Pneumatically Actuated Microfluidic Platform for Reconstituting 3D Vascular Tissue Compression
Abstract
1. Introduction
2. Results and Discussion
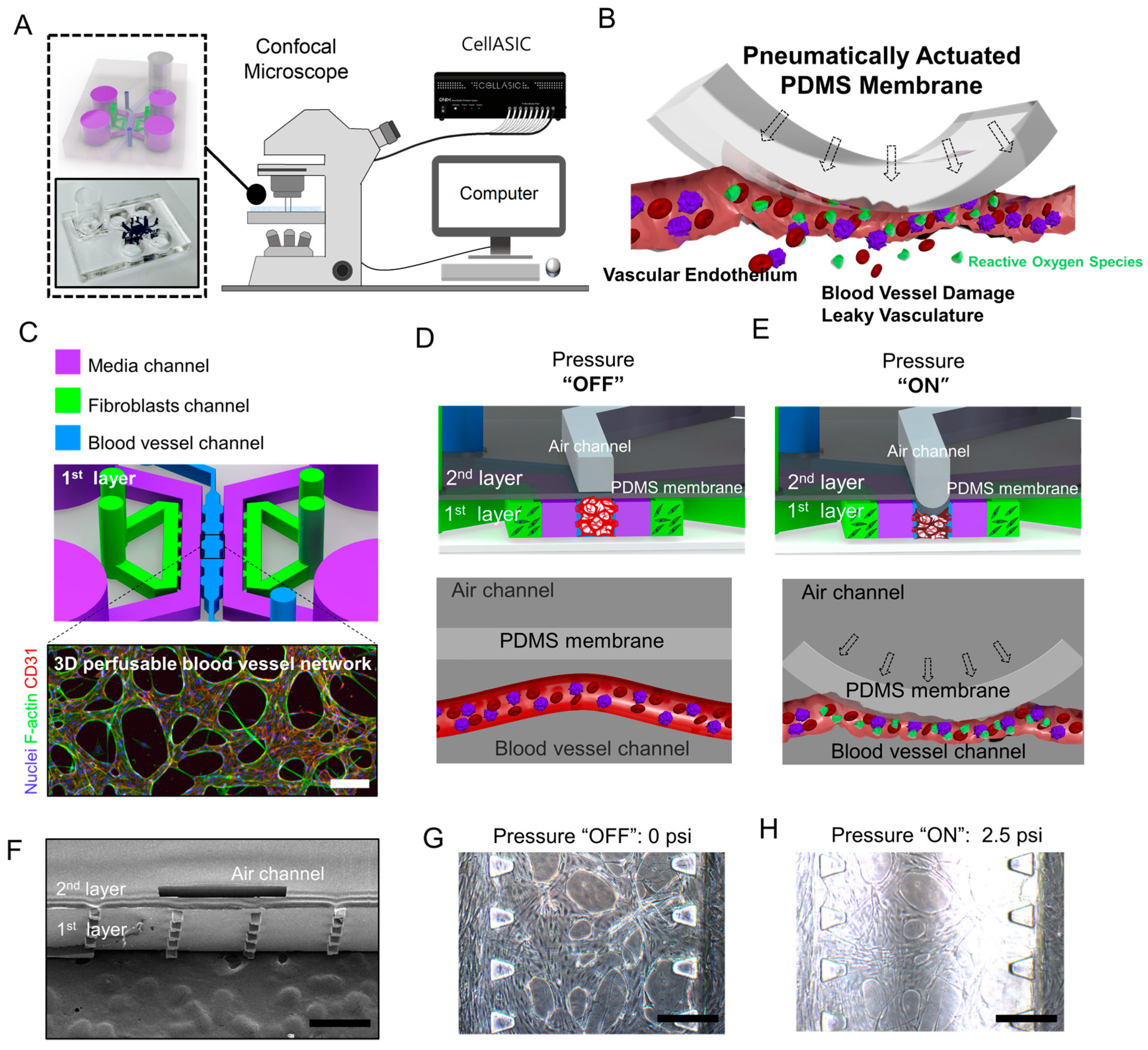
2.1. Microfluidic Chip Design and Experimental Design
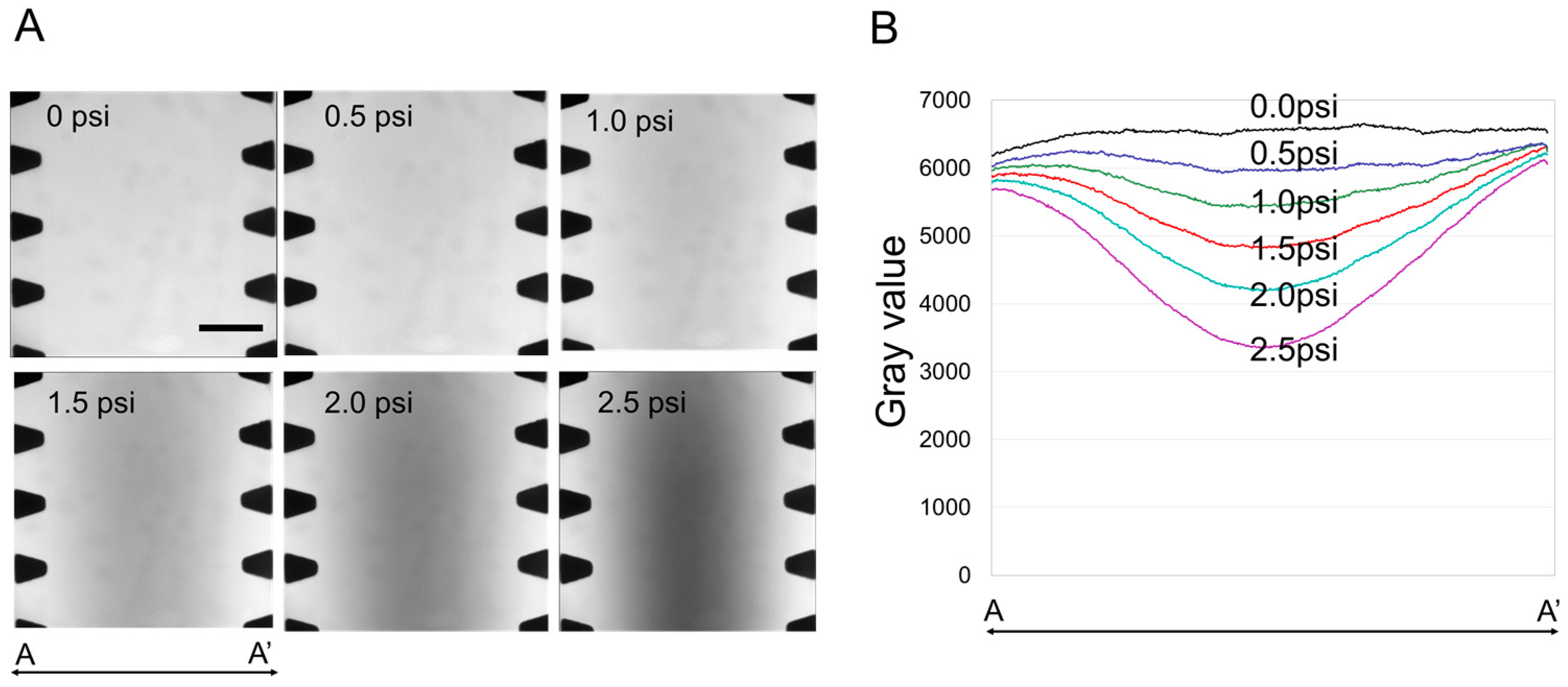
2.2. Finite Element Analysis Model of a Compressed Blood Vessel
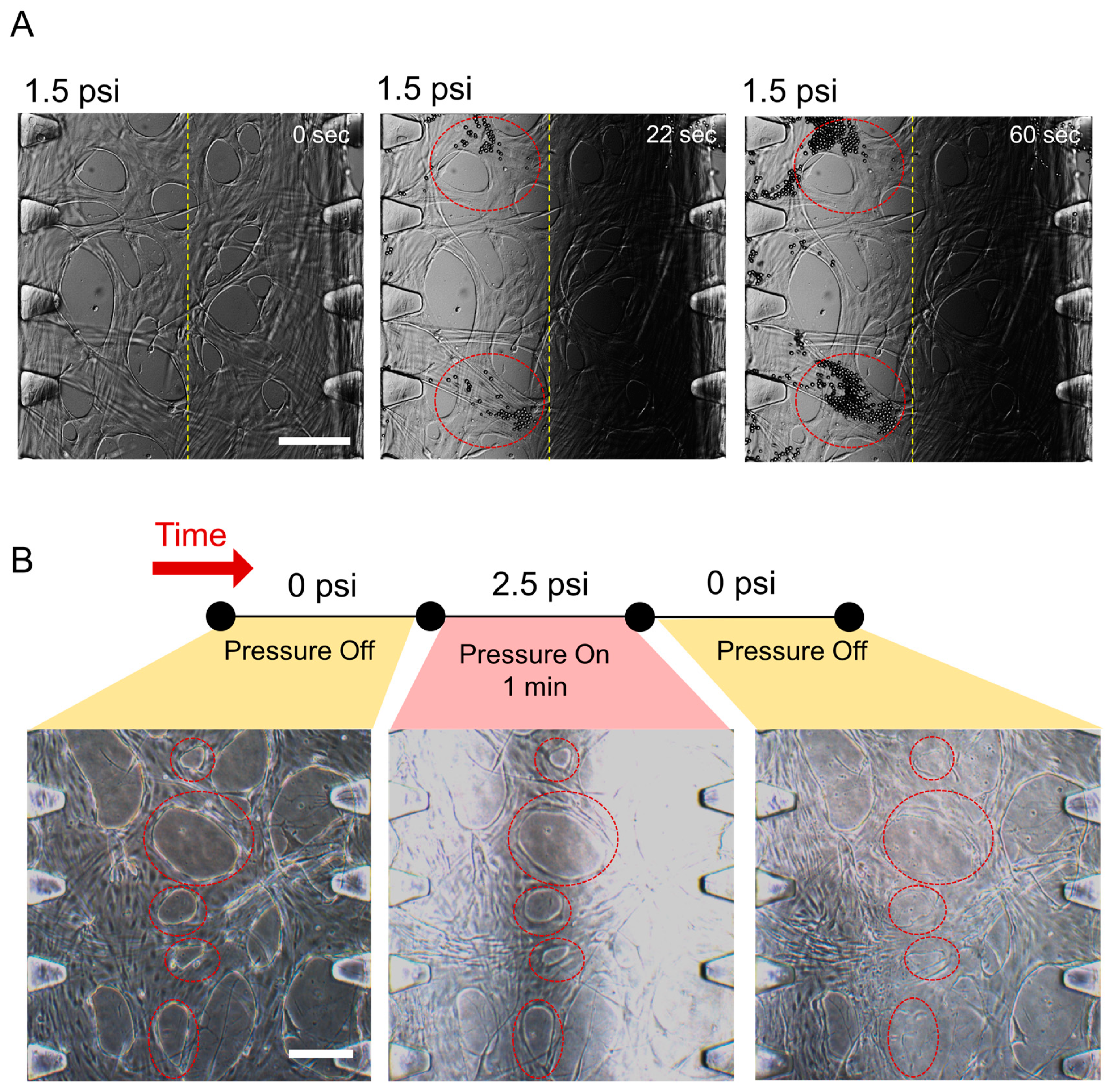
2.3. The Effect of Compressive Stress on a 3D Perfusable Microvessel Network
2.4. The Effect of Compressive Stress on Endothelial Cell Viability
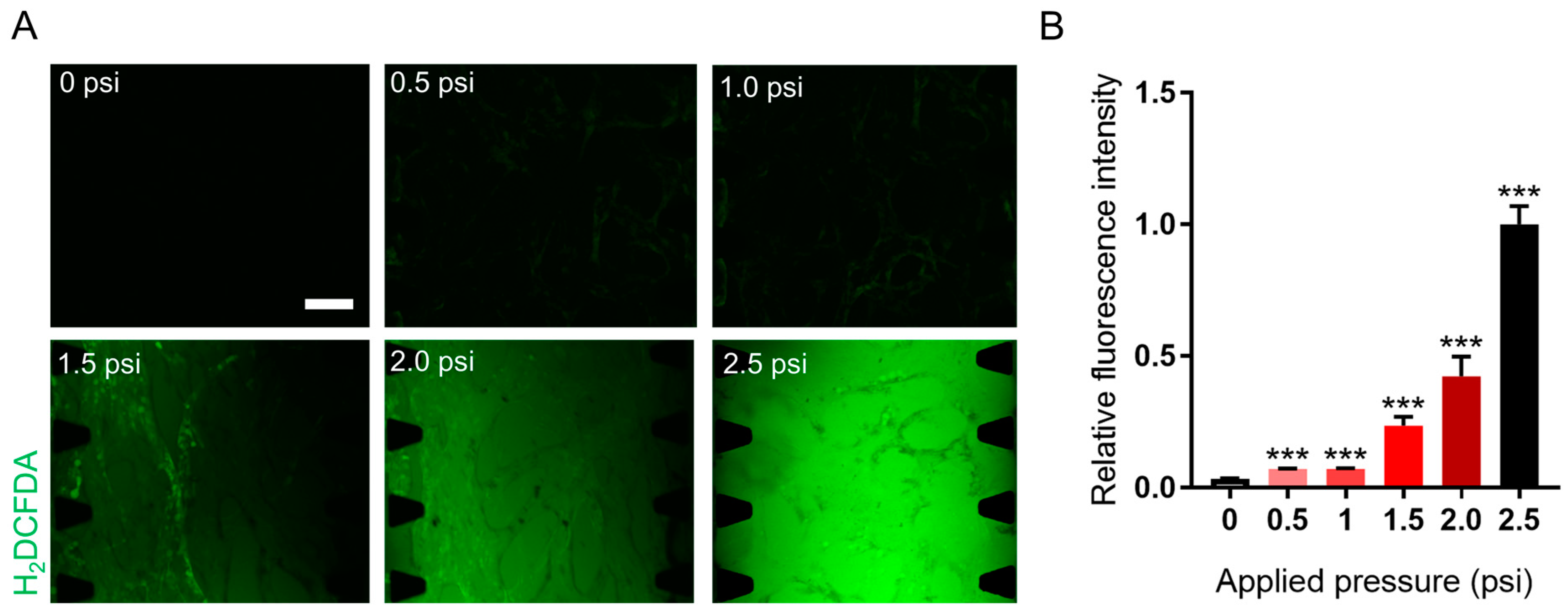
2.5. The Effect of Solid Stress on Reactive Oxygen Species Generation
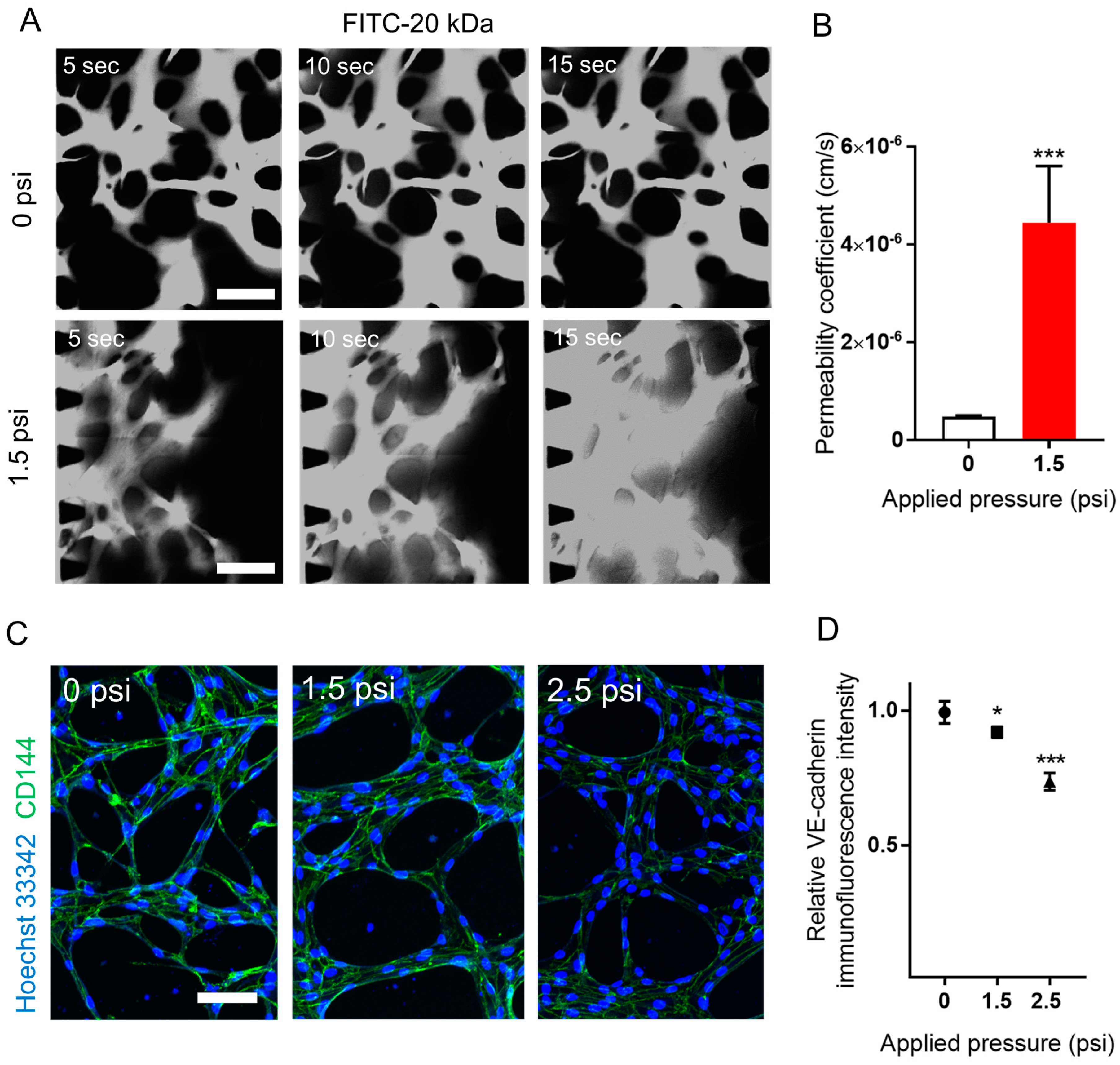
2.6. The Effect of Compressive Stress on Vascular Permeability and Vascular Endothelial (VE)-Cadherin Expression
3. Conclusions
4. Materials and Methods
4.1. Design and Fabrication of the Microfluidic Chip
4.2. Cell Culture
4.3. Hydrogel and Cell Loading into a Microfluidic Chip
4.4. Live/Dead Assay
4.5. Measurement of ROS Using H2DCFDA
4.6. Immunostaining
4.7. Permeability Coefficient Measurement
4.8. Finite Element Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hahn, C.; Schwartz, M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.; Chung, M.; Jeon, N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013, 13, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, M.; Jeon, N.L. Three-dimensional biomimetic model to reconstitute sprouting lymphangiogenesis in vitro. Biomaterials 2016, 78, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Ko, J.; Lee, S.; Yu, J.; Kim, Y.; Jeon, N.L. Microfluidics in nanoparticle drug delivery; From synthesis to pre-clinical screening. Adv. Drug Deliv. Rev. 2018, 128, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Wang, X.; Ren, L.; Wang, X.; Wang, J.; Wang, J. An integrated microfluidic system for studying cell-microenvironmental interactions versatilely and dynamically. Lab Chip 2010, 10, 1717–1724. [Google Scholar] [CrossRef]
- Gao, Y.; Majumdar, D.; Jovanovic, B.; Shaifer, C.; Lin, P.C.; Zijlstra, A.; Li, D. A versatile valve-enabled microfluidic cell co-culture platform and demonstration of its applications to neurobiology and cancer biology. Biomed. Microdevices 2011, 13, 539–548. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Bao, N.; Lu, C. A microfluidic cell array with individually addressable culture chambers. Biosens. Bioelectron. 2008, 24, 613–617. [Google Scholar] [CrossRef]
- Wang, Z.; Kim, M.-C.; Marquez, M.; Thorsen, T. High-density microfluidic arrays for cell cytotoxicity analysis. Lab Chip 2007, 7, 740–745. [Google Scholar] [CrossRef]
- Yap, Y.C.; Dickson, T.C.; King, A.E.; Breadmore, M.C.; Guijt, R.M. Microfluidic culture platform for studying neuronal response to mild to very mild axonal stretch injury. Biomicrofluidics 2014, 8, 044110. [Google Scholar] [CrossRef]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 2014, 16, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Friedland, J.C.; Lee, M.H.; Boettiger, D. Mechanically activated integrin switch controls α5β1 function. Science 2009, 323, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.P.; Coukos, G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef]
- Blann, A.D. How a damaged blood vessel wall contibutes to thrombosis and hypertenasion. Pathophysiol. Haemost. Thromb. 2003, 33, 445–448. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Salvetti, G.; Salvetti, A. Endothelial dysfunction in hypertension. J. Nephrol. 1999, 13, 205–210. [Google Scholar]
- Kim, Y.C.; Kang, J.H.; Park, S.-J.; Yoon, E.-S.; Park, J.-K. Microfluidic biomechanical device for compressive cell stimulation and lysis. Sens. Actuators B Chem. 2007, 128, 108–116. [Google Scholar] [CrossRef]
- Armstrong, J.; Whiteman, M. Measurement of reactive oxygen species in cells and mitochondria. Mitochondria 2007, 80, 355. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Chien, S. Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell. Am. J. Physiology-Heart Circ. Physiol. 2007, 292, H1209–H1224. [Google Scholar] [CrossRef] [PubMed]
- Abaci, H.E.; Drazer, G.; Gerecht, S. Recapitulating the Vascular Microenvironment in Microfluidic Platforms. Nano Life 2013, 3, 1340001. [Google Scholar] [CrossRef]
- Rouleau, L.; Copland, I.B.; Tardif, J.-C.; Mongrain, R.; Leask, R.L. Neutrophil adhesion on endothelial cells in a novel asymmetric stenosis model: Effect of wall shear stress gradients. Ann. Biomed. Eng. 2010, 38, 2791–2804. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Davies, P.F.; Tripathi, S.C. Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ. Res. 1993, 72, 239–245. [Google Scholar] [CrossRef]
- Wang, B.; Caluch, A.; Fodil, R.; Fereol, S.; Zadigue, P.; Pelle, G.; Isabey, D. Force control of endothelium permeability in mechanically stressed pulmonary micro-vascular endothelial cells. Bio-Med. Mater. Eng. 2012, 22, 163–170. [Google Scholar] [CrossRef]
- Yuan, F.; Leunig, M.; Berk, D.A.; Jain, R.K. Microvascular permeability of albumin, vascular surface area, and vascular volume measured in human adenocarcinoma LS174T using dorsal chamber in SCID mice. Microvasc. Res. 1993, 45, 269–289. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.; Chung, M.; Kim, J.H.; Jeon, N.L. A bioengineered array of 3D microvessels for vascular permeability assay. Microvasc. Res. 2014, 91, 90–98. [Google Scholar] [CrossRef]
- Johnston, I.; McCluskey, D.; Tan, C.; Tracey, M. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromechanics Microengineering 2014, 24, 035017. [Google Scholar] [CrossRef]
- Choi, A.; Zheng, Y. Estimation of Young’s modulus and Poisson’s ratio of soft tissue from indentation using two different-sized indentors: Finite element analysis of the finite deformation effect. Med. Biol. Eng. Comput. 2005, 43, 258–264. [Google Scholar] [CrossRef]
- Levental, I.; Georges, P.C.; Janmey, P.A. Soft biological materials and their impact on cell function. Soft Matter 2007, 3, 299–306. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.; Lee, H.; Kang, H.; Choi, H.; Son, K.; Yu, J.; Lee, J.; Lim, J.; Park, D.; Cho, M.; et al. Pneumatically Actuated Microfluidic Platform for Reconstituting 3D Vascular Tissue Compression. Appl. Sci. 2020, 10, 2027. https://doi.org/10.3390/app10062027
Ahn J, Lee H, Kang H, Choi H, Son K, Yu J, Lee J, Lim J, Park D, Cho M, et al. Pneumatically Actuated Microfluidic Platform for Reconstituting 3D Vascular Tissue Compression. Applied Sciences. 2020; 10(6):2027. https://doi.org/10.3390/app10062027
Chicago/Turabian StyleAhn, Jungho, Hyeok Lee, Habin Kang, Hyeri Choi, Kyungmin Son, James Yu, Jungseub Lee, Jungeun Lim, Dohyun Park, Maenghyo Cho, and et al. 2020. "Pneumatically Actuated Microfluidic Platform for Reconstituting 3D Vascular Tissue Compression" Applied Sciences 10, no. 6: 2027. https://doi.org/10.3390/app10062027
APA StyleAhn, J., Lee, H., Kang, H., Choi, H., Son, K., Yu, J., Lee, J., Lim, J., Park, D., Cho, M., & Jeon, N. L. (2020). Pneumatically Actuated Microfluidic Platform for Reconstituting 3D Vascular Tissue Compression. Applied Sciences, 10(6), 2027. https://doi.org/10.3390/app10062027




