [27].
Thus, the purpose of this study is to assess long-term effects of tramadol hydrochloride on the survival, development and histopathology (on the development of histopathological changes in selected organs—gill, kidney, liver, brain and skin) of juvenile zebrafish (Danio rerio), as well as its related effects on selected indices of oxidative stress. Thus, the activities of the enzymes glutathione S-transferase (GST), glutathione reductase (GR), glutathione peroxidase (GPx) and catalase (CAT), and the products of the lipid peroxidation (thiobarbituric acid reactive substances—TBARS) were determined.
2. Materials and Methods
2.1. Design of Experiment
The toxicity tests were carried out according to the Organisation for European Economic Cooperation (OECD) Guideline No. 215 Fish, Juvenile Growth Test [28]. Zebrafish (Danio rerio), at the age of 30 days, were chosen as the model organism for our study. This model organism belongs to the one of the most commonly used model species [29,30,31,32,33].
Moreover, the zebrafish is among the recommend organisms to perform Fish Juvenile Growth Test [28]. The fish used for the experiment were at the age of 30 days, as at this period of time, they were all considered to be at the juvenile stage [34].
Experimental organisms were exposed to a range of concentrations of tramadol hydrochloride (0.2, 2, 20, 200 and 600 µg/L). The duration of the subchronic toxicity test was 28 days. According to OECD guideline No. 215, five concentrations of the test substance are required. The two lowest concentrations were established with respect to the reported environmentally relevant concentration of this substance in surface water [7,23]. Additionally, 10×, 100× and 300× higher concentrations were also tested, in order to better understand the toxic effects of the substance tested.
The control and all experimental concentrations were tested in duplicate. The experiment was approved by the ethical committee of University of Veterinary and Pharmaceutical Sciences Brno (Czech Republic) and was in compliance with national legislation (Act No. 246/1992 Coll., on the Protection of Animals Against Cruelty, as amended, and Decree No. 419/2012 Coll., on the Protection, Breeding and Use of Experimental Animals, as amended).
A total of 600 fish were randomly divided into twelve 30-litre glass aquaria in dechlorinated tap water (50 in each aquarium). A toxicity test was performed in a flow-through system, with bath exchange every 12 h. Testing solutions of tramadol hydrochloride (chemical purity ≥ 99%, Sigma-Aldrich, Czech Republic) were prepared every day, and an ultrasonic bath was used to dissolve the substance. The initial body weight and total length were 11.34 ± 1.53 mg and 10.60 ± 4.83 mm (mean ± standard error of the mean), respectively. The fish were fed with commercial dried Artemia salina, without nutshells, at the total rate of 8% body weight. After fourteen days of toxicity test, the fish were weighed again, and the feed amount was recalculated. At the end of our experiment, the fish were euthanized (MS 222) and weighted, and their total lengths and tank-average specific growth rates (r) were determined.
During the toxicity tests, conditions, water quality and the number of dead fish were recorded in each aquarium, at 12-hour intervals. The values of water quality were as follows: temperature 25 ± 1 °C; oxygen saturation above 84 %; and pH from 7.94 to 8.24.
2.2. Histopathological Examination
Ten fish in each experimental group were used for histopathological examination. Fish were fixed in buffered 10% neutral formalin, dehydrated, embedded in paraffin wax, sectioned on a microtome at 4 μm and stained with hematoxylin and eosin. The qualitative histopathology analysis of selected tissues, such as gill, kidney, liver and intestine, was examined by light microscopy. Occurrence of abnormalities in experimental groups was compared to the control group.
2.3. Analysis of Selected Biomarkers in Whole-Body Homogenate
Whole-body homogenates were used for analysis of selected antioxidant enzymes (CAT, GR and GPx) [35,36,37], a detoxifying enzyme (GST) [38] and lipid peroxidation, using thiobarbituric acid (TBARS) [39]. At first, individual fish samples (ten in each experimental group) were mixed with phosphate buffer (pH 7.2) and homogenized (1:10; w/v). Lipid peroxidation was analyzed in pure homogenate. Enzyme analysis was performed in supernatant samples, which were obtained after centrifugation of homogenate (10,500 g, 4 °C, 20 min). All samples were immediately frozen and stored at -85 °C, until analysis. Analyses of all biomarkers were determined spectrophotometrically by a Varioskan Flash multimode microplate reader (Thermo Fisher Scientific, USA). Catalytic activities of all analyzed enzymes were normalized to protein concentration, determined by using bicinchoninic acid [40]. Amount of lipid peroxidation was expressed as nmol per gram wet weight of tissue.
2.4. Analysis of Tramadol Hydrochloride in Water
Tramadol hydrochloride was determined by high-performance liquid chromatography coupled with electrospray ionization–tandem mass spectrometry (HPLC–ESI–MS/MS). Water samples from each concentration were collected twice a day. Sample preparation included filtration through a 0.45 µm nylon filter (Millipore Billerica, MA), and a 10 µL aliquot of the final sample extract was injected. A Thermo Scientific HPLC Accela 1250 pump drew the mobile phase through a Hypersil C18 column (2.1 mm × 50 mm, 1.9 µm), into a mass spectrometer, Thermo Fisher Scientific TSQ Quantum Access MAX Triple Quadrupole Instrument (Thermo Fisher Scientific, USA). The mobile phase consisted of 0.1 mol/L ammonium formate:acetonitrile (90:10;v/v). Mass spectrometer used electrospray ionization probe (ESI) for ionization of the samples (Table 1). The ESI was operated in the positive-ion mode, under the following conditions: capillary temperature 375.0 °C; vaporizer temperature 300.0 °C; sheath gas pressure 35.0 psi; drying gas 10 a.u.; and capillary voltage 3500 V. The limit of detection was 0.145 µg/L. The internal standard was used as internal control.

Table 1.
LC/ESI/MS/MS analysis parameters for tramadol hydrochloride in water samples.
2.5. Statistical Evaluation
Statistical evaluation was performed by using statistical software—Unistat for Excel 5.6 (Unistat Ltd., GB). At first, all data were tested for normal distribution, using the Shapiro–Wilk test, and for homogeneity of variance, using the Levene test. The one-way analysis of variance, followed by post hoc Dunnett’s test, was used to determine the differences between the control and experimental groups. Level of significance was accepted at p < 0.05. All data are expressed as a mean ± standard deviation.
3. Results and Discussion
3.1. Mortality Rate
There was no increase in total cumulative mortality found between treated groups and the control. Among groups, mortality did not exceed 5% during the whole test. This result supports the finding from the previous study performed by Sehonova et al. [27], who tested similar concentrations of tramadol hydrochloride (from 10 to 200 µg/L) on zebrafish (Danio rerio) embryos and also on embryos and larvae of common carp (Cyprinus carpio). Likewise, Bachour et al. [26] did not record embryotoxicity of tramadol on zebrafish larvae. After 144 h of exposure (fish were exposed to tramadol concentrations from 24 to 6250 µg/L), no adverse impact of the tested substance was observed. Bachour et al. [26] also did not notice any effect, even after treatment with a mixture of tramadol and antidepressant citalopram. However, in a test performed by Sehonova et al. [12], where common carp embryos and larvae were exposed to a mixture of tramadol hydrochloride and naproxen sodium for a period of 32 days, increased mortality (50%) was already observed in the group of 200 µg/L.
Various authors have reported on the impact of tramadol on the behavior of aquatic organisms [14,15,26]. During our test, fish in all treated groups exhibited normal behavior. However, it is necessary to point out that monitoring of effects of tramadol on fish behavior was not the goal of our research, and thus no validated method on fish-behavior assessment was followed. Bachour et al. [26] monitored the swimming activity of zebrafish larvae and found out that a tramadol concentration of 320 µg/L had a significant anxiolytic effect (hypoactivity during dark conditions). Even lower concentrations can affect the behavior of some other aquatic organisms. An environmentally relevant concentration of tramadol (1 µg/L) caused crayfish to spend more time in shelters, and both their swimming velocity and swimming distance were affected [14,15].
3.2. Fish Growth
The effect of exposure of Danio rerio to tramadol hydrochloride on fish development is given in Table 2. Neither body weight at the beginning of the experiment nor body weight on last day of the experiment was found to be significantly different among groups. Similarly, no significant differences in average body weight, total length and specific growth rate were observed. In contrast, Sehonova et al. [27] reported inhibition of specific growth rate of common carp among groups exposed to tramadol. Carp larvae were also found to be significantly shorter after tramadol exposure (on day 28 and 32), with significantly lower body weight. Likewise, the inhibition of specific growth rate was found by Sehonova et al. [12], in groups exposed to a mixture of tramadol and naproxen, at the concentrations of 10, 50 and 100 µg/L. In contrast, higher body weight and significantly longer body were found after exposure to the highest tested concentration (200 µg/L) of tramadol and naproxen mixture. The authors discuss the possible effect of increased mortality in this group and, with it, related better life conditions of survivors compared to other groups (more space in the testing vessel and higher food availability). Moreover, Sehonova et al. [27] reported that tramadol exposure (including environmentally relevant concentration) caused a hatching delay in the fish embryos of both zebrafish and common carp.

Table 2.
Results of morphological parameters at the end of experiment. Data are expressed as mean ± standard deviation. No significant differences (p > 0.05) were found between control and experimental groups.
3.3. Histopathology
As a result of our experiment, only histopathological changes in liver (abnormal glycogen accumulation) were observed among tested groups, apart from the lowest concentration of 0.2 µg/L (Figure 1). In contrast, no histopathological changes in liver of common carp after tramadol hydrochloride exposure were described by Sehonova et al. [27].
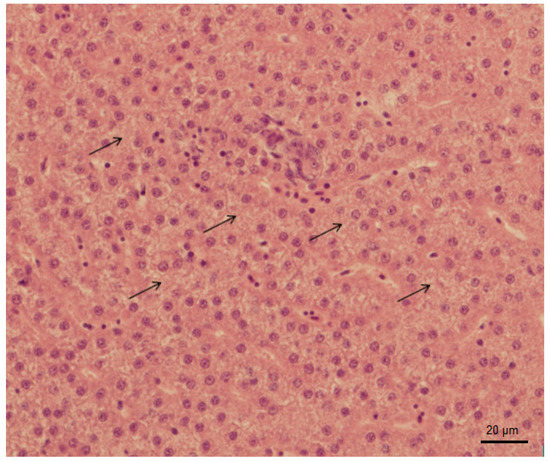
Figure 1.
Abnormal glycogen accumulation in liver of Danio rerio after exposure to 600 µg/L of tramadol hydrochloride (hematoxylin and eosin, 400x).
No significant histological changes were observed in all analyzed organs (skin, gill, kidney and brain). In contrast, Sehonova et al. [27] described that histopathological examination of early life stages of common carp revealed gill hyperemia. They also noticed an increased number of the skin mucous cells of fish in tested groups. Comparably, similar findings have been published by Sehonova et al. [12], where no changes in liver after tramadol-and-naproxen-mixture exposure in early life stages of common carp were observed. Similarly, there was an elevated amount of skin mucous cells. In addition, histopathological examination showed deformations of gill lamellas.
3.4. Oxidative Stress Indices
The potentially negative impacts of zebrafish subchronic exposure to tramadol hydrochloride were also studied by conducting an analysis of selected oxidative stress indices. Most often, induction of oxidative stress in aquatic animals is assessed by using changes in various enzyme activities, which play crucial roles in antioxidant defense. The role of the first-line defense enzyme antioxidants is primarily provided by superoxide dismutase, CAT and GPx, which suppress or prevent the formation of free radicals or reactive species in cells. In the next defense line, the oxidized form of glutathione, created as a result of the reaction catalyzed by GPx, is regenerated to the reduced form of glutathione by GR [41,42,43]. An important enzyme in antioxidant protection is also multifunctional phase II biotransformation enzyme – GST, which is involved in the xenobiotic detoxification and in elimination of reactive oxygen species, as well as products of lipoperoxidation [44]. One of the significant manifestations of antioxidant defense disruption is lipid peroxidation, which is often used as a useful oxidative stress indicator [40].
In our study, changes in various antioxidant enzyme activities (CAT, GR, GPx and GST) and lipid peroxidation were evaluated. Obtained results are given in Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6. In our study, fish exposed to tramadol hydrochloride did not show any considerable changes in GR, GPx or CAT activities between control and experimental groups. Surprisingly, a significant increase in GST activity was recorded only at the concentration of 2 µg/L (p < 0.01), compared to the control group. No changes were recorded in higher concentrations. An increase in GST activity, mainly in low environmentally relevant concentrations, was also found by Zivna et al. [45]. They studied the effect of salicylic acid on zebrafish, after 28 days of exposure. An increase in activity of the detoxification enzyme GST probably indicates an activation of antioxidant system against xenobiotic. A similar study was performed by Sehonova et al. [27]. They examined effects of tramadol hydrochloride on common carp (Cyprinus carpio) embryos and larvae. No changes in GR and GST activities after tramadol hydrochloride exposure were reported. In contrast, they observed a significant reduction in GPx activity at the highest concentrations of 100 and 200 µg/L of tramadol hydrochloride. Furthermore, Sehonova et al. [12] did not find differences in GR activity after exposure of a mixture of tramadol hydrochloride and naproxen sodium in common carp. They noticed a significant drop in GST activity, but only at a concentration of 100 µg/L. Likewise, they recorded a decrease in GPx activity among various groups exposed to a mixture of tramadol hydrochloride and naproxen sodium (50, 100 and 200 µg/L).
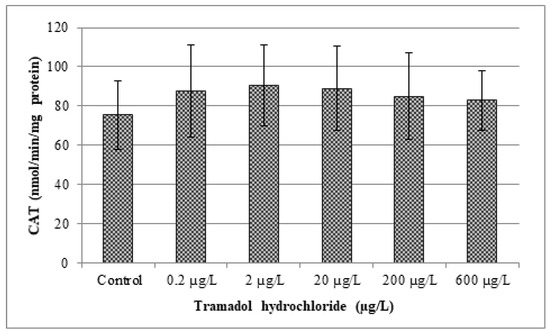
Figure 2.
Analysis of catalase (CAT). Data are expressed as mean ± standard deviation. No significant differences (p > 0.05) were found between control and experimental groups.
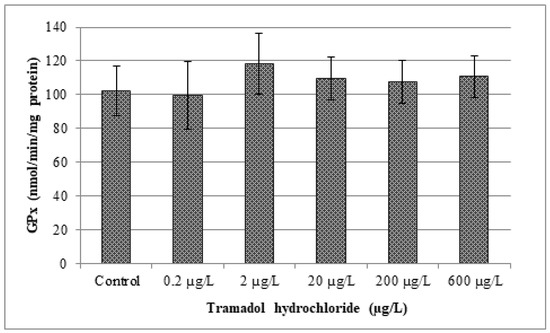
Figure 3.
Analysis of glutathione peroxidase activity (GPx) Data are expressed as mean ± standard deviation. No significant differences (p > 0.05) were found between control and experimental groups.
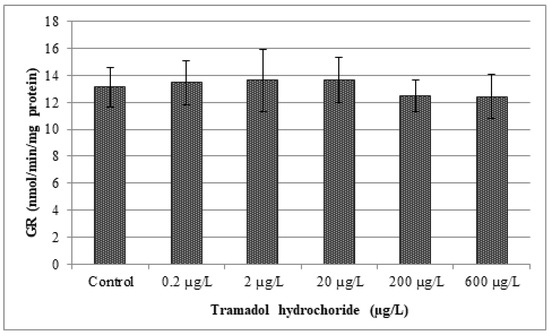
Figure 4.
Analysis of glutathione reductase activity (GR). Data are expressed as mean ± standard deviation. No significant differences (p > 0.05) were found between control and experimental groups.
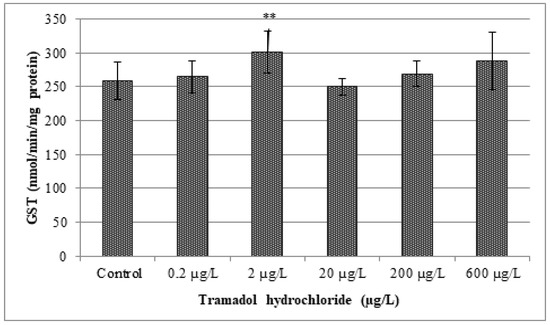
Figure 5.
Analysis of glutathione S-transferase activity (GST). Data are expressed as mean ± standard deviation. Significant differences between control and experimental groups are indicated by ** (p < 0.01).
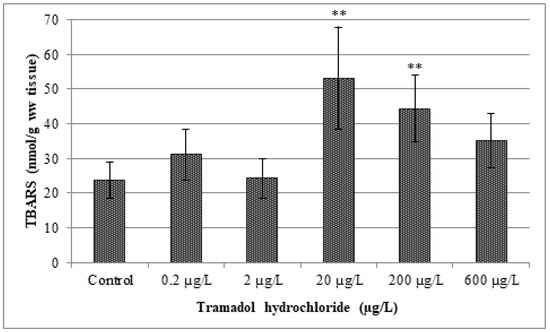
Figure 6.
Analysis of thiobarbituric acid reactive substance levels (TBARS). Data are expressed as mean ± standard deviation. Significant differences between control and experimental groups are indicated by ** (p < 0.01).
The results of our study indicate a significant increase in lipid peroxidation after exposure to tramadol hydrochloride. In particular, TBARS levels were increased among groups in comparison to the control, but a statistically significant (p < 0.01) difference was observed only in concentrations of 20 and 200 µg/L of tramadol hydrochloride. Surprisingly, TBARS values were not significantly increased in the highest tested concentration, i.e., 600 µg/L. The explanation for these results might be, as suggested by Sehonova et al. [12], that higher concentrations of tramadol hydrochloride may induce an antioxidant effect.
4. Conclusions
Since the purification of wastewater from tramadol is not perfect and the knowledge on its effects on non-target organisms is limited, we decided to assess the long-term impact of tramadol hydrochloride on survival, development and histopathology of juvenile zebrafish (Danio rerio), as well as the effects on selected indices of oxidative stress, represented by changes in GST, GR, GPx and CAT activity, and formation of lipid peroxidation products (TBARS).
The results of this study indicate that 28-day exposure can negatively influence selected indices of oxidative stress. A significant increase was observed in GST activity in the experimental group exposed to 2 µg/L, as compared to the control group. Moreover, increases in lipid peroxidation were observed in groups exposed to 20 and 200 µg/L, in comparison to the control group. Only slight changes in liver tissue were revealed by histopathological examination. On the contrary, not any of our tested concentrations of tramadol hydrochloride affected fish mortality, growth and activities of antioxidant enzymes, such as GR, GPx and CAT.
Author Contributions
L.P., performing of experiment and preparing of manuscript; P.S., analysis of oxidative stress indices; J.B., statistical analysis; V.D., analysis of tramadol hydrochloride in water samples; F.T. histopathological analysis; C.F., supervisor of experiment; P.B., performing of experiment; Z.S., preparing of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Our research was supported by institutional research funds of the Department of Animal Protection and Welfare and Veterinary Public Health of the University of Veterinary and Pharmaceutical Sciences Brno (Czech Republic).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sehonova, P.; Plhalova, L.; Blahova, J.; Doubkova, V.; Marsalek, P.; Prokes, M.; Tichy, F.; Skladana, M.; Fiorino, E.; Mikula, P.; et al. Effects of selected tricyclic antidepressants on early-life stages of common carp (Cyprinus carpio). Chemosphere 2017, 185, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Aliko, V.; Mehmeti, E.; Qirjo, M.; Faggio, C. Drink and sleep like a fish- goldfish as a behavior model to study pharmaceutical effects in freshwater ecosystem. J. Biol. Res. 2019, 92, 1–4. [Google Scholar] [CrossRef]
- Freitas, R.; Silvestro, S.; Coppola, F.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.V.M.; Pretti, C.; Faggio, C. Biochemical and physiological responses induced in Mytilus galloprovincialis after a chronic exposure to Salicylic Acid. Aquat. Toxicol. 2019, in press. [Google Scholar] [CrossRef]
- Freitas, R.; Silvestro, S.; Coppola, F.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.V.M.; Pretti, C.; Faggio, C. Combined effects of salinity changes and salicylic acid exposure in Mytilus galloprovincialis. Sci. Total. Environ. 2020, in press. [Google Scholar] [CrossRef]
- Freitas, R.; Silvestro, S.; Coppola, F.; Costa, S.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.V.M.; Pretti, C.; Faggio, C. Toxic impacts induced by sodium lauryl sulfate in Mytilus galloprovincialis. Comp. Biochem. Phys. A 2020, in press. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugsduring wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef]
- Dai, G.; Huang, J.; Chen, W.; Wang, B.; Yu, G.; Deng, S. Major pharmaceuticals and personal care products (PPCPs) in wastewater treatment plant and receiving water in Beijing, China, and associated ecological risks. Bull. Environ. Contam. Toxicol. 2014, 92, 655–661. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abdallah, M.A.-E.; Harrada, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Grabicova, K.; Grabic, R.; Fedorova, G.; Fick, J.; Cerveny, D.; Kolarova, J.; Turek, J.; Zlabek, V.; Randak, T. Bioaccumulation of psychoactive pharmaceuticals in fish in an effluent dominated stream. Water Res. 2017, 124, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Plhalova, L.; Blahova, J.; Doubkova, V.; Prokes, M.; Tichy, F.; Fiorino, E.; Faggio, C.; Svobodova, Z. Toxicity of naproxen sodium and its mixture with tramadol hydrochloride on fish early life stages. Chemosphere 2017, 188, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.J. Pollution from drug manufacturing: review and perspectives. Philos. Trans. R. Soc. B 2014, 369, 20130571. [Google Scholar] [CrossRef] [PubMed]
- Buřič, M.; Grabicová, K.; Kubec, J.; Kouba, A.; Kuklina, I.; Kozák, P.; Grabic, R.; Randák, T. Environmentally relevant concentrations of tramadol and citalopram alter behaviour of an aquatic invertebrate. Aquat. Toxicol. 2018, 200, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ložek, F.; Kuklina, I.; Grabicová, K.; Kubec, J.; Buřič, M.; Grabic, R.; Randák, T.; Císař, P.; Kozák, P. Behaviour and cardiac response to stress in signal crayfish exposed to environmental concentrations of tramadol. Aquat. Toxicol. 2019, 213, 105217. [Google Scholar] [CrossRef]
- Vazzana, M.; Andreani, T.; Fangueiro, J.; Faggio, C.; Silva, C.; Santini, A.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Tramadol hydrochloride: Pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems. Biomed. Pharmacother. 2015, 70, 234–238. [Google Scholar] [CrossRef]
- Zhuo, H.Q.; Huang, L.; Huang, H.Q.; Cai, Z. Effects of chronic tramadol exposure on the zebrafish brain: a proteomic study. J. Proteom. 2012, 75, 3351–3364. [Google Scholar] [CrossRef]
- Grond, S.; Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef]
- Pypendop, B.H.; Ilkiw, J.E. Pharmacokinetics of tramadol, and its metabolite O-desmethyl-tramadol, in cats. J. Vet. Pharmacol. Ther. 2008, 31, 52–59. [Google Scholar] [CrossRef]
- Rúa-Gómez, P.C.; Püttmann, W. Occurrence and removal of lidocaine, tramadol, venlafaxine, and their metabolites in German wastewater treatment plants. Environ. Sci. Pollut. Res. Int. 2012, 19, 689–699. [Google Scholar]
- Rúa-Gómez, P.C.; Püttmann, W. Impact of wastewater treatment plant discharge of lidocaine, tramadol, venlafaxine and their metabolites on the quality of surface waters and groundwater. J. Environ. Monit. 2012, 14, 1391–1399. [Google Scholar]
- Fedorova, G.; Randak, T.; Golovko, O.; Kodes, V.; Grabicova, K.; Grabic, R. A passive sampling method for detecting analgesics, psycholeptics, antidepressants and illicit drugs in aquatic environments in the Czech Republic. Sci. Total. Environ. 2014, 487, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.; Fink, G.; Joss, A.; Siegrist, H.; Ternes, T.A. Fate of beta blockers andpsycho-active drugs in conventional wastewater treatment. Water Res. 2009, 43, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, R.; Margiotta-Casaluci, L.; Huerta, B.; Runnalls, T.J.; Eguchi, A.; Nomiyama, K.; Kunisue, T.; Tanabe, S.; Sumpter, J.P. Protecting the environment from psychoactive drugs: Problems for regulators illustrated by the possible effects of tramadol on fish behaviour. Sci. Total. Environ. 2019, 664, 915–926. [Google Scholar] [CrossRef]
- Richmond, E.K.; Rosi, E.J.; Walters, D.M.; Fick, J.; Hamilton, S.K.; Brodin, T.; Sundelin, A.; Grace, M.R. A diverse suite of pharmaceuticals contaminates stream and riparian food webs. Nat. Commun. 2018, 9, 4491. [Google Scholar] [CrossRef]
- Bachour, R.L.; Golovko, O.; Kellner, M.; Pohl, J. Behavioral effects of citalopram, tramadol, and binary mixture in zebrafish (Danio rerio) larvae. Chemosphere 2020, 238, 124587. [Google Scholar] [CrossRef]
- Sehonova, P.; Plhalova, L.; Blahova, J.; Berankova, P.; Doubkova, V.; Prokes, M.; Tichy, F.; Vecerek, V.; Svobodova, Z. The effect of tramadol hydrochloride on early life stages of fish. Environ. Toxicol. Phar. 2016, 44, 151–157. [Google Scholar] [CrossRef]
- OECD. Guideline for Testing of Chemicals: Fish, Juvenile Growth Test; OECD 215: Paris, France, 2000; 16p. [Google Scholar]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef]
- Segner, H. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2009, 149, 187–195. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef]
- Hoo, J.Y.; Kumari, Y.; Shaikh, M.F.; Hue, S.M.; Goh, B.H. Zebrafish: A Versatile Animal Model for Fertility Research. Biomed. Res. Int. 2016, 2016, 9732780. [Google Scholar] [CrossRef] [PubMed]
- Bambino, K.; Chu, J. Zebrafish in Toxicology and Environmental Health. Curr. Top. Dev. Biol. 2017, 124, 331–367. [Google Scholar] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Emryonic Development of the Zebrafish. Develop. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jacoby, W.B. Glutathione S-transferases. First enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V.; Lushchak, O.V.; Storey, J.M.; Storey, K.B. Hypoxia and recovery perturb free radical processes and antioxidant potential in common carp (Cyprinus carpio) tissues. Int. J. Biochem. Cell. Biol. 2005, 37, 1319–1330. [Google Scholar] [CrossRef]
- Lushchak, V.; Semchyshyn, H.M. Oxidative Stress—Molecular Mechanisms and Biological Effects; IntechOpen: Rijeka, Croatia, 2012; 362p. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria. Med. J. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, X.; Xie, H.; Wang, J.; Su, J.; Yu, C. DNA damage and effects on glutathione-S-transferase activity induced by atrazine exposure in zebrafish (Danio rerio). Environ. Toxicol. 2011, 26, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Zivna, D.; Blahova, J.; Siroka, Z.; Plhalova, L.; Marsalek, P.; Doubkova, V.; Zelinska, G.; Vecerek, V.; Tichy, F.; Sehonova, P.; et al. The effects of salicylic acid on juvenile zebrafish Danio rerio under flow-through conditions. Bull. Environ. Contam. Toxicol. 2016, 97, 323–330. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).