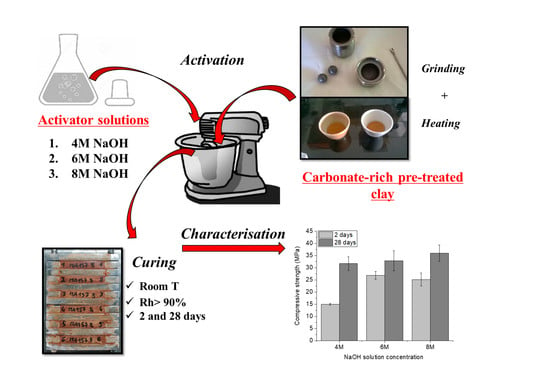
Effect of Alkali Concentration on the Activation of Carbonate-High Illite Clay
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
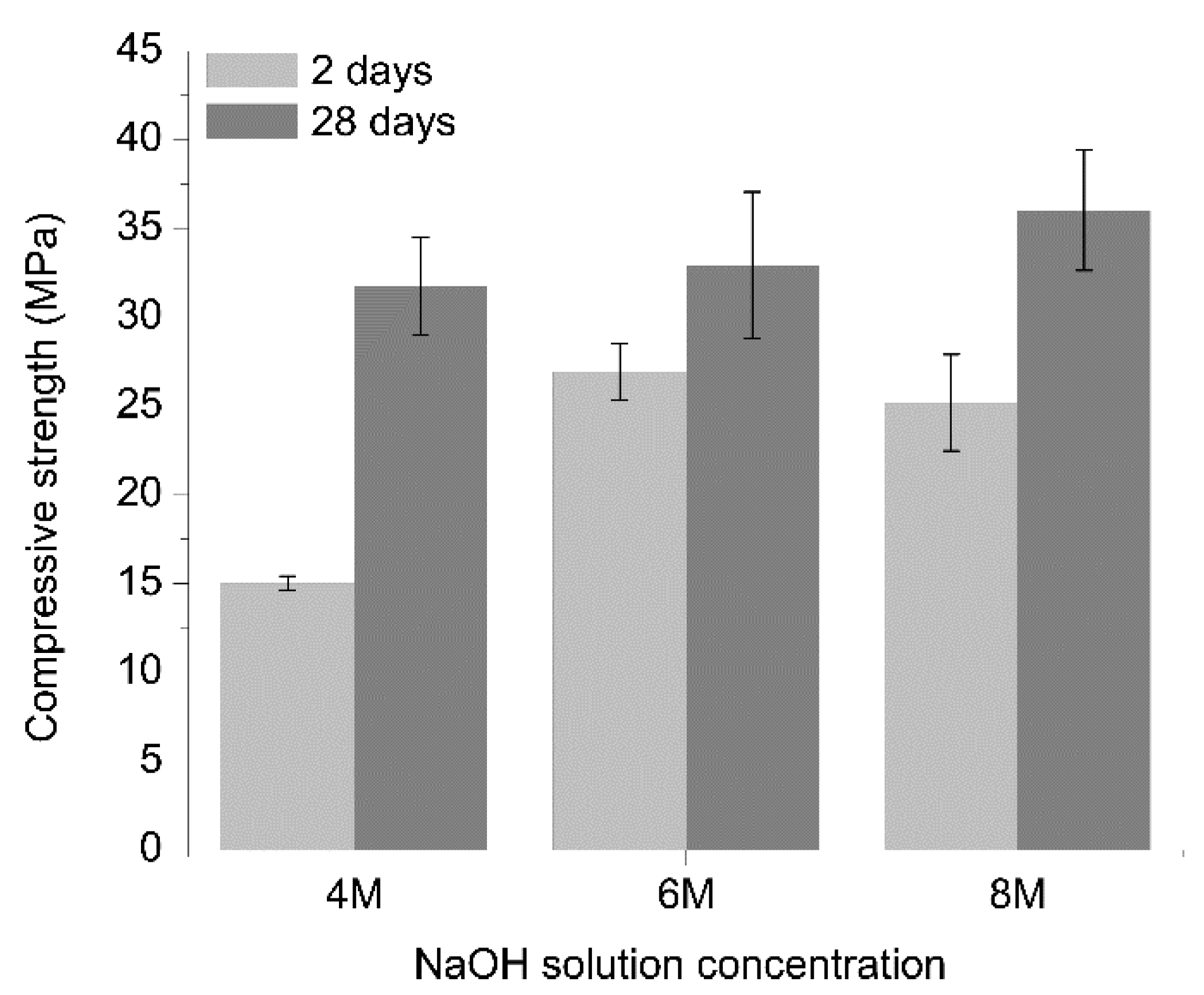
3.1. Mechanical Strength
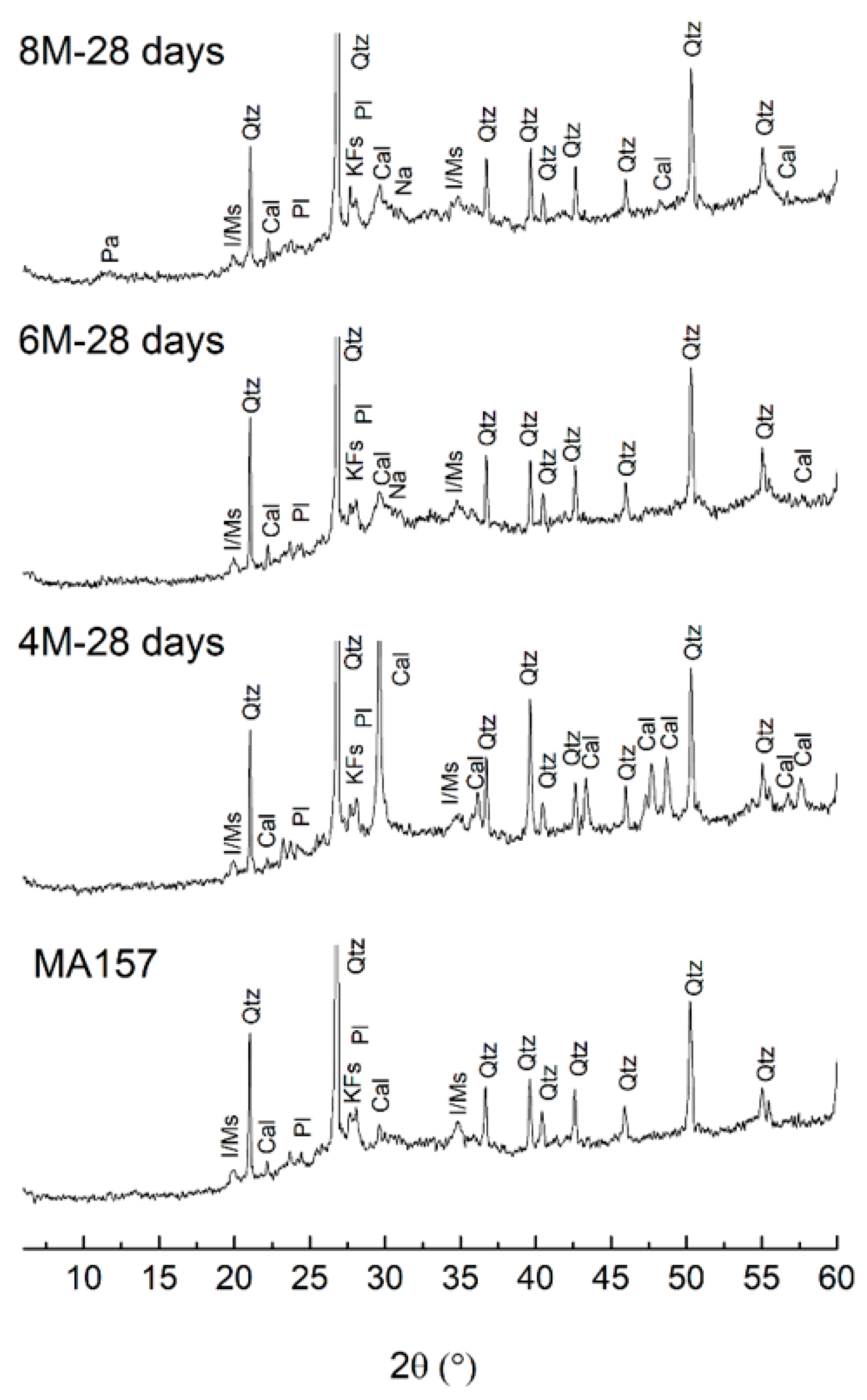
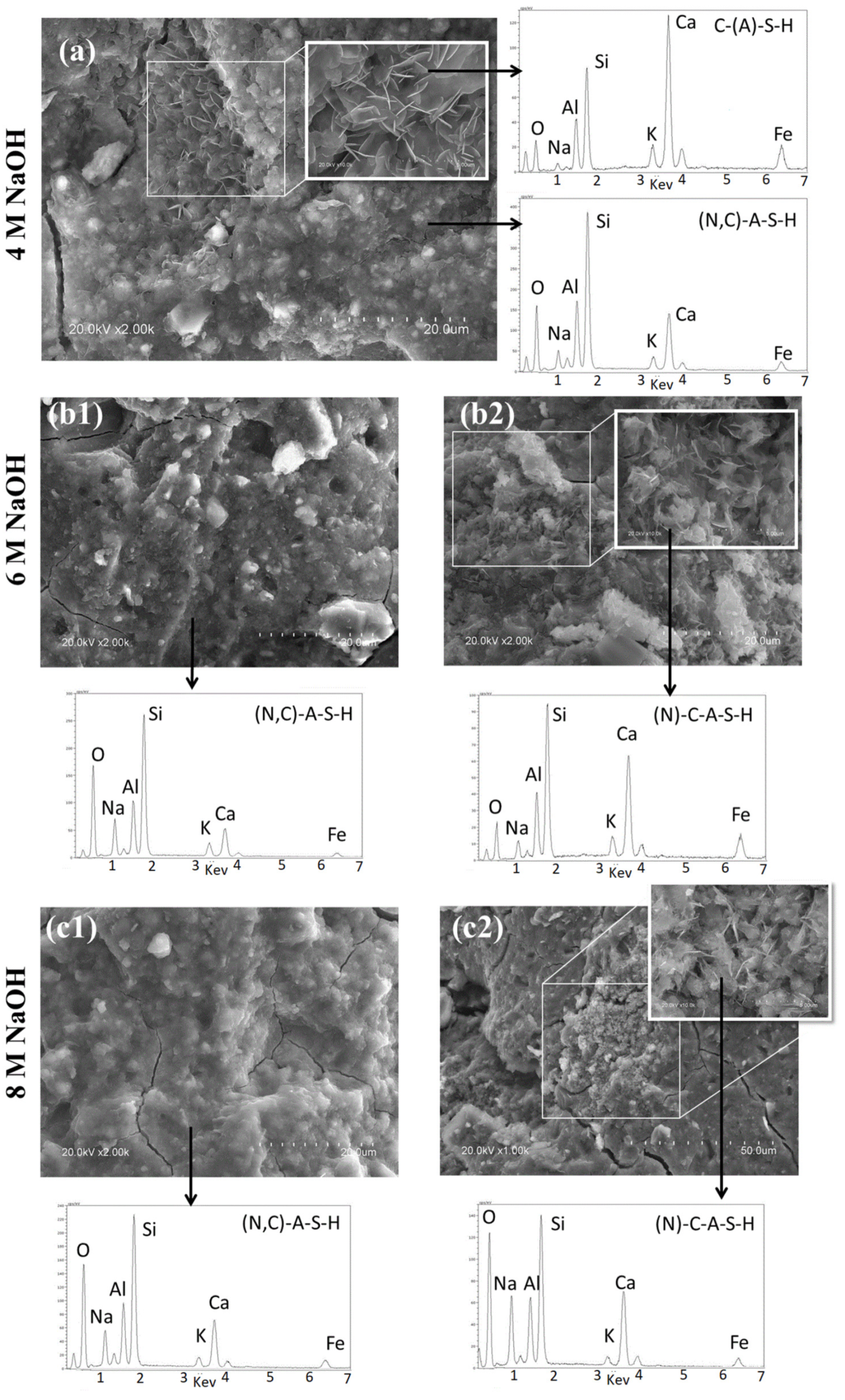
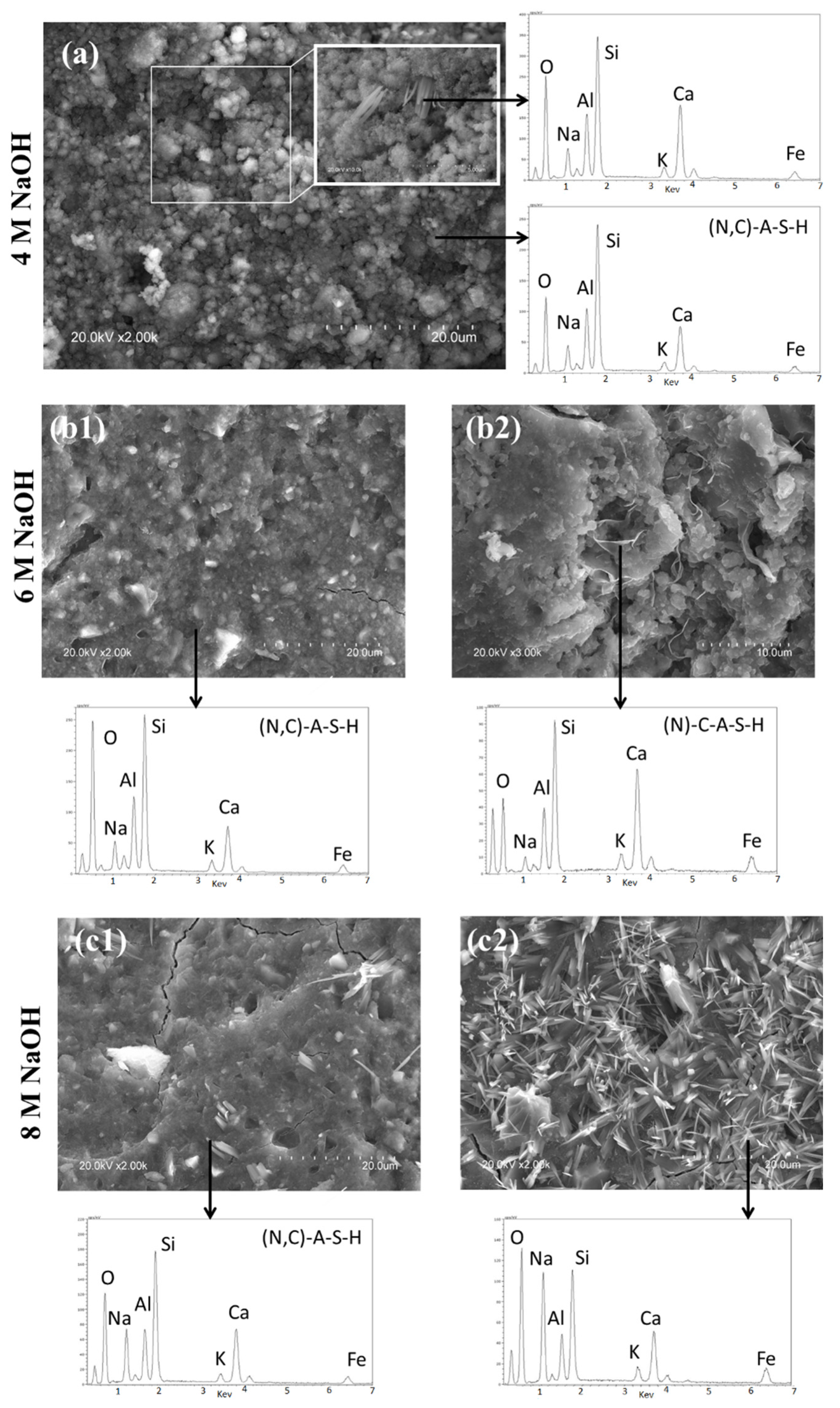
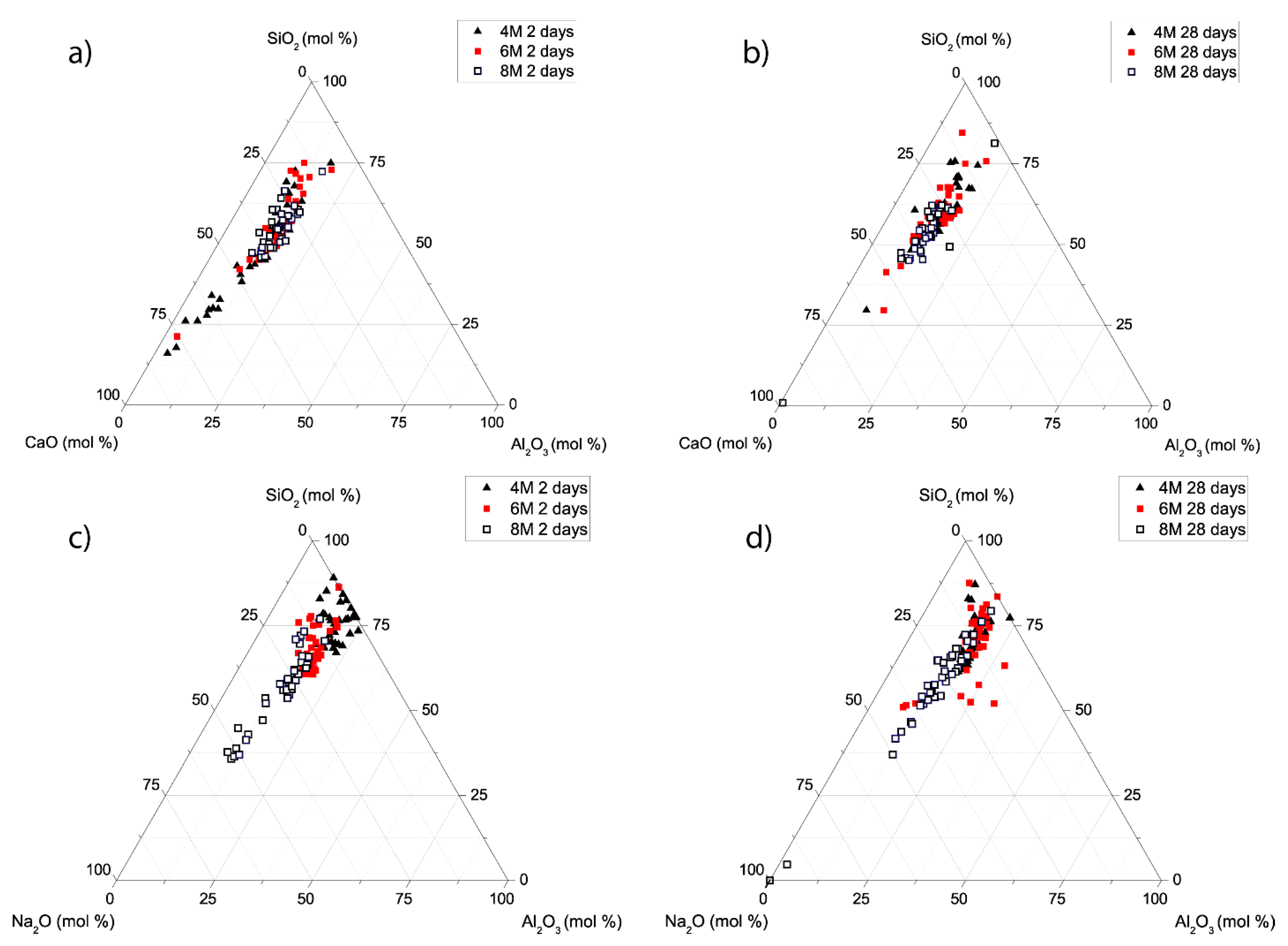
3.2. Reaction Products
4. Conclusions
- NaOH (at concentrations of 4, 6 and 8 M) activation of a carbonate-high illite clay contributes to paste hardening at ambient temperature. The presence of reactive calcium in the starting clay induces co-precipitation of a mix of gels: an aluminium-enriched C-S-H gel (C-A-S-H) and a N-A-S-H gel, in which sodium is partially replaced by calcium (N,C)-A-S-H.
- When the activator is a 4 M NaOH solution, the C-A-S-H gel is more intensely carbonated than with 6 or 8 M activators.
- Pastes prepared with higher (6 or 8 M) activator concentrations exhibit a more compact matrix than the ones prepared with 4 M NaOH. Given the comparability of the findings for the 6 and 8 M NaOH-activated pastes and the cost effectiveness of using a lower concentration activator, the 6 M NaOH solution may be deemed to be the most suitable of those studied here. Activating a mechanically and thermally pre-treated carbonate-high illite clay with a 6 M NaOH solution can deliver a binder with two days compressive strength of >20 MPa and 28 days strength of >30 MPa.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, H.; van Deventer, J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- Granizo, M.L.; Alonso, S.; Blanco-Varela, M.T.; Palomo, A. Alkaline Activation of Metakaolin: Effect of Calcium Hydroxide in the Products of Reaction. J. Am. Ceram. Soc. 2002, 85, 225–231. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Monzo, M.; Vincent, M.B.A.; Palomo, A. Alkaline activation of metakaolin-fly ash mixtures: Obtain of Zeoceramics and Zeocements. Microporous Mesoporous Mater. 2008, 108, 41–49. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Use of reservoir clay sediments as raw materials for geopolymer binders. Adv. Appl. Ceram. 2013, 112, 184–189. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic Polymeric New Materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Sakthivel, T.; Santhanam, M.; Gettu, R. Pillai Mechanical properties and durability performance of concretes with limestone calcined clay cement (LC3). Cem. Concr. Res. 2018, 107, 136–151. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Alvarez-Pinazo, G.; Santacruz, I.; Aranda, M.A.; De la Torre, A.G. Hydration of belite–ye’elimite–ferrite cements with different calcium sulfate sources. Adv. Cem. Res. 2016, 28, 1–15. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; García-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A review on alkaline activation: New analytical perspectives. Materiales Construcción 2014, 64, e022. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. (Eds.) Alkali-Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, H.J.; Zhou, C.H.; Wang, H. Geopolymer from kaolin in China: An overview. Appl. Clay Sci. 2016, 119, 31–41. [Google Scholar] [CrossRef]
- Kuenzel, C.; Vandeperre, L.J.; Donatello, S.; Boccaccini, A.R.; Cheeseman, C. Ambient Temperature Drying Shrinkage and Cracking in Metakaolin-Based Geopolymers. J. Am. Ceram. Soc. 2012, 95, 3270–3277. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Mustafa, A.B.M.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and Mineralogy of Clay Minerals. In Handbook of Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Oxford, UK, 2013; pp. 21–81.1148. [Google Scholar] [CrossRef]
- Murray, H.H. Applied Clay Mineralogy: Occurrences, Processing and Application of Kaolins, Bentonites, Palygorskite-Sepiolite, and Common Clays; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Seiffarth, T.; Hohmann, M.; Posern, K.; Kaps, C. Effect of thermal pre-treatment conditions of common clays on the performance of clay-based geopolymeric binders. Appl. Clay Sci. 2013, 7, 35–41. [Google Scholar] [CrossRef]
- Ferone, C.; Liguori, B.; Capasso, I.; Colangelo, F.; Cioffi, R.; Cappelletto, E.; di Maggio, R. Thermally treated clay sediments as geopolymer source material. Appl. Clay Sci. 2015, 107, 195–204. [Google Scholar] [CrossRef]
- D’Elia, A.; Pinto, D.; Eramo, G.; Giannossa, L.C.; Ventruti, G.; Laviano, R. Effects of processing on the mineralogy and solubility of carbonate-rich clays for alkaline activation purpose: Mechanical, thermal activation in red/ox atmosphere and their combination. Appl. Clay Sci. 2018, 152, 9–21. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Granizo, M.L.; Blanco-Varela, M.T.; Palomo, A. Influence of the starting kaolin on alkali-activated materials based on metakaolin. Study of the reaction parameters by isothermal conduction calorimetry. J. Mater. Sci. 2000, 35, 6309–6315. [Google Scholar] [CrossRef]
- Palomo, A.; Blanco-Varela, M.T.; Granizo, M.L.; Puertas, F.; Vazquez, T.; Grutzeck, M.W. Chemical stability of cementitious materials based on metakaolin. Cem. Concr. Res. 1999, 29, 997–1004. [Google Scholar] [CrossRef]
- Buchwald, A.; Hohmann, M.; Posern, K.; Brendler, E. The suitability of thermally activated illite/smectite clay as raw material for geopolymer binders. Appl. Clay Sci. 2009, 46, 300–304. [Google Scholar] [CrossRef]
- Essaidi, N.; Samet, B.; Baklouti, S.; Rossignol, S. Effect of calcination temperature of Tunisian clay on the properties of geopolymers. Ceramics Silikáty 2013, 57, 251–257. [Google Scholar]
- Ruiz-Santaquiteria, C.; Fernández-Jiménez, A.; Skibsted, J.; Palomo, A. Clay reactivity: Production of alkali activated cements. Appl. Clay Sci. 2013, 73, 11–16. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Cherfa, N.; Zibouche, F.; Fernández-Jimenez, A.; Palomo, A. The role of aluminium in alkali-activated bentonites. Mater. Struct. 2015, 48, 585–597. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, A.; Fernandez-Jimenez, A. Palomo Hybrid Alkaline Cements: Bentonite-Opc Binders. Minerals 2018, 8, 137. [Google Scholar] [CrossRef]
- Khater, H.M. Effect of Calcium on Geopolymerization of Aluminosilicate Wastes. J. Mater. Civ. Eng. 2012, 24, 92–101. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O-CaO-Al2O3-SiO2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Alahrache, S.; Winnefeld, F.; Champenois, J.B.; Hesselbarth, F.; Lothenbach, B. Chemical activation of hybrid binders based on siliceous fly ash and Portland cement. Cem. Concr. Compos. 2006, 66, 10–23. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Donatello, S.; Fernández-Jiménez, A.; Palomo, A. Hydration of Hybrid Alkaline Cement Containing a Very Large Proportion of Fly Ash: A Descriptive Model. Materials 2016, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.M.; Reynolds, R.C., Jr. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Komljenović, M.; Bascarević, Z.; Bradić, V. Mechanical and microstructural properties of alkali-activated fly ash geopolymers. J. Hazard. Mater. 2010, 181, 35–42. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Fernández-Jiménez, A. Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; Provis, J.L. Phase diagrams for alkali-activated slag binders. Cem. Concr. Res. 2017, 95, 30–38. [Google Scholar] [CrossRef]
- Cwirzen, A.; Provis, J.L.; Penttala, V.; Habermehl-Cwirzen, K. The effect of limestone on sodium hydroxide-activated metakaolin-based geopolymers. Constr. Build. Mater. 2014, 66, 53–62. [Google Scholar] [CrossRef]
- Rees, C.; Provis, J.L.; Luckey, G.C.; van Deventer, J.S.J. In Situ ATR-FTIR Study of the Early Stages of Fly Ash Geopolymer Gel Formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio Part I: FTIR study. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Joshi, S.; Kalyanasundaram, S.; Balasubramanian, V. Quantitative Analysis of Sodium Carbonate and Sodium Bicarbonate in Solid Mixtures Using Fourier Transform Infrared Spectroscopy (FT-IR). Appl. Spectrosc. 2013, 67, 841–845. [Google Scholar] [CrossRef]
- Yip, C.; Lukey, G.; Provis, J.; van Deventer, J. Effect of calcium silicate sources on geopolymerisation. Cem. Concr. Res. 2008, 38, 554–564. [Google Scholar] [CrossRef]
- Yip, C.; Provis, J.; Lukey, G.; van Deventer, J. Carbonate mineral addition to metakaolin-based geopolymers. Cem. Concr. Compos. 2008, 30, 979–985. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Vazquez, T. Carbonation process of alkali-activated slag mortars. J. Mater. Sci. 2006, 41, 3071–3082. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Zibouche, F.; Boudissa, N.; García-Lodeiro, I.; Abadlia, M.T.; Palomo, A. Metakaolin-Slag-Clinker Blends. The role of Na+ or K+ as Alkaline Activators of Theses Ternary Blends. J. Am. Ceram. Soc. 2013, 96, 1–8. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Hydration kinetics in hybrid binders: Early reaction stages. Cem. Concr. Compos. 2013, 39, 82–92. [Google Scholar] [CrossRef]
- Liu, B.; Ray, A.S.; Thomas, P.S. Strength development in autoclaved aluminosilicate rich industrial waste cement systems containing reactive magnesia. J. Aust. Ceram. Soc. 2007, 43, 82–87. [Google Scholar]
- Bernal, S.A.; Provis, J.L.; Walkley, B.; Nicolas, R.S.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.; Duxson, P.; van Deventer, J.S.J. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; de Gutiérrez, R.M.; van Deventer, J.S.J. Accelerated carbonation testing of alkali-activated slag/metakaolin blended concretes: Effect of exposure conditions. Mater. Struct. 2015, 48, 653–669. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Brice, D.G.; Kilcullen, A.; Duxson, P.; van Deventer, J.S.J. Accelerated carbonation testing of alkali-activated binders significantly underestimate the real service life: The role of the pore solution. Cem. Concr. Res. 2012, 42, 1317–1326. [Google Scholar] [CrossRef]
- Bernal, S.A. Effect of the activator dose on the compressive strength and accelerated carbonation resistance of alkali silicate-activated slag/metakaolin blended materials. Constr. Build. Mater. 2015, 98, 217–226. [Google Scholar] [CrossRef]
- Bauchy, M.; Laubie, H.; Qomi, M.A.; Hoover, C.G.; Ulm, F.J.; Pellenq, R.M. Fracture toughness of calcium–silicate–hydrate from molecular dynamics simulations. J. Non-Cryst. Solids 2015, 419, 58–64. [Google Scholar] [CrossRef]
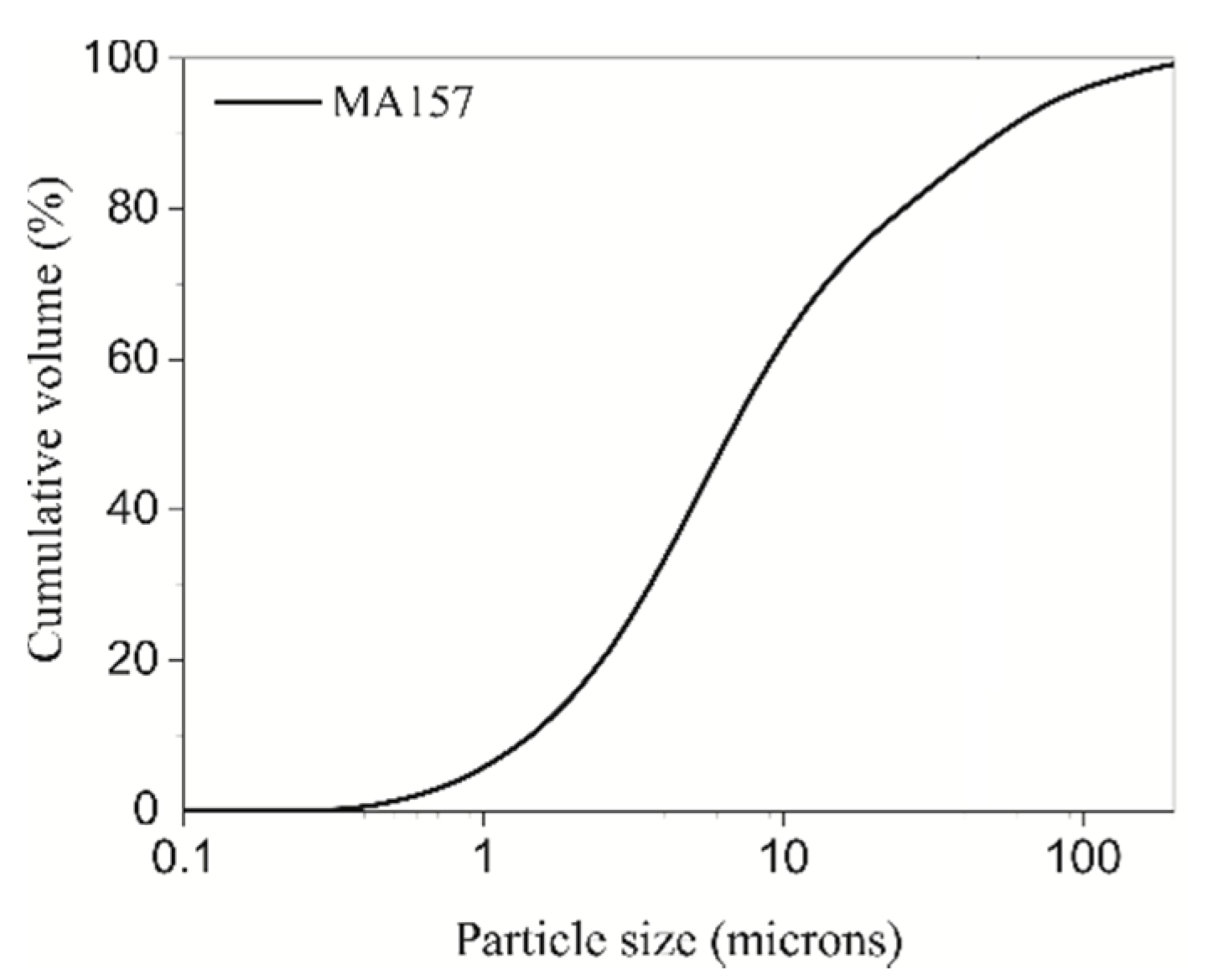

| Chemical Composition (wt%) XRF | Mineralogical Composition (wt%, 3σ) | ||||
|---|---|---|---|---|---|
| Untreated Clay | MA157 1 | Untreated Clay | MA157 1 | ||
| SiO2 | 35.67 | 43.05 | Quartz | 16.3 (0.7) | 20.6 (1.4) |
| Al2O3 | 11.47 | 13.14 | Calcite | 31.2 (1.3) | 5.4 (1.1) |
| CaO | 21.15 | 29.97 | Illite/Muscovite | 3.8 (0.5) | 3.5 (1.0) |
| K2O | 1.73 | 2.28 | R0 illite(0.3)/smectite mixed layers 2 | 24.5 (2.9) | - |
| Na2O | 0.34 | 0.36 | Kaolinite | 5.9 (1.0) | |
| Fe2O3 | 6.67 | 9.34 | K-feldspar | 4.2 (1.0) | 10.6 (1.7) |
| MgO | 1.91 | 2.22 | Albite | 3.2 (0.5) | 3.4 (0.8) |
| MnO | 0.15 | 0.21 | Goethite | 1.7 (0.2) | - |
| TiO2 | 0.53 | 0.73 | Hematite | - | 2.8 (0.5) |
| P2O5 | 0.07 | 0.09 | Dolomite | 1.0 (0.2) | - |
| LoI | 20.32 | 0.61 | Chlorite | 1.0 (0.3) | - |
| Amorphous matter | 7.1 (6.3) | 53.9 (3.6) | |||
| Tot C.M. | 35.2 (3.7) | 3.5 (1.0) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Elia, A.; Pinto, D.; Eramo, G.; Laviano, R.; Palomo, A.; Fernández-Jiménez, A. Effect of Alkali Concentration on the Activation of Carbonate-High Illite Clay. Appl. Sci. 2020, 10, 2203. https://doi.org/10.3390/app10072203
D’Elia A, Pinto D, Eramo G, Laviano R, Palomo A, Fernández-Jiménez A. Effect of Alkali Concentration on the Activation of Carbonate-High Illite Clay. Applied Sciences. 2020; 10(7):2203. https://doi.org/10.3390/app10072203
Chicago/Turabian StyleD’Elia, Angela, Daniela Pinto, Giacomo Eramo, Rocco Laviano, Angel Palomo, and Ana Fernández-Jiménez. 2020. "Effect of Alkali Concentration on the Activation of Carbonate-High Illite Clay" Applied Sciences 10, no. 7: 2203. https://doi.org/10.3390/app10072203
APA StyleD’Elia, A., Pinto, D., Eramo, G., Laviano, R., Palomo, A., & Fernández-Jiménez, A. (2020). Effect of Alkali Concentration on the Activation of Carbonate-High Illite Clay. Applied Sciences, 10(7), 2203. https://doi.org/10.3390/app10072203