Evaluation of the In Vitro Wound-Healing Activity and Phytochemical Characterization of Propolis and Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey and Propolis Samples
2.2. Propolis Extracts
2.3. Mixtures of Honey with Propolis
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Phytochemical Characterization
2.5.1. Total Phenolic Compounds Determination
2.5.2. Flavonoid Determination
2.6. Antioxidant Activity Evaluation
DPPH Free Radical Scavenging Assay
β-Carotene Bleaching Test
2.7. Assessment of In Vitro Anti-Inflammatory Activity
2.8. Evaluation of the In Vitro Wound-Healing Activity
2.8.1. Cell Culture
2.8.2. Wound Scratch Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. FTIR Analysis of the Samples of Honey, Propolis, and Propolis Extracts
3.2. Phytochemical Characterization
3.3. Antioxidant and Anti-Inflammatory Activities
3.4. Wound-Healing Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Osés, S.M.; Pascual-Maté, A.; Fernández-Muiño, M.A.; López-Díaz, T.M.; Sancho, M.T. Bioactive properties of honey with propolis. Food Chem. 2016, 196, 1215–1223. [Google Scholar] [CrossRef]
- Liu, J.R.; Ye, Y.L.; Lin, T.Y.; Wang, Y.W.; Peng, C.C. Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honeys in Taiwan. Food Chem. 2013, 139, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Sforcin, J.M. Propolis and the immune system: A review. J. Ethnopharmacol. 2007, 113, 1–14. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghram, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A complex natural product with a plethora of biological activities that can be explored for drug development. Evid.-Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Propolis: A new frontier for wound healing? Burn. Trauma 2015, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, N.; Konteles, S.J.; Troullidou, E.; Mourtzinos, I.; Karathanos, V.T. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009, 116, 452–461. [Google Scholar] [CrossRef]
- Felician, F.F.; Yu, R.; Li, M.; Li, C.; Chen, H.; Jiang, Y.; Tang, T.; Qi, W.; Xu, H. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Sousa, S.; Duarte, A.P.; Pereira, L.; Domingues, F. Phytochemical characterization, and evaluation of rheological and antioxidant properties of commercially available juices of berries. J. Berry Res. 2018, 8, 11–23. [Google Scholar] [CrossRef]
- Luís, Â.; Neiva, D.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Stumps of Eucalyptus globulus as a source of antioxidant and antimicrobial polyphenols. Molecules 2014, 19, 16428–16446. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.P.; Pereira, L.; Domingues, F. Interactions between the major bioactive polyphenols of berries: Effects on antioxidant properties. Eur. Food Res. Technol. 2018, 244, 175–185. [Google Scholar] [CrossRef]
- Luís, Â.; Sousa, S.; Wackerlig, J.; Dobusch, D.; Duarte, A.P.; Pereira, L.; Domingues, F. Star anise (Illicium verum Hook. f.) essential oil: Antioxidant properties and antibacterial activity against Acinetobacter baumannii. Flavour Fragr. J. 2019, 34, 260–270. [Google Scholar] [CrossRef]
- Luís, Â.; Breitenfeld, L.; Ferreira, S.; Duarte, A.P.; Domingues, F. Antimicrobial, antibiofilm and cytotoxic activities of Hakea sericea Schrader extracts. Pharmacogn. Mag. 2014, 10, S6–S13. [Google Scholar]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; Oliveira, F.C.S.; Passos, T.M.; Quilty, B.; Thiré, R.M.S.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Rev. Mater. 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIR-ATR spectroscopy to the quantification of sugar in honey. Food Chem. 2015, 169, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Orsák, M.; Hejtmánková, A.; Kovářová, E. Evaluation of antioxidant activity and total phenolics of selected Czech honeys. LWT Food Sci. Technol. 2010, 43, 52–58. [Google Scholar] [CrossRef]
- Kassim, M.; Achoui, M.; Mustafa, M.R.; Mohd, M.A.; Yusoff, K.M. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr. Res. 2010, 30, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Domingues, F.; Gil, C.; Duarte, A.P. Antioxidant activity of extracts of Portuguese shrubs: Pterospartum tridentatum, Cytisus scoparius and Erica spp. J. Med. Plants Res. 2009, 3, 886–893. [Google Scholar]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef]
- Watanabe, M.A.E.; Amarante, M.K.; Conti, B.J.; Sforcin, J.M. Cytotoxic constituents of propolis inducing anticancer effects: A review. J. Pahrmacy Pharmacol. 2011, 63, 1378–1386. [Google Scholar] [CrossRef]
- Martinotti, S.; Pellavio, G.; Laforenza, U.; Ranzato, E. Propolis induces AQP3 expression: A possible way of action in wound healing. Molecules 2019, 24, 1544. [Google Scholar] [CrossRef]
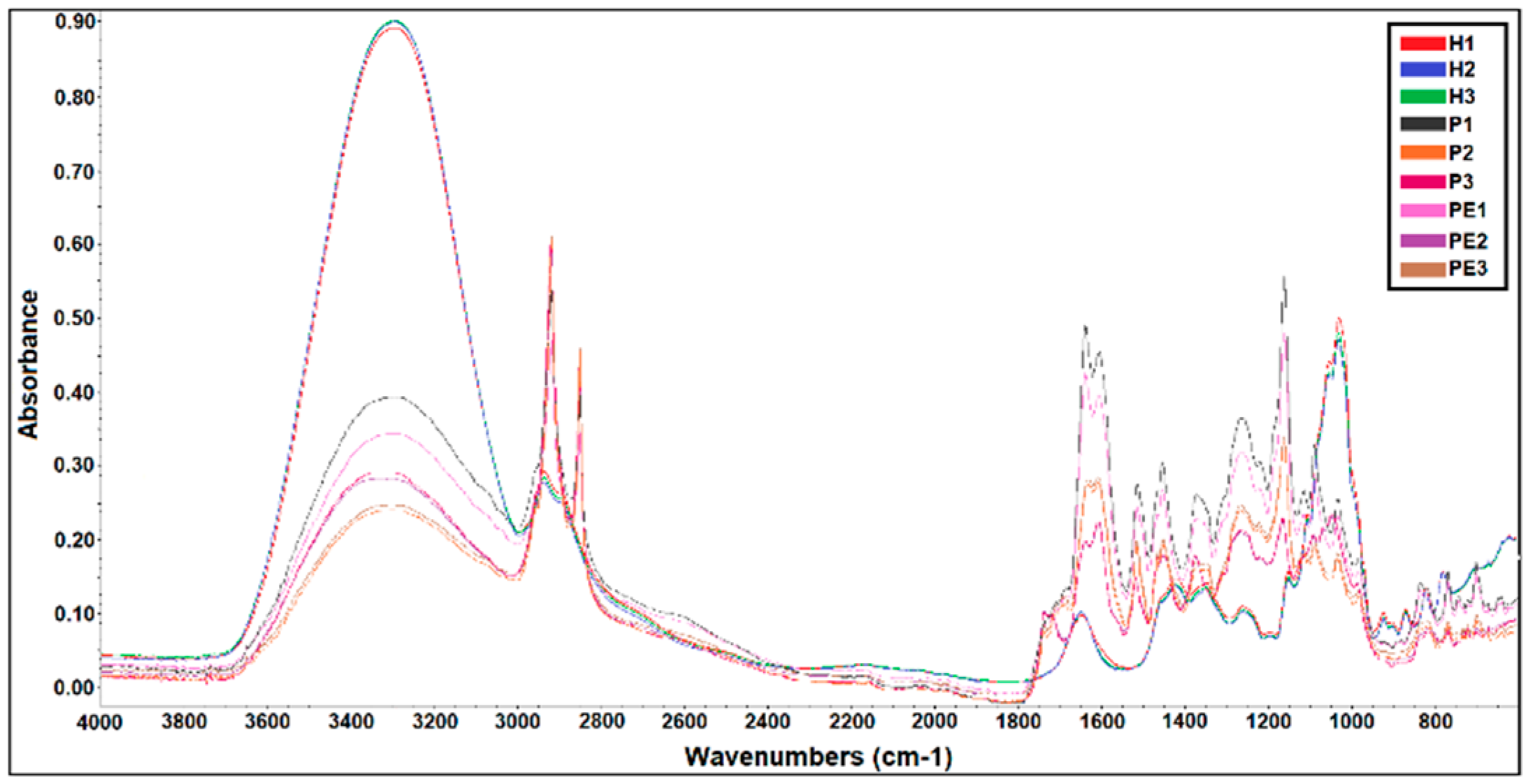
| Samples | Total Phenolic Compounds | Flavonoids |
|---|---|---|
| (g GAE/100 g Sample) 1 | (g QE/100 g Sample) 1 | |
| H1 | 0.107 ± 0.016 | 0.007 ± 0.001 |
| H2 | 0.046 ± 0.005 | Not detected |
| H3 | 0.029 ± 0.003 | Not detected |
| PE1 | 28.947 ± 1.329 | 5.494 ±0.335 |
| PE2 | 21.747 ± 1.062 | 1.786 ± 0.029 |
| PE3 | 28.667 ± 0.774 | 4.280 ± 0.123 |
| H1PE1 0.3% | 2.394 ± 0.227 | 0.290 ± 0.007 |
| H1PE1 0.5% | 3.324 ± 0.044 | 0.452 ± 0.012 |
| H1PE2 0.3% | 1.219 ± 0.049 | 0.115 ± 0.005 |
| H1PE2 0.5% | 1.750 ± 0.076 | 0.199 ± 0.018 |
| H1PE3 0.3% | 1.969 ± 0.071 | 0.054 ± 0.011 |
| H1PE3 0.5% | 3.506 ± 0.257 | 0.308 ± 0.006 |
| Samples | DPPH | β-Carotene Bleaching Test | Anti-Inflammatory Activity |
|---|---|---|---|
| % Inhibition/100 g Sample 1 | % Inhibition/100 g Sample 1 | % Inhibition/100 g Sample 1 | |
| H1 | 0.431 ± 0.023 | 0.809 ± 0.042 | 20.625 ± 0.884 |
| H2 | 0.133 ± 0.019 | 0.684 ± 0.032 | 23.438 ± 3.094 |
| H3 | Not detected | 0.349 ± 0.028 | Not detected |
| PE1 | 92.506 ± 1.249 | 51.441 ± 4.477 | 15.000 ± 3.536 |
| PE2 | 92.012 ± 0.258 | 48.660 ± 1.876 | 31.250 ± 1.768 |
| PE3 | 93.245 ± 0.687 | 53.909 ± 2.328 | 48.750 ± 1.768 |
| H1PE1 0.3% | 8.271 ± 0.044 | 3.027 ± 0.070 | 36.408 ± 6.865 |
| H1PE1 0.5% | 8.119 ± 0.040 | 3.929 ± 0.105 | 28.571 ± 0.001 |
| H1PE2 0.3% | 7.921 ± 0.097 | 2.878 ± 0.096 | 40.049 ± 1.716 |
| H1PE2 0.5% | 8.154 ± 0.158 | 3.108 ± 0.049 | 45.238 ± 0.001 |
| H1PE3 0.3% | 8.165 ± 0.026 | 2.919 ± 0.106 | 36.408 ± 3.433 |
| H1PE3 0.5% | 8.396 ± 0.321 | 3.596 ± 0.089 | 40.476 ± 6.734 |
Representative Image of the Cells at the Initial Moment (0 h) | |||
| Samples | 2 h | 24 h | 36 h |
| Control |  |  |  |
| H1 |  |  |  |
| H2 |  |  |  |
| H3 | 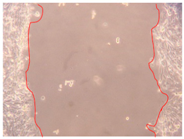 | 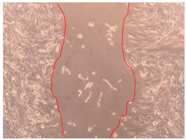 | 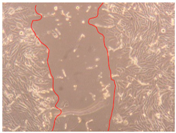 |
| Samples | 2 h | 24 h | 36 h |
|---|---|---|---|
| PE1 0.3% |  |  | 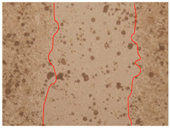 |
| PE1 0.5% | 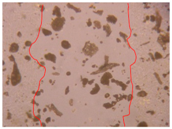 |  |  |
| PE2 0.3% | 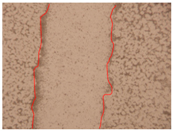 |  |  |
| PE2 0.5% |  |  |  |
| PE3 0.3% |  |  |  |
| PE3 0.5% | 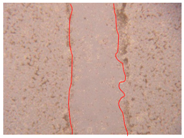 |  |  |
| Samples | 2 h | 24 h | 36 h |
|---|---|---|---|
| H1PE1 0.3% |  |  |  |
| H1PE1 0.5% |  |  |  |
| H1PE2 0.3% |  |  |  |
| H1PE2 0.5% |  |  |  |
| H1PE3 0.3% |  |  |  |
| H1PE3 0.5% | 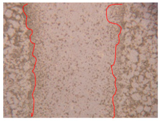 |  |  |
| Samples | 0 h 1 | 2 h 1 | p-Value | 24 h 1 | p-Value | 36 h 1 | p-Value |
|---|---|---|---|---|---|---|---|
| Control | 1.00 ± 0.05 | 0.99 ± 0.05 | 0.818 | 0.99 ± 0.05 | 1.000 | 0.98 ± 0.05 | 0.817 |
| H1 | 0.71 ± 0.04 | 0.002 * | 0.59 ± 0.03 | 0.001 * | 0.48 ± 0.02 | 0.001 * | |
| H2 | 0.83 ± 0.04 | 0.011 * | 0.37 ± 0.02 | 0.001 * | 0.33 ± 0.02 | 0.001 * | |
| H3 | 0.90 ± 0.05 | 0.062 | 0.57 ± 0.03 | 0.001 * | 0.49 ± 0.02 | 0.001 * | |
| PE1 0.3% | 0.80 ± 0.04 | 0.006 * | 0.78 ± 0.04 | 0.005 * | 0.69 ± 0.03 | 0.002 * | |
| PE1 0.5% | 0.84 ± 0.04 | 0.014 * | 0.81 ± 0.04 | 0.009 * | 0.78 ± 0.04 | 0.005 * | |
| PE2 0.3% | 0.54 ± 0.03 | 0.001 * | 0.39 ± 0.02 | 0.001 * | 0.34 ± 0.02 | 0.001 * | |
| PE2 0.5% | 0.66 ± 0.03 | 0.001 * | 0.52 ± 0.03 | 0.001 * | 0.27 ± 0.01 | 0.001 * | |
| PE3 0.3% | 0.74 ± 0.04 | 0.003 * | 0.62 ± 0.03 | 0.001 * | 0.53 ± 0.03 | 0.001 * | |
| PE3 0.5% | 0.45 ± 0.02 | 0.001 * | 0.41 ± 0.02 | 0.001 * | 0.39 ± 0.02 | 0.001 * | |
| H1PE1 0.3% | 0.74 ± 0.04 | 0.003 * | 0.63 ± 0.03 | 0.001 * | 0.55 ± 0.03 | 0.001 * | |
| H1PE1 0.5% | 0.43 ± 0.02 | 0.001 * | 0.43 ± 0.02 | 0.001 * | 0.42 ± 0.02 | 0.001 * | |
| H1PE2 0.3% | 0.54 ± 0.03 | 0.001 * | 0.37 ± 0.02 | 0.001 * | 0.49 ± 0.02 | 0.001 * | |
| H1PE2 0.5% | 0.64 ± 0.03 | 0.001 * | 0.58 ± 0.03 | 0.001 * | 0.46 ± 0.02 | 0.001 * | |
| H1PE3 0.3% | 0.80 ± 0.04 | 0.006 * | 0.44 ± 0.02 | 0.001 * | 0.31 ± 0.02 | 0.001 * | |
| H1PE3 0.5% | 0.74 ± 0.04 | 0.001 * | 0.45 ± 0.02 | 0.001 * | 0.34 ± 0.02 | 0.001 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Afonso, A.; Gonçalves, J.; Luís, Â.; Gallardo, E.; Duarte, A.P. Evaluation of the In Vitro Wound-Healing Activity and Phytochemical Characterization of Propolis and Honey. Appl. Sci. 2020, 10, 1845. https://doi.org/10.3390/app10051845
M. Afonso A, Gonçalves J, Luís Â, Gallardo E, Duarte AP. Evaluation of the In Vitro Wound-Healing Activity and Phytochemical Characterization of Propolis and Honey. Applied Sciences. 2020; 10(5):1845. https://doi.org/10.3390/app10051845
Chicago/Turabian StyleM. Afonso, Alexandra, Joana Gonçalves, Ângelo Luís, Eugenia Gallardo, and Ana Paula Duarte. 2020. "Evaluation of the In Vitro Wound-Healing Activity and Phytochemical Characterization of Propolis and Honey" Applied Sciences 10, no. 5: 1845. https://doi.org/10.3390/app10051845
APA StyleM. Afonso, A., Gonçalves, J., Luís, Â., Gallardo, E., & Duarte, A. P. (2020). Evaluation of the In Vitro Wound-Healing Activity and Phytochemical Characterization of Propolis and Honey. Applied Sciences, 10(5), 1845. https://doi.org/10.3390/app10051845








