Abstract
Cutaneous melanoma is the most aggressive type of skin cancer with a not-sufficient clinical outcome. High tumor mutation rates often hamper a remedial treatment, creating the need for palliative care in many patients. To reduce pain and burden, local palliation often includes cryo-ablation, immunotherapy via injection of IL2, or electrochemotherapy. Yet, a fraction of patients and lesions do not respond to those therapies. To reach even these resistances in a redox-mediated way, we treated skin biopsies from human melanoma ex vivo with cold physical plasma (kINPen MED plasma jet). This partially ionized gas generates a potent mixture of reactive oxygen species (ROS). Physical plasmas have been shown to be potent antitumor agents in preclinical melanoma and clinical head and neck cancer research. The innovation of this technology lies in its ease-of-use without anesthesia, as the “cold” plasma temperature of the kINPen MED does not exceed 37 °C. In metastatic melanoma skin biopsies from six patients, we identified a marked increase of apoptosis with plasma treatment ex vivo. This had an impact on the chemokine/cytokine profile of the cultured biopsies, e.g., three of six patient-derived biopsy supernatants showed an apparent decrease in VEGF compared to non-plasma treated specimens. Moreover, the baseline release levels of 24 chemokines/cytokines investigated may serve as a useful tool for future research on melanoma skin biopsy treatments. Our findings suggest a clinically useful role of cold physical plasma therapy in palliation of cutaneous melanoma lesions, possibly in a combinatory setting with other immune therapies.
1. Introduction
With its high mutational load [1], its increasing incidence [2], and a majority of patients not responding to therapy [3], cutaneous melanoma is the ideal model tumor for testing emerging therapeutic avenues. It is established that harnessing the power of antitumor immunity, e.g., through checkpoint inhibition, provides a powerful tool to target cutaneous melanoma. While unleashed T cell responses against tumor cells is an important arm of this therapy [4], the tumor microenvironment (TME) is another key factor that governs therapeutic outcomes and relates to chemokines, cytokines [5], and reactive oxygen species (ROS) [6]. A novel modality of generating various kinds of ROS is cold physical plasma. This partially ionized gas can be applied to cells and tissues without causing thermal damage. Importantly, plasmas were shown to initiate immunogenic cancer cell death in melanoma cells in vitro and in vivo [7,8,9]. First results with plasma treatment of a small cohort of palliative patients suffering from advanced head and neck cancer indicated a marked benefit in survival and tumor regression in some cases [10]. Yet, the effects of plasma treatment in human melanoma tissue were not explored so far. In this study, we collected punch biopsies of patient-derived metastatic skin melanoma lesions to investigate the effect of cold physical plasma ex vivo. Lesional skin punch biopsies from patients suffering from metastatic melanoma were ex vivo treated with cold physical plasma generated by an atmospheric pressure argon plasma jet accredited as medical device class IIa in Europe [11]. Samples were analyzed for the induction of apoptosis and their secretory products (24 different chemokines and cytokines) released into the medium during culture ex vivo. Besides some clear changes in secretion of these molecules after plasma treatment capable of locally modulating inflammation, the baseline expression levels obtained in this study may serve as a valuable resource for future studies on human cutaneous melanoma sample treatments ex vivo.
2. Materials and Methods
2.1. Cutaneous Melanoma Biopsies and Exposure to Cold Physical Plasma
Metastatic lesions from six patients suffering from malignant melanoma stage IV (one female and five male, mean age 59 (50–86 years, ±12 years), metastases from, e.g., cervical, axillary or inguinal tissue) were surgically removed, and punch biopsies (diameter approximately 3 mm) were generated ex vivo. The study was approved by the Institutional Review Board of the University of Duisburg-Essen (ethical committee of the University of Duisburg-Essen) under the Institutional Review Board protocol no. 12-4961-BO. Patient samples were retrieved upon informed consent to perform the experiments and publish the data anonymously. Biopsies were inserted in small tips placed into a 96-well plate to ensure their stability during subsequent exposure to cold physical plasma (Figure 1a) for 120s (kINPen MED operated at a frequency of 1 MHz and five standard liters of argon gas per minute; neoplas tools, Germany) or left untreated with argon gas only as controls. Immediately afterward, samples were transferred to the cell culture medium (RPMI1640 supplemented with 2% penicillin/streptomycin; Thermo Fisher Scientific, Germany) and incubated at 37 °C. Twentyfour hours later, supernatants were collected and stored at −80 °C. Likewise, tissues were embedded in OCT (VWR, Germany) in disposable base molds (Thermo Fisher Scientific, Germany), snap-frozen in liquid nitrogen, and stored at −80 °C.
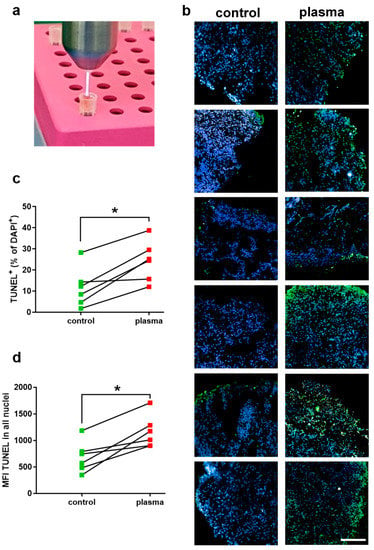
Figure 1.
(a) Cold physical plasma treatment of cutaneous melanoma tissue was performed ex vivo with an atmospheric pressure argon plasma jet (kINPen MED); (b) nuclei (blue) and apoptotic cells (green) in control and plasma-treated tissue sections at 24 h; (c, d) percentage of apoptotic cells (c) and mean fluorescence intensity (MFI) of TUNEL in all nuclei (d) as calculated using nuclei segmentation and quantitative image analysis of several sections of control and plasma-treated tissue. Data are mean of results from 6 patients; asterisks indicate a significant difference (p < 0.05) between groups as identified with Wilcoxon matched-pairs rank test. Scale bar is 200 µm.
2.2. Multiplex Chemokine and Cytokine Analysis
Multiplex chemokine/cytokine analysis was performed using a bead-based assay (BioLegend, London, UK) according to the vendor’s instructions. Briefly, supernatants of melanoma biopsies were incubated with beads, and mean fluorescence intensities (MFI) of each bead population (representing a single analyte) were determined using flow cytometry (CytoFLEX S; Beckman-Coulter, USA). Total chemokine/cytokine concentrations in picogram per milliliter were calculated against a known standard and using 5-log fitting with dedicated software (VigeneTech, USA). Statistical analysis was performed by multiple t-tests for each analyte and patient with Holm-Sidak testing.
2.3. Immunofluorescence and Imaging
Samples were sectioned vertically (thickness: 6 µm) using a cryomicrotome (Leica Microsystems, Germany) and placed on glass microscope slides (Thermo Fisher Scientific, Germany). After DAKO (Dako, Germany) block, nuclei were stained with 4′,6-Diamidin-2-phenylindol (DAPI; Sigma, Germany). Apoptosis was detected using TUNEL (Roche Diagnostics, Switzerland) according to the manufacturer’s instructions. Slides were mounted using Vectashield (Biozol, Germany). Tissue sections were evaluated using a high content imaging device (Operetta CLS; PerkinElmer, Germany). For each tissue section, areas of the same size were used to compare the plasma-effect on the treated with that of the untreated samples. Data acquisition and analyses were performed with the respective software of the device (Harmony 4.8, PerkinElmer, Germany). Student’s t-test or Wilcoxon matched-pairs rank test was performed to compare untreated and treated groups.
3. Results
In this study, the effect of cold physical plasma-derived ROS on metastatic skin melanoma punch biopsies was investigated ex vivo (Figure 1a). Tissues were sectioned, stained with DAPI (staining for nuclei), and assessed for apoptosis induction using TUNEL. Qualitative analysis showed an increase in apoptotic cells after exposure to physical plasma (green cells in Figure 1b). Quantitative image analysis over dozens of fields of view was performed. Elaborating the percentage of apoptotic cells, a marked increase was observed in the plasma treatment group compared to untreated control tissue (Figure 1c). The mean increase in apoptotic cells after plasma treatment was 10%. To validate these results with a second quantitative image analysis strategy, the mean fluorescence intensity (MFI) was identified in the perinuclear regions of tissues. This analysis also revealed a marked increase in TUNEL signal in the plasma-treated samples compared to untreated tissue (Figure 1d). These results suggest an increase of apoptosis in cutaneous melanoma samples exposed to cold physical plasma ex vivo. As not only apoptosis but also ROS engage in the modulation of inflammation, we performed a chemokine/cytokine measurement spanning 24 target molecules secreted from human cutaneous melanoma tissue sample into the culture medium after 24 h. These secretory patterns in untreated samples alone are a valuable resource for future research to investigate inflammatory responses after melanoma treatment ex vivo. Specifically, all metastatic skin melanoma tissue samples showed high secretion levels of CCL2, G-CSF, IL1β, IL6, IL8, PDGF, and VEGF (Figure 2). By contrast, secretion of other target molecules was mostly (in > 66% of the samples) absent, including sFAS, sFASL, granzyme B, IL2, IL4, IFNγ, perforin, and TNFα. In physical plasma-treated samples, secretory changes were observed for some of the 24 targets. However, an overall similar and distinguishable pattern could not be identified for the treated melanoma tissue compared to untreated tissue over the six patients investigated. With regard to similar changes in equal or more than one-third of the patients’ tissue, a marked secretory decrease after plasma treatment was observed for CCL2, IL10, and VEGF while the secretion of CCL5, EGF, and granulysin was increased after plasma treatment. For all other chemokines/cytokines, there was no change of expression after plasma treatment, at least in samples of at least two different patients.
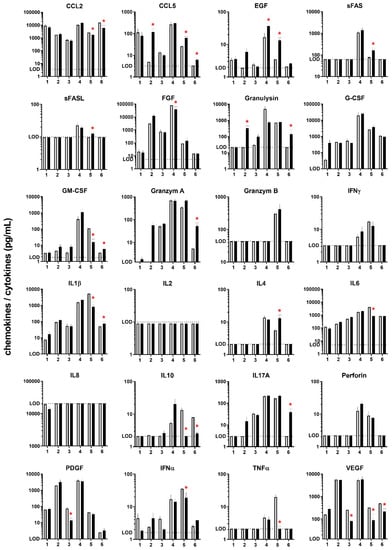
Figure 2.
Chemokine/cytokine secretory profile (in pg/mL) of 24 targets measured in culture medium from cutaneous melanoma punch biopsy cultures 24 h after exposure to cold physical plasma (dark bars) or left untreated (grey bars). LOD= lower limit of detection, except for IL8 (upper limit of detection); red asterisks demark significant difference (p < 0.05) between plasma and control samples for each patient (1–6); given are mean and the standard error of three technical replicates per patient and sample.
4. Discussion
Exposure of human cutaneous melanoma biopsies to cold physical plasma ex vivo increased the number of apoptotic cells in the treated tissue. This is in line with previous in vitro findings showing induction of cellular senescence [12] and apoptosis [13] in melanoma cells with plasma treatment. Plasma-induced apoptosis in melanoma is dependent on caspase activation and can be abrogated by prior addition of radical scavengers [14], which underscores the notion of the strong ROS-based effects of physical plasmas. Of note, apoptosis of cancer cells following plasma treatment is not restricted to melanoma cells but also includes a variety of other tumor cell types [15]. ROS, as well as cell demise, can be both the source and target of an altered inflammatory signature in the tumor microenvironment (TME). To this end, we profiled the supernatants of cultured tumor samples quantitatively for the secretion of 24 different chemokines/cytokines to understand how plasma treatment may directly or indirectly modify the TME of human melanoma samples ex vivo. Following exposure to plasma, markedly increased (CCL5, EGF, granulysin) or decreased (CCL2, IL10, VEGF) chemokine/cytokine secretions were observed in at least two out of six patients with a similar trend. It is well-known that VEGF and IL10 but also CCL2 support immunosuppression in the TME, leading to senescence of antitumor immunity (IL10), angiogenesis (VEGF), education of tumor-associated macrophages (CCL2), and subsequently augmented tumor growth [16]. Hence, plasma treatment may constitute a therapeutic approach to make “cold tumors hot”, i.e., to induce a mild inflammation that is the prerequisite for antitumor immune responses. That the Nobel Prize in Medicine 2018 was awarded for the identification of checkpoint inhibition exemplifies the importance and magnitude of T-cells specifically targeting tumor cells in antitumor therapies. Yet, many patients still not benefit from such antibodies masking, e.g., PD1/2, PD-L1/2, and/or CTL4, and it is discussed that other immunosuppressive mechanisms in the TME may abrogate T-cell activity [17]. Therefore, plasma treatment may possibly add efficacy to immunotherapies in cutaneous melanoma metastases. EGF release was significantly upregulated in two out of six patient samples after plasma treatment. The activation of the EGF receptor is a therapeutic target in many cancer entities. The significant increase of CCL5, seen in three out of six patient-derived melanoma samples after plasma exposure, has been well described to support the infiltration of tumor-toxic NK cells [18]. NK cells lyse their target cells using, e.g., granulysin. Interestingly, in two of the three samples with increased CCL5 levels after plasma treatment, we also observed a 10- and 20-fold increase in granulysin. Perforin, another lytic molecule in NK cells, was, however, not increased. This could be explained by the fact that skin-resident NK cells preferentially lyse target cells via granulysin [19]. Altogether, the plasma treatment decreased immunosuppressive and increased immune-supportive chemokine/cytokine secretions in 33–50% of the patient-derived melanoma samples investigated. Despite using a highly standardized plasma treatment and sample culture protocol, the heterogeneity of our responses observed may also relate to differences in the biopsies’ location and microenvironment as well as the patients’ health status. In our hands, the small sample size also does not allow for correlations between changes in chemokine and cytokine secretion and the level of apoptosis. Nevertheless, the baseline chemokine/cytokine expression profile of the 24 targets presented may serve as a valued baseline resource for future studies investigating human cutaneous melanoma samples ex vivo.
5. Conclusions
Exposure of human melanoma skin metastases to cold physical plasma led to the induction of apoptosis and to potentially beneficial modulation of the chemokine/cytokine expression signature towards an increase of immunogenic cell death. Plasma therapy may serve as an option to enhance antitumor effects in combination with checkpoint inhibition.
Author Contributions
Conceptualization, S.B., I.H. and I.S.; Methodology, S.B., I.H. and I.S.; Software, S.B.; Formal Analysis, S.B. and J.M.; Resources, S.B., I.H., K.-D.W. and I.S.; Writing – Original Draft Preparation, S.B.; Writing – Review & Editing, S.B., J.M., I.H., L.B., K.-D.W., S.E., H.-R.M., I.S. and T.v.W.; Visualization, S.B.; Supervision, S.B.; Project Administration, S.B., J.M., I.H. and I.S.; Funding Acquisition, S.B., I.H., K.-D.W., H.-R.M., I.S. and T.v.W. All authors have read and agreed to the published version of the manuscript.
Funding
S.B. and J.M. were supported by grants funded by the German Federal Ministry of Education and Research (BMBF), grant number 03Z22DN11. This study was supported by the European Social Fund, grant numbers ESF/14-BM-A55-0001/18 (L.B., S.E.), ESF/14-BM-A55-0005/18 (H.-R.M.), and ESF/14-BM-A55-0006/18 (S.B. and T.v.W.).
Acknowledgments
The authors acknowledge technical support from Felix Niessner.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Little, E.G.; Eide, M.J. Update on the current state of melanoma incidence. Dermatol. Clin. 2012, 30, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, A.; Cabanas, L.; Espinosa, E.; Fernandez-de-Misa, R.; Martin-Algarra, S.; Martinez-Cedres, J.C.; Rios-Buceta, L.; Rodriguez-Peralto, J.L. Melanoma: Diagnosis, staging, and treatment. Consensus group recommendations. Adv. Ther. 2014, 31, 945–960. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B.; et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017, 545, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.; Byles, V.; Horng, T. ROS sets the stage for macrophage differentiation. Cell Res. 2013, 23, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Rodder, K.; Fregin, B.; Otto, O.; Lippert, M.; Weltmann, K.D.; Wende, K.; Schmidt, A.; Gandhirajan, R.K. Toxicity and Immunogenicity in Murine Melanoma following Exposure to Physical Plasma-Derived Oxidants. Oxid. Med. Cell. Longev. 2017, 2017, 4396467. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.G.; Xiang, B.; Merlino, D.J.; Baybutt, T.R.; Sahu, J.; Fridman, A.; Snook, A.E.; Miller, V. Non-thermal plasma induces immunogenic cell death in vivo in murine CT26 colorectal tumors. Oncoimmunology 2018, 7, e1484978. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Shirakawa, Y.; Sakamoto, T.; Ishizaki, H.; Nishijima, Y.; Ono, R. Plasma-Induced Suppression of Recurrent and Reinoculated Melanoma Tumors in Mice. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 353–359. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kINPen—A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A.K. Cold atmospheric plasma causes a calcium influx in melanoma cells triggering CAP-induced senescence. Sci. Rep. 2018, 8, 10048. [Google Scholar] [CrossRef] [PubMed]
- Sensenig, R.; Kalghatgi, S.; Cerchar, E.; Fridman, G.; Shereshevsky, A.; Torabi, B.; Arjunan, K.P.; Podolsky, E.; Fridman, A.; Friedman, G.; et al. Non-thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species. Ann. Biomed. Eng. 2011, 39, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, M.; Kumar, S.; Varinli, H.; Han, Z.J.; Rider, A.E.; Evans, M.D.; Murphy, A.B.; Ostrikov, K. Atmospheric gas plasma-induced ROS production activates TNF-ASK1 pathway for the induction of melanoma cancer cell apoptosis. Mol. Biol. Cell. 2014, 25, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Mgrditchian, T.; Arakelian, T.; Paggetti, J.; Noman, M.Z.; Viry, E.; Moussay, E.; Van Moer, K.; Kreis, S.; Guerin, C.; Buart, S.; et al. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc. Natl. Acad. Sci. USA 2017, 114, E9271–E9279. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.; Jackson, C.; Cooper, A. Review of Toxic Epidermal Necrolysis. Int. J. Mol. Sci. 2016, 17, 2135. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).