Development of Bionanocomposites Based on PLA, Collagen and AgNPs and Characterization of Their Stability and In Vitro Biocompatibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA Bionanocomposites
2.3. Methods
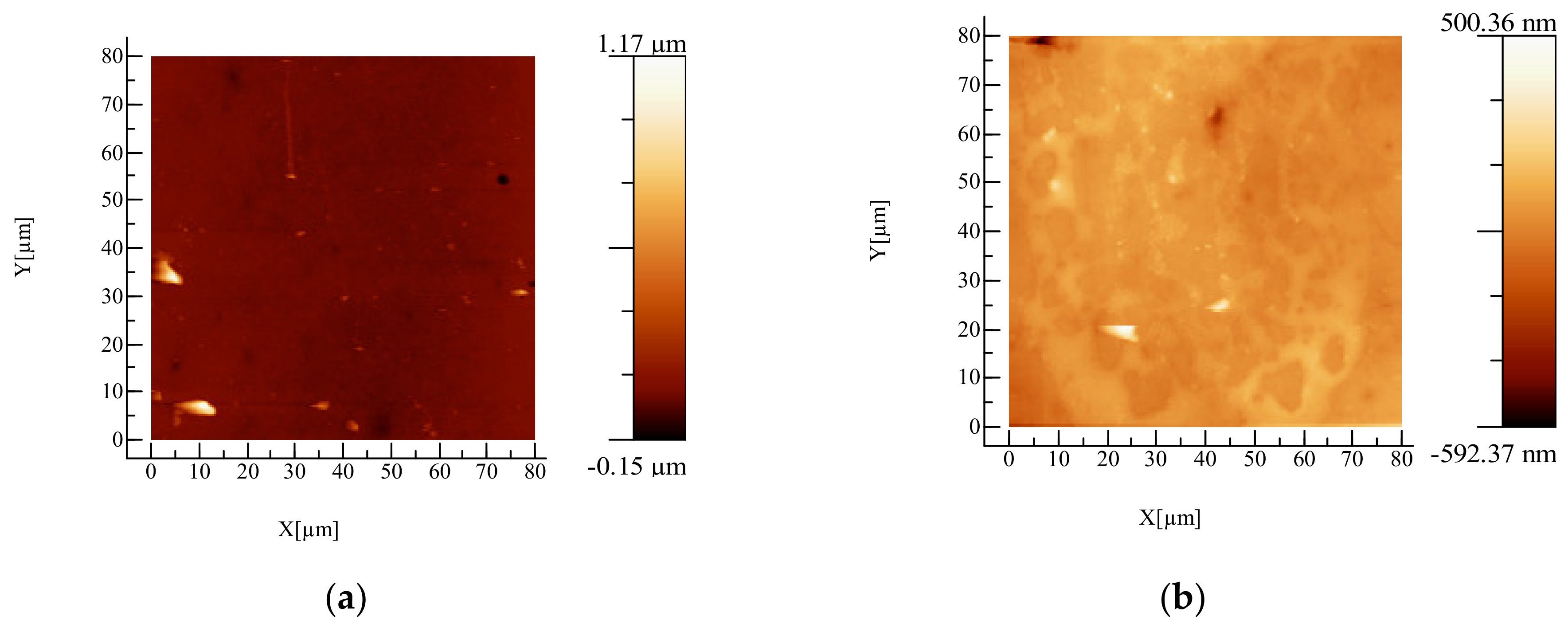
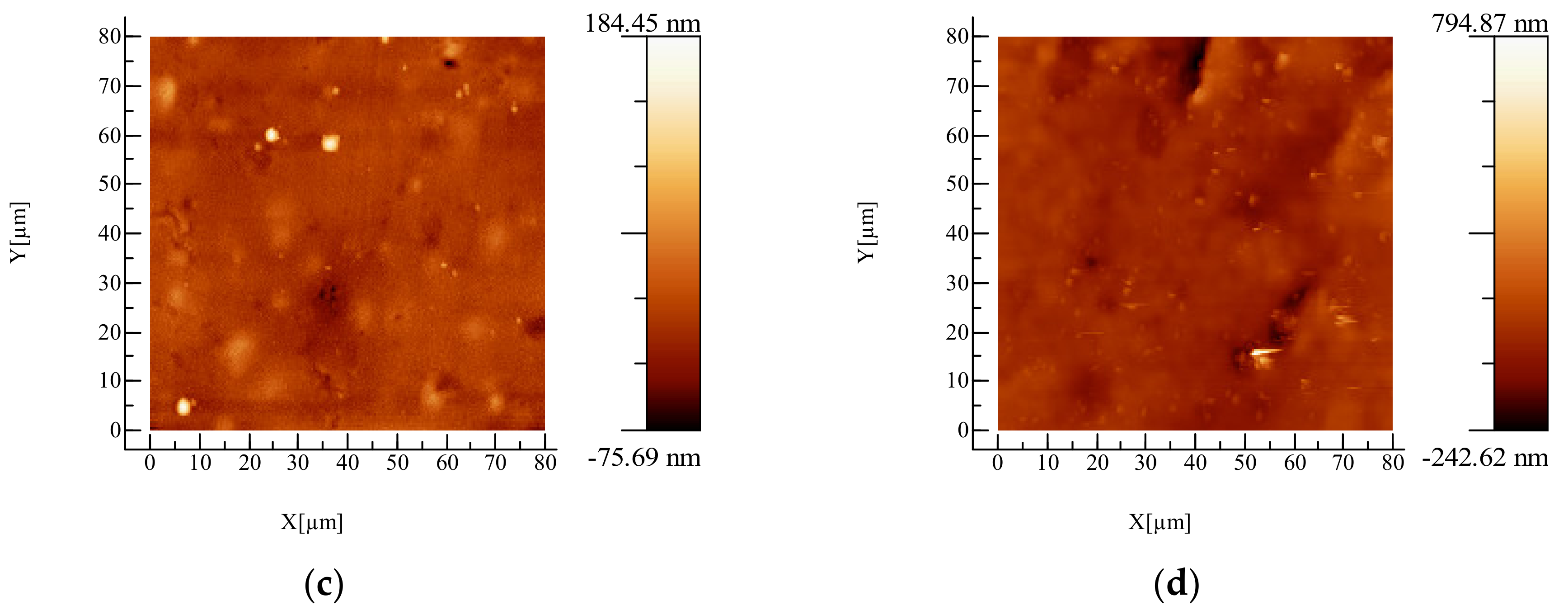
2.3.1. Atomic Force Microscopy (AFM) Examination
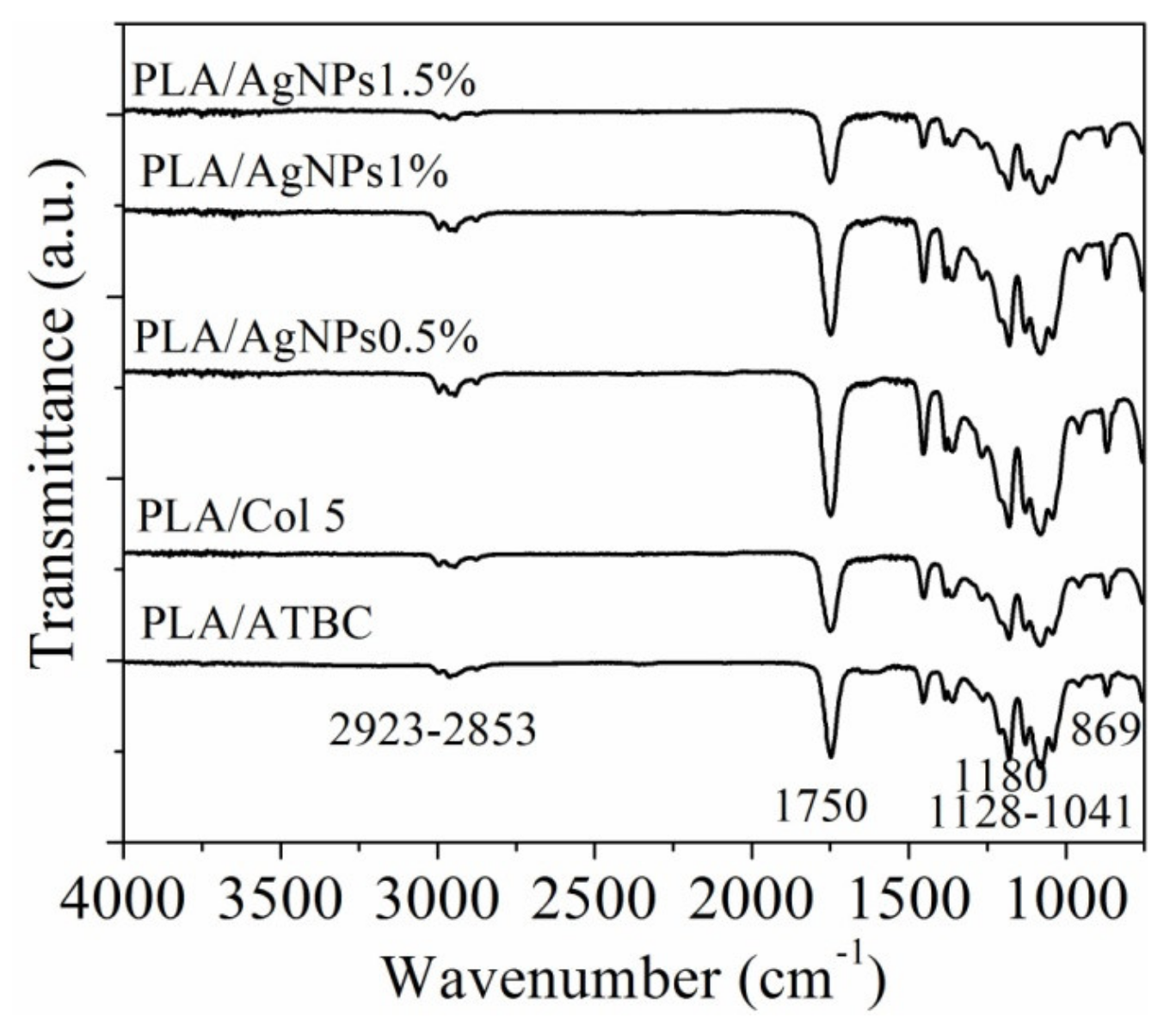
2.3.2. Infrared Spectroscopy (ATR-FT-IR)
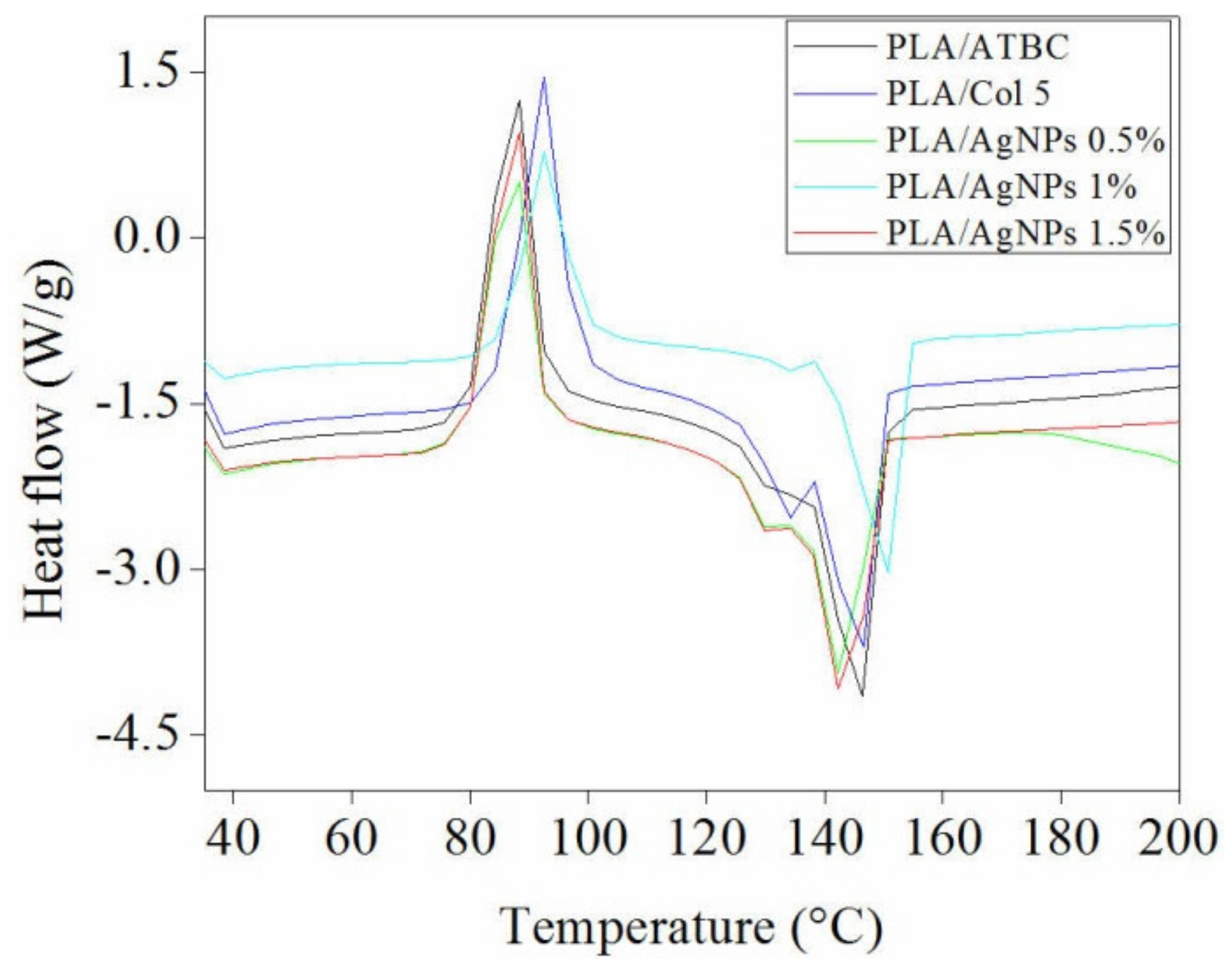
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Chemiluminescence Analysis
2.3.5. In Vitro Cytotoxicity Tests
2.3.6. Cell Morphology
2.3.7. Cell Cycle Analysis by Flow Cytometry
3. Results
3.1. AFM Examination
3.2. Infrared Spectroscopy Analysis
3.3. Differential Scanning Calorimetry (DSC)
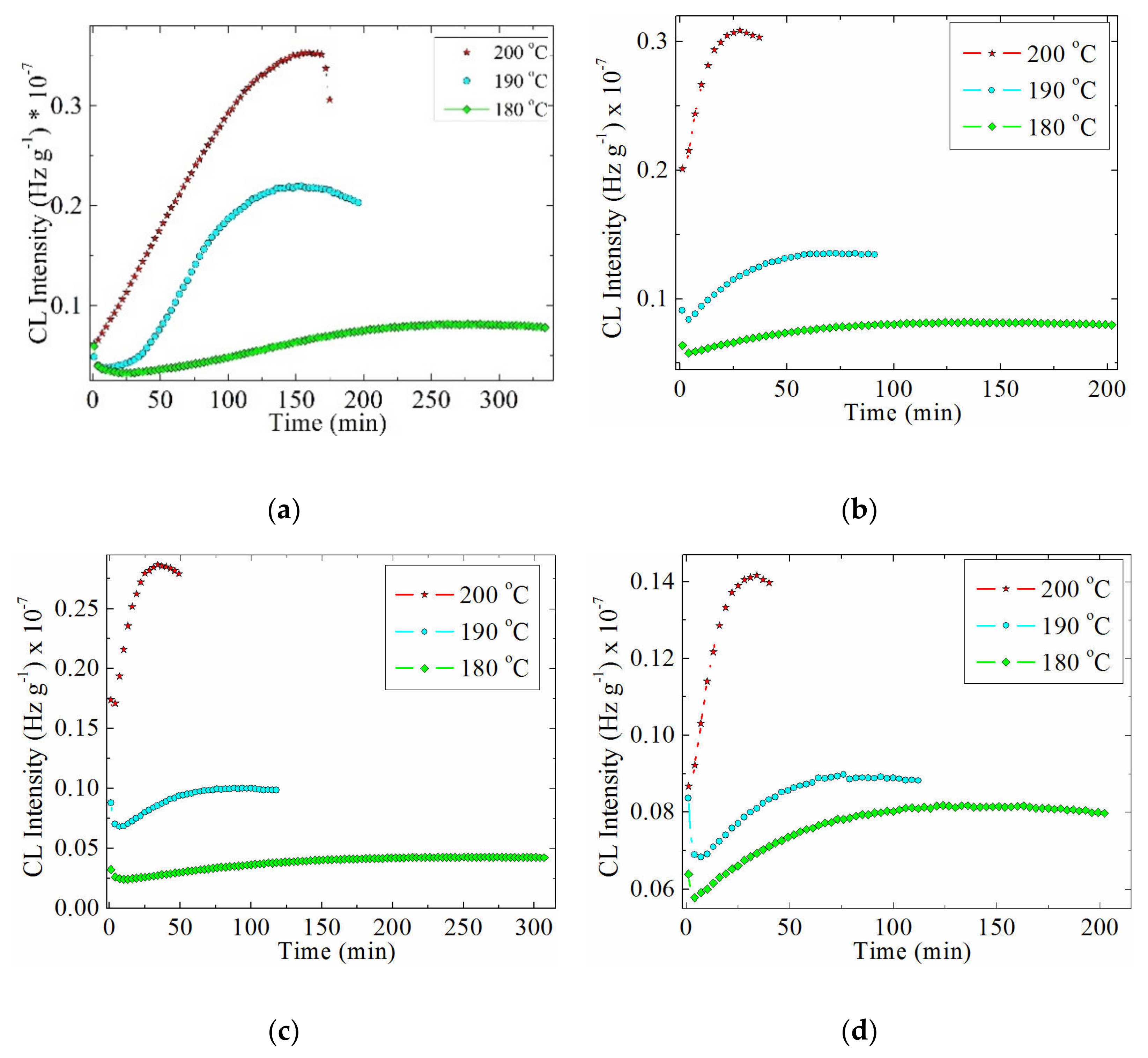
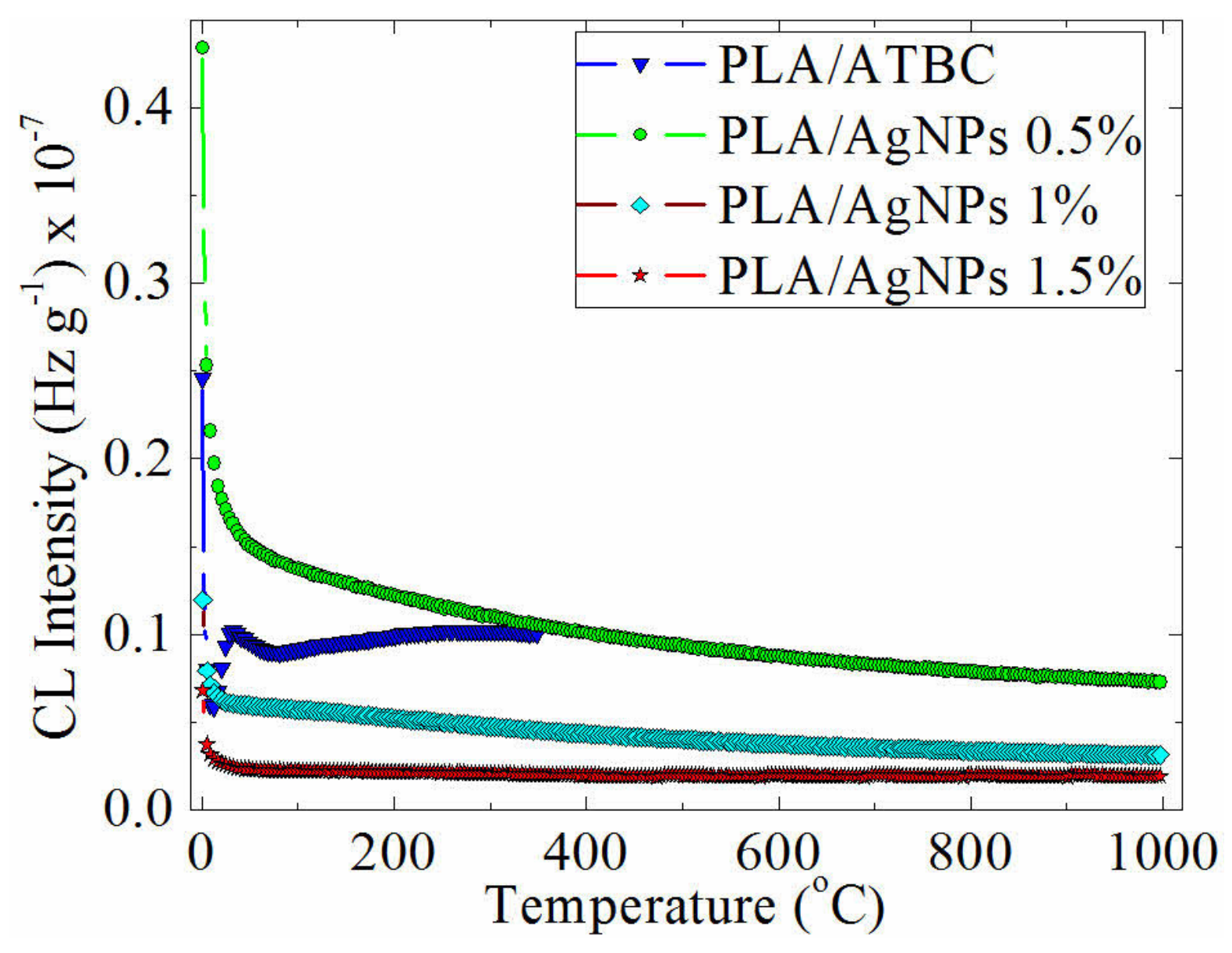
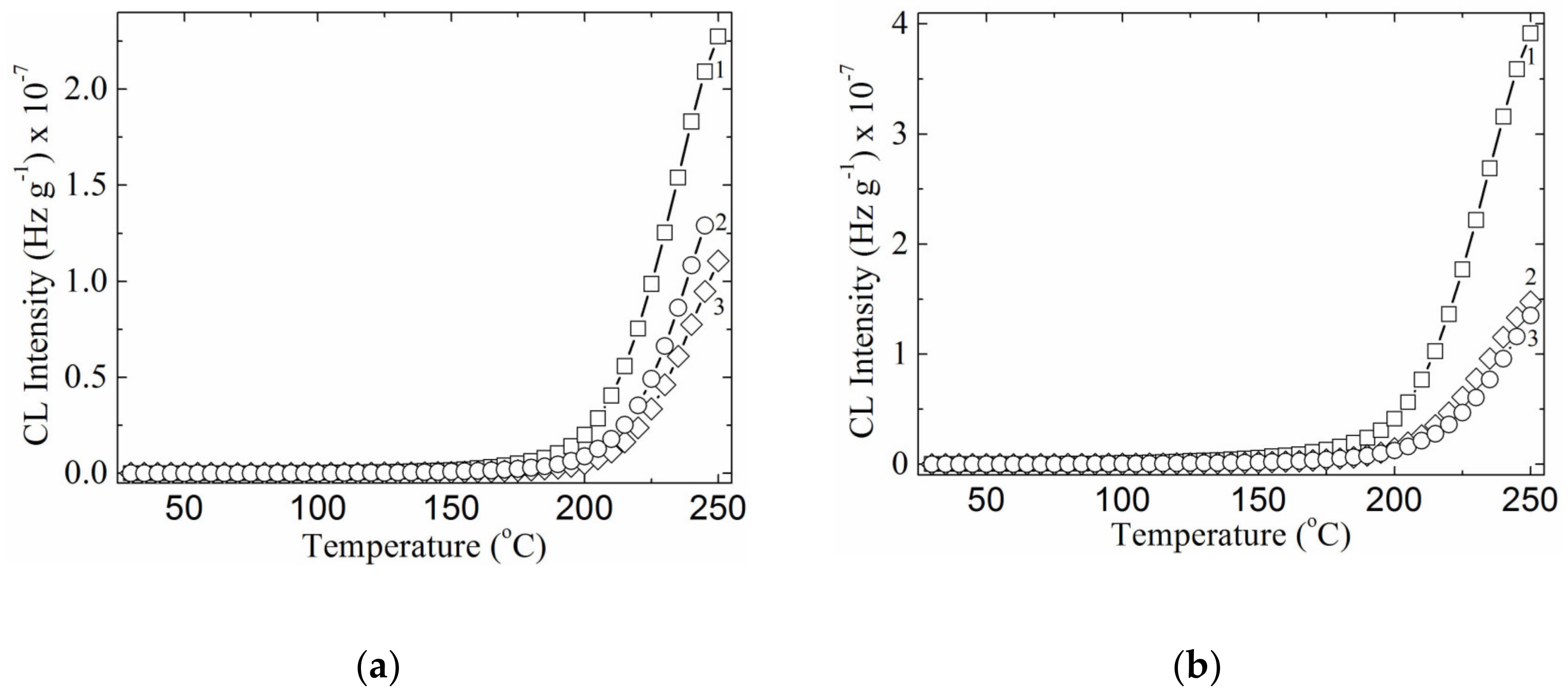
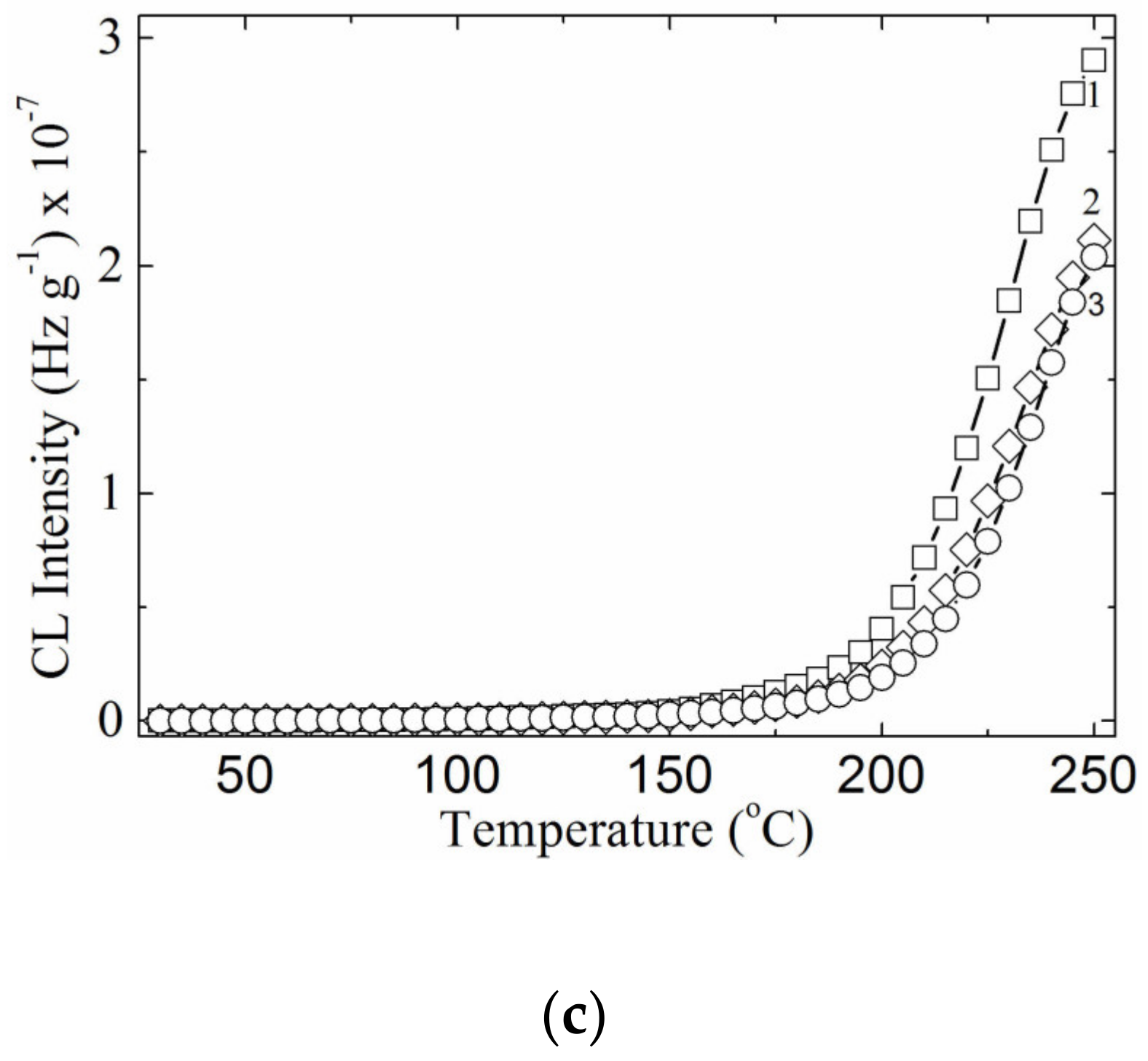
3.4. Chemiluminescence Analysis
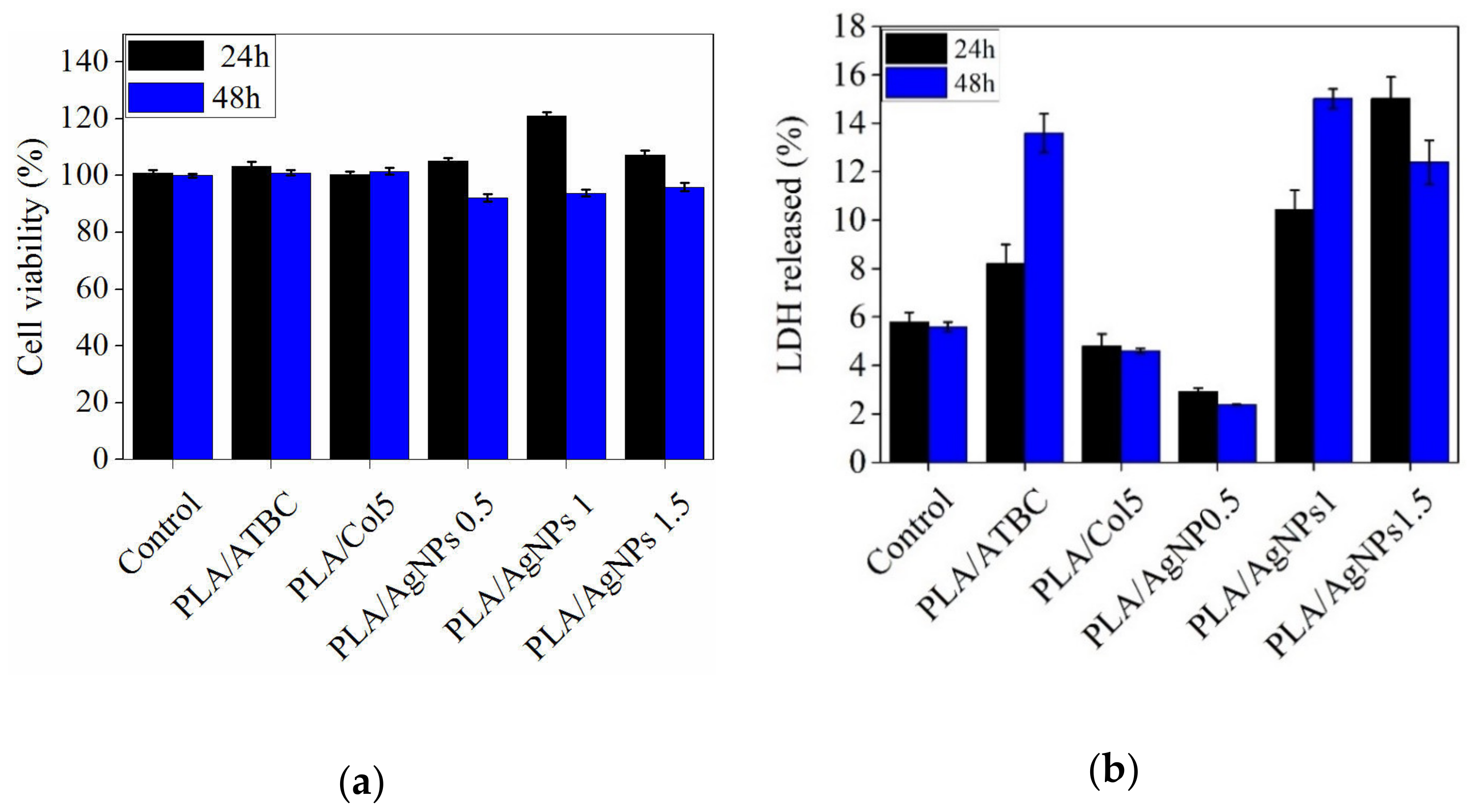
3.5. In Vitro Cytotoxicity Evaluation
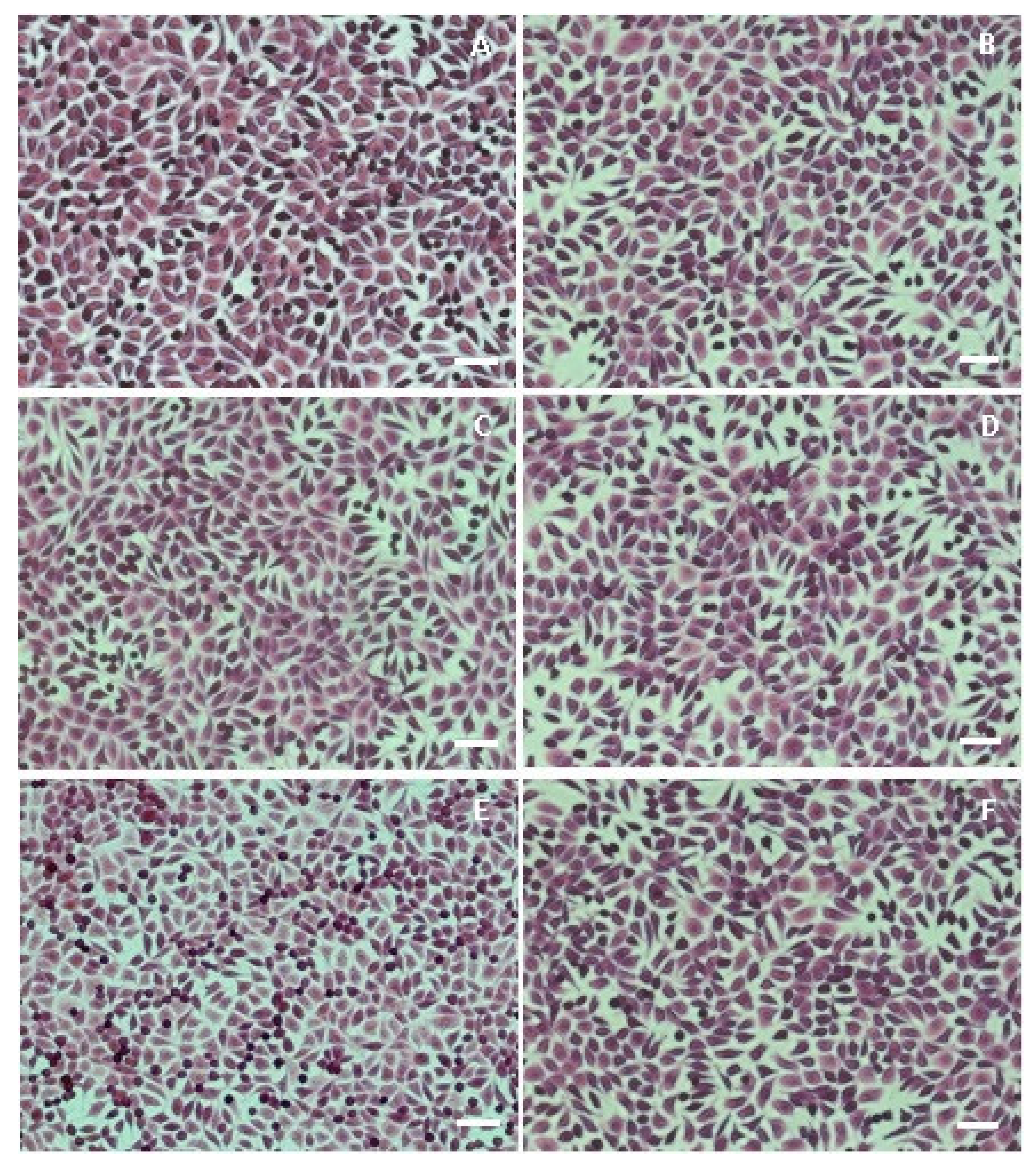
3.6. Cell Morphology
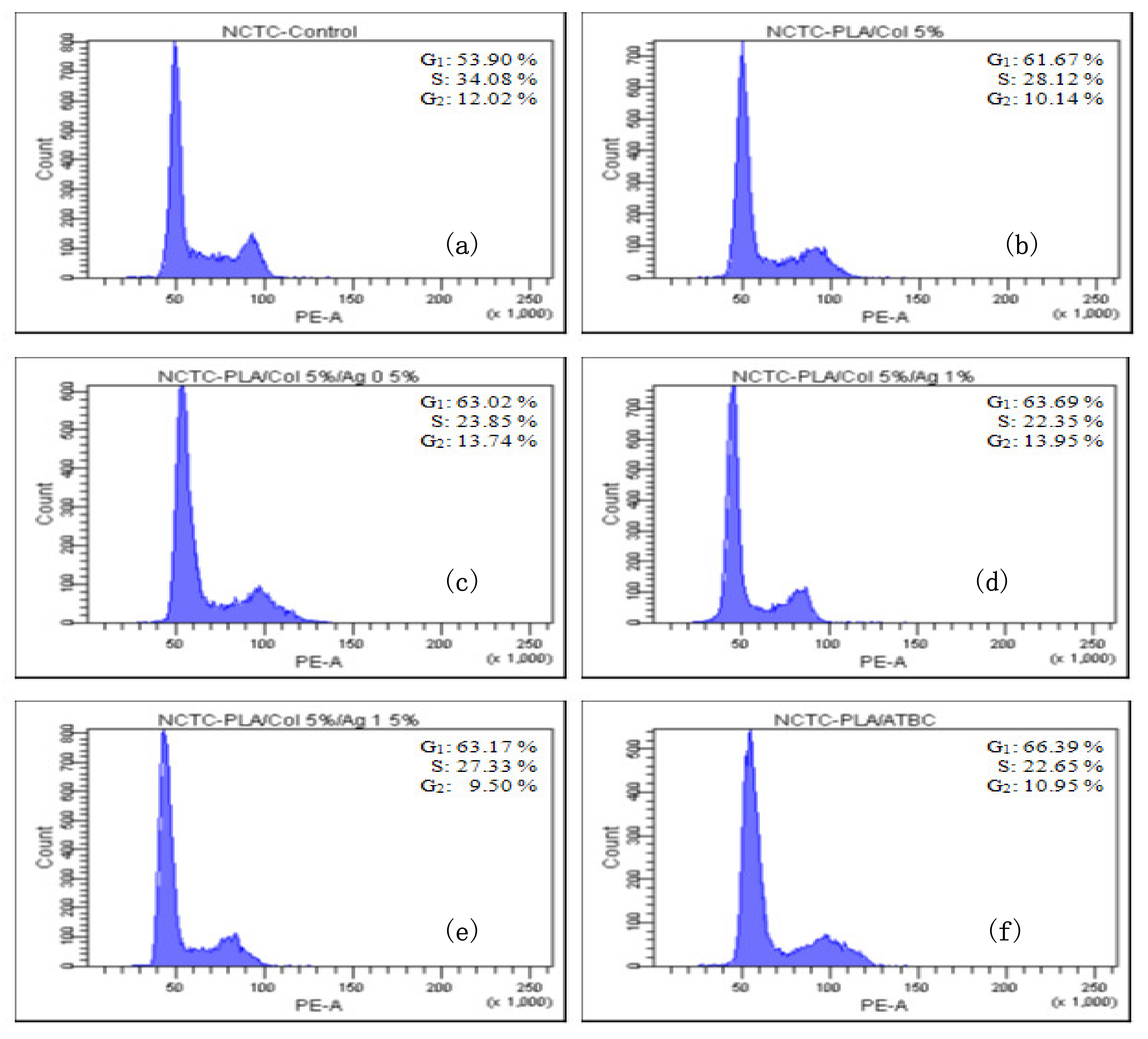
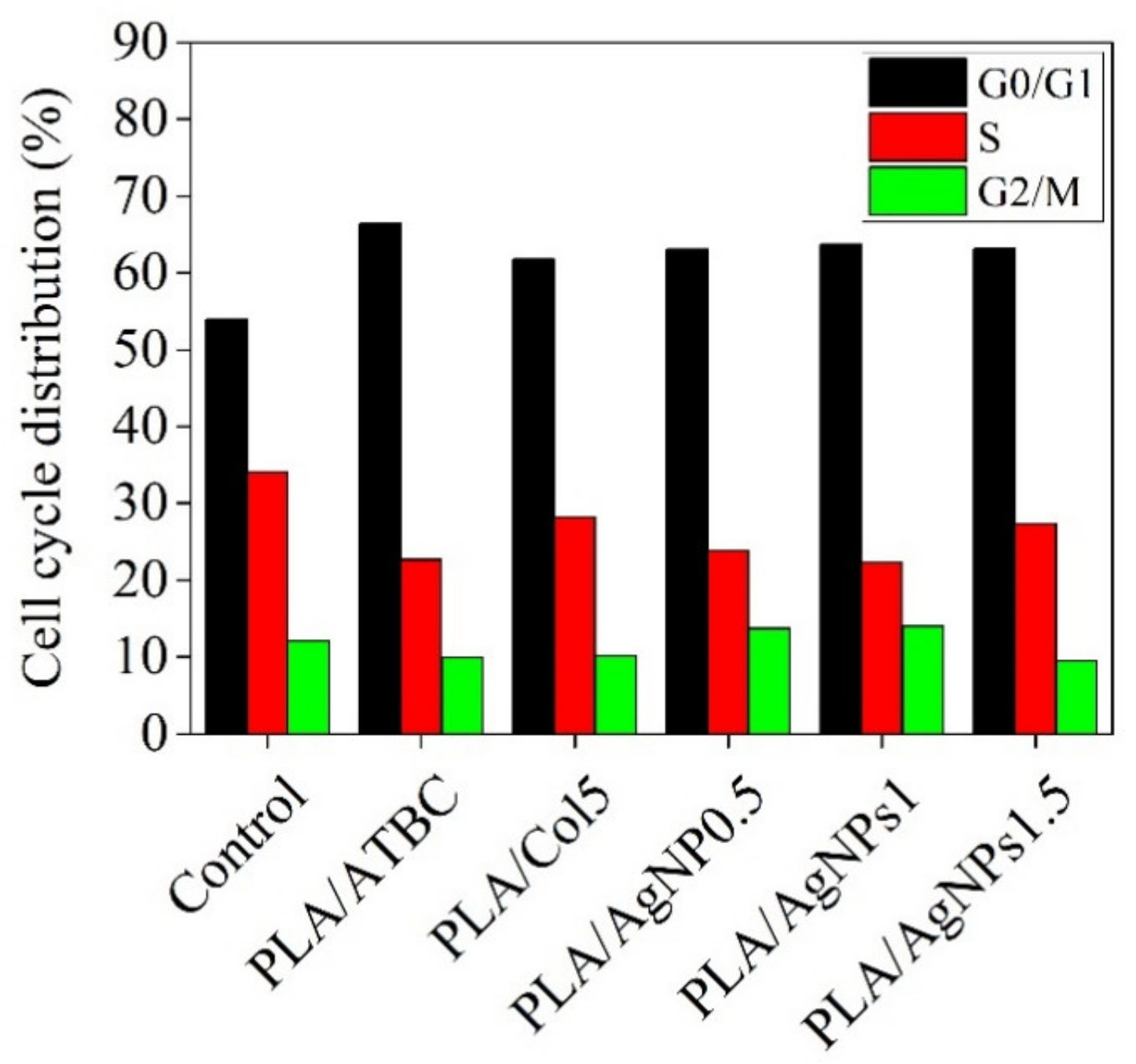
3.7. Effect of Bionanocomposites on Cell Cycle Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feldman, D. Polymer nanocomposites in medicine. J. Macromol. Sci. A 2016, 53, 55–62. [Google Scholar] [CrossRef]
- Giammona, G.; Craparo, E.F. Biomedical applications of polylactide (PLA) and its copolymers. Molecules 2018, 23, 980. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Yeh, W.L.; Chao, C.L.; Hsu, Y.H.; Yu, Y.H.; Chen, J.K.; Liu, S.J. Enhancement of tendon–bone healing via the combination of biodegradable collagen-loaded nanofibrous membranes and a three-dimensional printed bone-anchoring bolt. Int. J. Nanomed. 2016, 11, 4173–4418. [Google Scholar] [CrossRef] [PubMed]
- Rapa, M.; Darie-Nita, R.N.; Preda, P.; Coroiu, V.; Tatia, R.; Vasile, C.; Matei, E.; Predescu, A.M.; Maxim, M.E. PLA/collagen hydrolysate/silver nanoparticles bionanocomposites for potential antimicrobial urinary drains. Polym. Plast. Technol. 2019, 1–16. [Google Scholar] [CrossRef]
- Rapa, M.; Stefan, L.M.; Preda, P.; Darie-Nita, R.N.; Gaspar-Pintiliescu, A.; Seciu, A.M.; Vasile, C.; Matei, E.; Predescu, A.M. Effect of hydrolyzed collagen on thermal, mechanical and biological properties of poly(lactic acid) bionanocomposites. Iran Polym. J. 2019, 28, 271–282. [Google Scholar] [CrossRef]
- Rapa, M.; Darie Nita, R.N.; Vasile, C. Influence of plasticizers over some physico-chemical properties of PLA. Mater. Plast. 2017, 54, 73–78. [Google Scholar] [CrossRef]
- Rapa, M.; Darie-Nita, R.N.; Irimia, A.M.; Sivertsvik, M.; Rosnes, J.T.; Trifoi, A.R.; Vasile, C.; Tanase, E.E.; Gherman, T.; Popa, M.E.; et al. Comparative analysis of two bioplasticizers used to modulate the properties of PLA biocomposites. Mater. Plast. 2017, 54, 610–615. [Google Scholar] [CrossRef]
- Nonato, R.C.; Mei, L.H.I.; Bonse, B.C.; Chinaglia, E.F.; Morales, A.R. Nanocomposites of PLA containing ZnO nanofibers made by solvent cast 3D printing: Production and characterization. Eur. Polym. J. 2019, 114, 271–278. [Google Scholar] [CrossRef]
- Zaharescu, T.; Râpă, M.; Lungulescu, E.M.; Butoi, N. Filler effect on the degradation of γ-processed PLA/vinyl POSS hybrid. Radiat. Phys. Chem. 2018, 153, 188–197. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, L.; Yang, B.; Li, J.; Ren, J. Preparation and characterization of polylactic acid (PLA) carbon nanotube nanocomposites. Polym. Test. 2018, 68, 34–38. [Google Scholar] [CrossRef]
- Babu, S.S.; Kalarikkal, N.; Thomas, S.; Radhakrishnan, E.K. Enhanced antimicrobial performance of cloisite 30B/poly (ε-caprolactone) over cloisite 30B/poly (l-lactic acid) as evidenced by structural features. Appl. Clay Sci. 2018, 153, 198–204. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C Mater. 2019, 101, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Mania, S.; Partyka, K.; Pilch, J.; Augustin, E.; Cieślik, M.; Ryl, J.; Jinn, J.R.; Wang, Y.J.; Michałowska, A.; Tylingo, R. Obtaining and Characterization of the PLA/chitosan foams with antimicrobial properties achieved by the emulsification combined with the dissolution of chitosan by CO2 saturation. Molecules 2019, 24, 4532. [Google Scholar] [CrossRef]
- Karakurt, I.; Ozaltin, K.; Vesela, D.; Lehocky, M.; Humpolícek, P.; Mozetic, M. Antibacterial activity and cytotoxicity of immobilized glucosamine/chondroitin sulfate on polylactic acid films. Polymers 2019, 11, 1186. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, M.P.; Prabhakaran, M.; Morshed, M.H.; Nasr-Esfahani, S.; Ramakrishna, S. Electrospun poly(epsilon-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Ramanathan, G.; Singaravelu, S.; Raja, M.D.; Nagiah, N.; Padmapriya, P.; Ruban, K.; Kaveri, K.; Natarajan, T.S.; Sivagnanam, U.T.; Perumal, P.T. Fabrication and characterization of a collagen coated electrospun poly(3-hydroxybutyric acid)–gelatin nanofibrous scaffold as a soft bio-mimetic material for skin tissue engineering applications. RSC Adv. 2016, 6, 7914–7922. [Google Scholar] [CrossRef]
- Kai, D.; Ren, W.; Tian, L.; Chee, P.L.; Liu, Y.; Ramakrishna, S.; Loh, X.J. Engineering poly(lactide)–lignin nanofibers with antioxidant activity for biomedical application. ACS Sustain. Chem. Eng. 2016, 4, 5268–5276. [Google Scholar] [CrossRef]
- Gaidau, C.; Petica, A.; Ciobanu, C.; Martinescu, T. Investigations on antimicrobial activity of collagen and keratin based materials doped with silver nanoparticles. Rom. Biotechnol. Lett. 2009, 14, 4665–4672. [Google Scholar]
- Turalija, M.; Bischof, S.; Budimir, A.; Gaan, S. Antimicrobial PLA films from environment friendly additives. Compos. Part B Eng. 2016, 102, 94–99. [Google Scholar] [CrossRef]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M.Q. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int. J. Nanomed. 2014, 9, 2399–2407. [Google Scholar] [CrossRef]
- Sharif, M.K.; Awan, K.A.; Butt, M.S.; Sharif, H.R. Nanotechnology: A Pioneering Rebellion for Food Diligence. In Impact of Nanoscience in the Food Industry; Chapter 2; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 29–56. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Rahman, R.A.; Jokar, M.; Darroudi, M. Silver/poly (lactic acid) nanocomposites: Preparation, characterization, and antibacterial activity. Int. J. Nanomed. 2010, 5, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Maróti, P.; Kocsis, B.; Ferencz, A. Differential thermal analysis of the antibacterial effect of PLA-based materials planned for 3D printing. J. Anal. Calorim. 2019. [Google Scholar] [CrossRef]
- Liu, C.; Shen, J.; Yeung, K.W.K.; Tjong, S.C. Development and Antibacterial Performance of Novel Polylactic Acid-Graphene Oxide-Silver Nanoparticle Hybrid Nanocomposite Mats Prepared By Electrospinning. ACS Biomater. Sci. Eng. 2017, 3, 471–486. [Google Scholar] [CrossRef]
- Cock, F.; Cuadri, A.A.; García-Morales, M.; Partal, P. Thermal, rheological and microstructural characterisation of commercial biodegradable polyesters. Polym. Test. 2013, 32, 716–723. [Google Scholar] [CrossRef]
- Allen, M.; Millett, P.; Dawes, E.; Rushton, N. Lactate dehydrogenase activity as a rapid and sensitive test for the quantification of cell numbers in vitro. Clin. Mater. 1994, 16, 189–194. [Google Scholar] [CrossRef]
- Korzeniewski, C.; Callewaert, D.M. An enzyme-release assay for natural cytotoxicity. J. Immunol. Methods 1983, 64, 313–320. [Google Scholar] [CrossRef]
- Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem. Cent. J. 2012, 6, 97. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Dominici, F.; Jiménez, A.; Del Carmen Garrigós, M.; Kenny, J.M. Influence of thymol and silver nanoparticles on the degradation of poly(lactic acid) based nanocomposites: Thermal and morphological properties. Polym. Degrad. Stab. 2014, 108, 158–165. [Google Scholar] [CrossRef]
- Zembouai, I.; Kaci, M.; Bruzaud, S.; Benhamida, A.; Corre, Y.M.; Grohens, Y. A study of morphological, thermal, rheological and barrier properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/polylactide blends prepared by melt mixing. Polym. Test. 2013, 32, 842–851. [Google Scholar] [CrossRef]
- Fehri, S.; Cinelli, P.; Coltelli, M.B.; Anguillesi, I.; Lazzeri, A. Thermal properties of plasticized poly (lactic acid) (PLA) containing nucleating agent. IJCEA 2016, 7, 85–88. [Google Scholar] [CrossRef]
- Bher, A.; Unalan, I.U.; Auras, R.; Rubino, M.; Schvezov, C.E. Toughening of poly(lactic acid) and thermoplastic cassava starch reactive blends using graphene nanoplatelets. Polymers 2018, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthi, J.; Ramasamy, P.; Shanmugam, V.; Shanmugam, A. Isolation and partial characterization of collagen from outer skin of Sepia pharaonis (Ehrenberg, 1831) from Puducherry coast. Biochem. Biophys. Rep. 2017, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, C.; Chi, H.; Li, L.; Lan, T.; Han, P.; Chen, H.; Qin, Y. Development of antimicrobial packaging film made from poly(lactic acid) incorporating titanium dioxide and silver nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef]
- Ma, P.; Jiang, L.; Yu, M.; Dong, W.; Chen, M. Green antibacterial nanocomposites from poly(lactide)/poly(butylene adipate-co-terephthalate)/nanocrystal cellulose–silver nanohybrids. ACS Sustain. Chem. Eng. 2016, 4, 6417–6426. [Google Scholar] [CrossRef]
- Rychý, J.; Rychlá, L.; Stloukal, P.; Koutný, M.; Pekarová, S.; Verney, V.; Fiedlorová, A. UV initiated oxidation and chemiluminescence from aromatic-aliphatic copolyesters and polylactic acid. Polym. Degrad. Stab. 2013, 98, 2556–2563. [Google Scholar] [CrossRef]
- Zaharescu, T.; Rapa, M.; Marinescu, V. Chemiluminescence kinetic analysis on the oxidative degradation of poly(lactic acid). J. Therm. Anal. Calorim. 2017, 128, 185–191. [Google Scholar] [CrossRef]
- Darie-Niță, N.; Vasile, C.; Stoleru, E.; Pamfil, D.; Zaharescu, T.; Tarțău, L.; Tudorachi, N.; Brebu, M.A.; Pricope, G.M.; Dumitriu, R.P.; et al. Evaluation of the rosemary extract effect on the properties of polylactic acid-based materials. Materials 2018, 11, 1825. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2020, 9, 24. [Google Scholar] [CrossRef]
- Guntur, S.R.; Sampath Kumar, N.S.; Hegde, M.M.; Dirisala, V.R. In vitro studies of the antimicrobial and free-radical scavenging potentials of silver nanoparticles biosynthesized from the extract of Desmostachya bipinnata. Anal. Chem. Insights 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Moteriya, P.; Padalia, H.; Chanda, S. Characterization, synergistic antibacterial and free radical scavenging efficacy of silver nanoparticles synthesized using Cassia roxburghii leaf extract. J. Genet. Eng. Biotechnol. 2017, 15, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Maddah, B.; Shamsi, J.; Barsang, M.J.; Rahimi-Nasrabadi, M. The chemiluminescence determination of 2-chloroethyl ethyl sulfide using luminol–AgNO3–silver nanoparticles system. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 142, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Chen, Z.; Chen, Z.; Zhang, A.; Yang, Z. Polylactic acid based nanocomposites: Promising safe and biodegradable materials in biomedical field. Int. J. Polym. Sci. 2016, 2016, 6869154. [Google Scholar] [CrossRef]
- Qiao, X.; Russell, S.J.; Yang, X.; Tronci, G.; Wood, D.J. Compositional and in vitro evaluation of nonwoven type I collagen/poly-dl-lactic acid scaffolds for bone regeneration. J. Funct. Biomater. 2015, 6, 667–686. [Google Scholar] [CrossRef]
- Shin, Y.C.; Lee, J.H.; Jin, L.; Kim, M.J.; Kim, Y.J.; Hyun, J.K.; Jung, T.G.; Hong, S.W.; Han, D.W. Stimulated myoblast differentiation on graphene oxide-impregnated PLGA-collagen hybrid fibre matrices. J. Nanobiotechnol. 2015, 13, 21. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, P.; Shi, X.; Zhang, G.; Wang, C. Multilevel structural stereocomplexpolylactic acid/collagen membranes by pattern electrospinning for tissue engineering. Polymer 2018, 156, 250–260. [Google Scholar] [CrossRef]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Soto, K.F.; Murr, L.E.; Garza, K.M. Cytotoxic responses and potential respiratory health effects of carbon and carbonaceous nanoparticulates in the Paso del Norte airshed environment. Int. J. Environ. Res. Public Health 2008, 5, 12–25. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Jin, G.; Prabhakaran, M.P.; Nadappuram, B.P.; Singh, G.; Kai, D.; Ramakrishna, S. Electrospun poly (L-lactic acid)-co-poly (ϵ-caprolactone) nanofibres containing silver nanoparticles for skin-tissue engineering. J. Biomater. Sci. Polym. Ed. 2012, 23, 2337–2352. [Google Scholar]
- Liu, C.; Chan, K.W.; Shen, J.; Wong, H.M.; Yeung, K.W.; Tjong, S.C. Melt-compounded polylactic acid composite hybrids with hydroxyapatite nanorods and silver nanoparticles: Biodegradation, antibacterial ability, bioactivity and cytotoxicity. RSC Adv. 2015, 5, 72288–72299. [Google Scholar] [CrossRef]
- Pazos-Ortiz, E.; Roque-Ruiz, J.H.; Hinojos-Márquez, E.A.; López-Esparza, J.; Donohué-Cornejo, A.; Cuevas-González, J.C.; Espinosa-Cristóbal, L.F.; Reyes-López, S.Y. Dose-dependent antimicrobial activity of silver nanoparticles on polycaprolactone fibers against gram-positive and gram-negative bacteria. J. Nanomater. 2017, 1–9. [Google Scholar] [CrossRef]
- Pauksch, L.; Rohnke, M.; Schnettler, R.; Lips, K.S. Silver nanoparticles do not alter human osteoclastogenesis but induce cellular uptake. Toxicol. Rep. 2014, 1, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Sivolella, S.; Stellini, E.; Brunello, G.; Gardin, C.; Ferroni, L.; Bressan, E.; Zavan, B. Silver nanoparticles in alveolar bone surgery devices. J. Nanomater. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.L.; Hazelwood, K.J. Exposure-Related Health Effects of Silver and Silver Compounds: A Review. Ann. Occup. Hyg. 2005, 49, 575–585. [Google Scholar] [CrossRef]
- Maki, D.G. In vitro studies of a novel antimicrobial luer-activated needleless connector for prevention of catheter-related bloodstream infection. Clin. Infect. Dis. 2010, 50, 1580–1587. [Google Scholar] [CrossRef]
| Sample | PLA (wt.%) | ATBC (wt.%) | Col (wt.%) | AgNPs (wt.%) |
|---|---|---|---|---|
| PLA/ATBC | 80 | 20 | - | - |
| PLA/Col5 | 76 | 19 | 5 | - |
| PLA/AgNPs 0.5% | 75.6 | 18.9 | 5 | 0.5 |
| PLA/AgNPs 1% | 75.2 | 18.8 | 5 | 1.0 |
| PLA/AgNPs 1.5% | 74.7 | 18.7 | 5 | 1.5 |
| Sample | Roughness, nm |
|---|---|
| Plasticized PLA | 34.4 |
| PLA/AgNPs 0.5% | 46.5 |
| PLA/AgNPs 1% | 49.3 |
| PLA/AgNPs 1.5% | 57.0 |
| Sample | Tg (°C) | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) | Xc, PLA (%) |
|---|---|---|---|---|---|---|
| PLA/ATBC | 42.3 | 86.7 | 20.5 | 145.8 131.7 | 22.1 | 29.6 |
| PLA/Col 5 | 46.5 | 92.0 | 22.7 | 145.50 133.23 | 21.2 | 29.9 |
| PLA/AgNPs 0.5% | 47.2 | 86.7 | 20.3 | 143.9 131.0 | 22.9 | 32.5 |
| PLA/AgNPs 1% | 48.0 | 92.5 | 22.9 | 150.8 134.3 | 23.8 | 33.9 |
| PLA/AgNPs 1.5% | 51.3 | 86.8 | 18.1 | 144.3 131.0 | 23.9 | 34.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Râpă, M.; Stefan, L.M.; Zaharescu, T.; Seciu, A.-M.; Țurcanu, A.A.; Matei, E.; Predescu, A.M.; Antoniac, I.; Predescu, C. Development of Bionanocomposites Based on PLA, Collagen and AgNPs and Characterization of Their Stability and In Vitro Biocompatibility. Appl. Sci. 2020, 10, 2265. https://doi.org/10.3390/app10072265
Râpă M, Stefan LM, Zaharescu T, Seciu A-M, Țurcanu AA, Matei E, Predescu AM, Antoniac I, Predescu C. Development of Bionanocomposites Based on PLA, Collagen and AgNPs and Characterization of Their Stability and In Vitro Biocompatibility. Applied Sciences. 2020; 10(7):2265. https://doi.org/10.3390/app10072265
Chicago/Turabian StyleRâpă, Maria, Laura Mihaela Stefan, Traian Zaharescu, Ana-Maria Seciu, Anca Andreea Țurcanu, Ecaterina Matei, Andra Mihaela Predescu, Iulian Antoniac, and Cristian Predescu. 2020. "Development of Bionanocomposites Based on PLA, Collagen and AgNPs and Characterization of Their Stability and In Vitro Biocompatibility" Applied Sciences 10, no. 7: 2265. https://doi.org/10.3390/app10072265
APA StyleRâpă, M., Stefan, L. M., Zaharescu, T., Seciu, A.-M., Țurcanu, A. A., Matei, E., Predescu, A. M., Antoniac, I., & Predescu, C. (2020). Development of Bionanocomposites Based on PLA, Collagen and AgNPs and Characterization of Their Stability and In Vitro Biocompatibility. Applied Sciences, 10(7), 2265. https://doi.org/10.3390/app10072265









