Abstract
During cold storage, packed red blood cells (PRBCs) undergo slow detrimental changes that are collectively termed storage lesion. The aging of the cells causes alterations in the composition of the storage-medium in the PRBC unit. In this paper, we present the comparison of the dielectric response of water in the primary (fresh) storage medium (citrate phosphate dextrose adenine solution, CPDA-1) versus the storage medium from three expired units of PRBCs. Dielectric response of the water molecules has been characterized by dielectric spectroscopy technique in the microwave frequency band (0.5–40 GHz). The dominant phenomenon is the significant increase of the dielectric strength and decrease the relaxation time τ for the samples of the stored medium in comparison with the fresh medium CPDA-1. Furthermore, we demonstrated that removing the ghosts from PRBC hemolysate did not cause the alteration of the dielectric spectrum of water. Thus, the contribution associated with water located near the cell membrane can be neglected in microwave dielectric measurements.
1. Introduction
Red blood cell (RBC) transfusion is a life-saving procedure whose primary objective is to sustain tissue and organ oxygenation in patients with massive bleeding or acute anemia. Packed red blood cell (PRBC) donations for transfusion are routinely stored for up to 35 to 42 days, depending on the preservation solution [1].
During cold storage, PRBCs undergo slow detrimental changes that are collectively termed storage lesion [1,2,3]. Aging-related processes (that are at least partially due to the sensitivity of cells to oxidative stress [4]) lead to significant metabolic and structural changes in red blood cells [5,6,7,8], and involve global biochemical and biophysical alteration, remodeling of the cell membrane, and cytoplasm composition [3,9]. The most studied changes include: adenosine triphosphate (ATP) and 2,3-bisphosphoglycerate (2.3-DPG) depletion [9], loss of cellular antioxidant capability [10,11], changes in K+ and Na+ concentration [12,13], loss of membrane and skeleton proteins [14,15], loss of membrane lipids, and changes in their in/out distribution, vesicle generation [16,17], oxidation and remodeling of skeleton proteins [18], clustering of band 3 proteins [19,20], alteration of nitric oxide signaling [21,22], decrease in antioxidant activity [23,24,25], etc.
A number of these changes are interrelated and initiate a cascade of biochemical and structural changes, which in turn lead to impairment in RBC functionality, specifically—alteration in the biophysical/mechanical properties of cells [5,7].
Each of the reported storage-induced alterations in PRBC properties occurs on its specific time-scale [26,27,28,29,30,31,32,33,34]. Some of these changes take place at an early stage of storage (during the first seven days), while others occur later.
Changes in the composition and property of the cell membrane begin at the very early stages of PRBCs storage [35,36,37,38]. Freitas Leal et al. summarized this cascade of changes as follows [36]: oxidative damage-induced, high-affinity binding of hemoglobin to the cytoplasmic domain of band 3, activation of Ca2+-permeable channels, phosphorylation-controlled alterations in morphology and metabolism affecting ATP production and redox status, degradation of band 3 and/or aggregation of band-3 fragments, binding of immunoglobulin-G, and micro vesicles generation. Obviously, such changes in the composition of the cell membrane, its shape, and properties must inevitably lead to a change in its hydrophobicity [39].
Furthermore, bio-active substances accumulate in storage medium [40]. The accumulation of immunomodulatory factors in PRBC concentrates [41] has been implicated as a potential cause of transfusion reactions associated with the use of PRBCs [42,43]. These include lipids (that prime recipient neutrophils and have been implicated in transfusion-related acute lung injury [44]), cytokines [26,41], arachidonic acid [45], and malondialdehyde (MDA, a marker of lipid peroxidation) [2]. In addition, PRBC lesion causes the alteration of sodium/potassium balance [46], accumulation of lactate [41,46], lactate dehydrogenase [2], iron [26,46,47], and free hemoglobin [2,41,46] in the supernatant in parallel with decreasing of glucose concentration [46] and reducing of medium pH [26,40].
Our recent Microwave Dielectric Spectroscopy (MDS) studies have shown that it is possible to evaluate the dielectric response of cytoplasmic water by measuring the suspension of stored red blood cells (RBC) [48]. From the analysis of the dielectric relaxation as a function of storage time (ex vivo aging), it was assumed that the behavior was rooted in the delicate interplay between bound and bulk water in the cellular interior. Based on these results, we hypothesized that the state of intracellular water might be used as a marker of the functionality of stored cells. Furthermore, the RBC aging is associated with a specific pattern of changes in intracellular water. It was shown the broadening parameter α of the main dielectric peak of cytosolic water shows a linear correlation with the storage time and allows to predict the RBC deformability [48]. This observation was recently supported by Petrovic et al. [49], who monitored changes of intracellular water dynamics (during packed RBC (PRBC) cold storage) by H1 NMR.
To calculate the dielectric spectrum of the cell interior, we used a Kraszewski mixed formula [48], where the spectrum of buffer was subtracted from the spectrum of RBC suspension. We assumed [48] that (1) storage medium does not change within the period of storage, and (2) the contribution associated with the water molecules located near the surface of the cell membrane can be neglected. However, the change in the composition (alteration of ions and dipoles concentration) of the medium described above should lead to a transformation in the water molecules state and will lead to variation in the number of bounded water molecules; these changes should come out in the alteration of dielectric water response. The present study was undertaken to examine this hypothesis.
Note that in our previous work [48], we did not take into account water in the vicinity of the membrane, which may affect the dielectric signal of the cell interior. Therefore, in this study, we also tested the role of water molecules located near the surface of the membrane on the dielectric response of water molecules in the cytoplasm of RBCs. All-in-all, in the presented study, we assessed the microwave response of water molecules, which are in the storage buffer and those located near to the cell membrane. Thus, for the first time, we evaluated the changes in the composition of the storage-medium caused by PRBCs aging using MDS. The results open up the avenues of non-invasive monitoring of RBC lesion during their storage in a blood bank.
2. Materials and Methods
2.1. Study Design
In the present study, we compared the dielectric response of the water in the primary (fresh) storage medium versus the storage medium from PRBC units and compared microwave dielectric response of water in hemolysate that contains cell membranes with dielectric behavior of water in membranes-free hemolysate. The dielectric response of the water molecules has been characterized by the MDS in the frequency range (0.5–40 GHz) at 25 °C.
2.2. Samples Preparation
The institutional review board approved the study for human experiments (Helsinki Committee Regulations Permit 98290, Hadassah Hospital, Jerusalem, Israel). To examine the alteration of citrate phosphate dextrose adenine solution (CPDA-1, Macopharma, Tourcoing, France) PRBC samples (7.0 mL) were taken from five outdated un-filtrated units (in CPDA-1) stored 35–37 days under the standard condition in the blood bank. Cells were mixed with an equal volume of fresh CPDA with the following separation by centrifugation under 500 g for 7 min. After centrifugation, the supernatant was separated and re-centrifuged to remove the cells completely. For examination, the dielectric response of water molecules in the vicinity of the membrane surface 20 mL of hemolysate was prepared by sequential freezing (at −78 °C) and thawing of the cells. After thawing 10 mL of hemolysate was centrifuged (14,000× g, 7 min.) to remove ghosts.
2.3. Microwave Dielectric Spectroscopy
Dielectric measurements were carried out in the frequency range from 500 MHz to 40 GHz using a microwave vector network analyzer N5234B PNA-L (Keysight, Santa Rosa, CA, USA), together with a flexible cable and slim-form probe (Keysight N1501A Dielectric Probe Kit). The calibration of the system was performed with the aid of three references: air, a Keysight standard short circuit, and pure water at 25 °C. Calibration was maintained by Ecal mode. A special stand for the slim-form probe was designed and combined with a sample cell holder for liquids (total volume 7.8 mL). The holder was enveloped by a thermal jacket and attached to a Julabo CF 41 (Julabo, Seelbach, Germany) oil-based heat circulatory system. The cell was held at 25 °C by the circulator-thermostat, with temperature fluctuations less than 0.1 °C. The whole measuring system was placed in an air-conditioned room maintained at 25 ± 1 °C. Each sample was measured at least six times. The real and imaginary parts ε′(ω) and ε″(ω) were evaluated using the Keysight N1500A Materials Measurement Software with an accuracy of Δε′/ε′ = 0.05, Δε″/ε″ = 0.05.
2.4. Statistical Analysis
The results are presented as mean ± SEM and tested for statistical significance using the nonparametric Mann–Whitney test. Statistical differences, examined with the SPSS 21 software package, were considered significant at p < 0.05.
3. Results and Discussion
The measurements of storage solutions were carried out at 25 °C. The dielectric spectrum of the storage-medium in comparison with fresh CPDA in the frequency band 0.5–40 GHz is presented in Figure 1a.
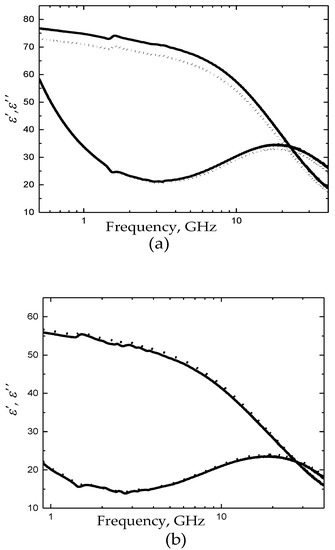
Figure 1.
(a) Dielectric spectra of fresh citrate phosphate dextrose adenine solution (CPDA-1) (dot line) and the storage-medium for sample C (black line). (b) Dielectric spectra of membrane-containing (H) (dot line) and membrane-free (MF-H) (black line) hemolysates for sample C.
Figure 1a shows the typical dielectric spectra of the storage medium (dielectric spectrum of sample C is plotted) versus fresh CPDA. The relaxation time τ, the broadening parameter α, and the relaxation amplitude Δε of water for fresh/pure and stored medium are demonstrated in Table 1 and Figure 2.

Table 1.
The mean values of relaxation times τ, the broadening parameter α, and the relaxation amplitude Δε of water for fresh CPDA-1 and stored-medium. Each datum is the mean ± SEM from six repeated measurements.
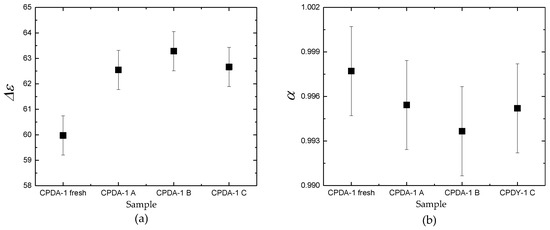
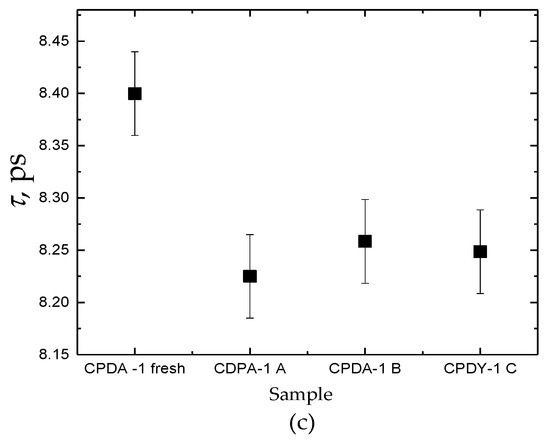
Figure 2.
Comparison of dielectric parameters Δε (a), α (b), τ (c) for fresh CPDA-1 with storage medium for three samples A, B, and C. Each datum is mean ± SEM from six repeated measurements.
In Figure 2, dielectric features of water molecules for all tested fluids are presented. It is shown that dielectric parameters of fresh/pure CPDA-1 and storage-medium that separated from PRBCs units are different. In Table 1, we summarized the obtained averaged dielectric parameters. For all samples of the stored-medium, the dominant feature is the significant increase of the dielectric strength Δε and decrease the relaxation time τ in comparison with the fresh medium CPDA-1.
Changes in dielectric response of water, observed in this study, can be assigned to alteration of ions and dipoles concentration that occur during the storage in the medium in the PRBC unit. We can assume that alteration of Na+/K+ balance, decreasing of pH, and formation of lactate ion in the medium (during the storage) can cause disarrays of the H-bond network of water. In this case, the water’s main relaxation peak shifts to lower relaxation times [50]. In parallel, the accumulation of cytokines, arachidonic acid, and free hemoglobin can induce ordering the H-bond network of water. It shifts the water main relaxation peak to the lower frequencies or higher relaxation times [50]. In this regard, special attention must be paid to the possible effect of the accumulation of MDA in the storage-medium. This is since the molecule of MDA molecule is small, polar, and highly water-soluble [50], and its accumulation in solution [2] should decrease water relaxation time as it was obtained in the experiment.
In this table, we show the average values of the primary dielectric parameters (that obtained for three samples of PRBC) for both types of hemolysates. Each value is presented as a median from six repeated measurements with standard error values. We have not observed a significant difference (pair test) between dielectric features that characterized water behavior in membrane-containing and membrane-free hemolysates.
As can be seen from Table 2, the presence of membranes does not affect the dielectric response of water in hemolysates. Thus, we can conclude that changes in the intracellular ionic composition can be attributed to the highest factors determining the dielectric response of water molecules in the cytoplasm of RBC.

Table 2.
Comparison of the relaxation time τ, the broadening parameter α, and the relaxation amplitude Δε of water for membrane-containing (H) and membrane-free (MF-H) hemolysates. Each datum is the mean ± SEM from six repeated measurements.
The phenomenon we discovered can be explained in the following way. Water present in biological tissues has been defined as bound or free water depending upon its proximity to neighboring macromolecules, membranes, or other interfaces. In a classical paper [51] entitled, “What retains water in living cells?” Ling & Walton concluded that cell proteins located on the surface of a biological membrane polarize most of the cell water in the form of multilayer layers. Water forming the hydration shell of the macromolecule is considered as bounded. The remaining water in the system is defined as bulk. As we can expect that the number of water layers in which the mobility of the molecules is limited (differs from) does not exceed 4–5. This should mean that the volume of such water, in comparison with the intracellular water volume, is insignificant. Therefore, the presence of a membrane in the hemolysate should not lead to a change in its dielectric spectrum.
Limitation of the study: It is well known that the PRBCs lesion is affected by the protocol of PRBCs preparation and storage [1,5,26]. The results presented here, were obtained for units of PRBCs that are non-leukoreduced and cells are stored in CPDA-1. In the future, we plan to examine PRBCs units that are produced with leukoreduction and gamma-irradiation, in which cells are storage in SAGM (Saline, Adenine, Glucose, Mannitol).
Thus, from the results demonstrated in this study, and based on our previously published data [48], it follows that:
- The main factors determining the microwave dielectric response of the PRBC suspension is the condition of water molecules in the cytosol and storage medium;
- The contribution associated with the water molecules located near the surface of the RBC membrane can be neglected in microwave dielectric measurements.
Thus, having summed up our results, we conclude that using MDS, it is possible to control the functionality of PRBCs by the response of water molecules located both inside and outside the cell.
4. Conclusions
We showed that MDS is sensitive to the alterations in the composition of the storage medium of the suspension of PRBC. Since CPDA-1 undergoes significant changes during the storage period, senescent buffer (buffer of the same age of PRBC suspension) has to be subtracted from the spectrum of PRBC suspension in Kraszewski’s formula [48]. Thereby it seems interesting to repeat the experiment carried out by Levy et al. [48] using the current knowledge of the storage medium. The results allow us to conclude that the contribution associated with the water molecules located near the surface of the cell membrane can be neglected in microwave dielectric measurements.
Author Contributions
Conceptualization, G.B., Y.F. and L.L.; methodology, G.B.; software, L.L.; validation, L.L., Y.F. and G.B.; formal analysis, G.B. and L.L.; investigation, L.L.; resources, D.A.; data curation, L.L.; writing—original draft preparation, L.L.; writing—review and editing, G.B., Y.F.; visualization, L.L.; supervision, Y.F.; project administration, Y.F.; funding acquisition, Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Swiss National Science Foundation CRSII5_180234 for the Research Project Premembrane and cytosolic water as a marker of red blood cell aging in vivo and in vitro and ISF 341/18. The role of water in the aging of Red Blood Cells.
Acknowledgments
We would like to thank Keysight Technologies Israel Ltd. and INTERLLIGENT RF & Microwave Solutions for the loan of Vector Network Analyzer Agilent N5234B PNA-L. We would like to thank Anna Bogdanova for her support and scientific assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hess, J.R. An update on solutions for red cell storage. Vox Sang. 2006, 91, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Katharia, R. Oxidative injury as contributory factor for red cells storage lesion during twenty eight days of storage. Blood Transfus. 2012, 10, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Obrador, R.; Musulin, S.; Hansen, B. Red blood cell storage lesion. J. Vet. Emerg. Crit. Care (San Antonio) 2015, 25, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaeinia, M.; Cluitmans, J.C.; Akel, A.; Dreischer, P.; Toulany, M.; Koberle, M.; Skabytska, Y.; Saki, M.; Biedermann, T.; Duszenko, M.; et al. The impact of erythrocyte age on eryptosis. Br. J. Haematol. 2012, 157, 606–614. [Google Scholar] [CrossRef]
- Barshtein, G.; Arbell, D.; Livshits, L.; Gural, A. Is it possible to reverse the storage-induced lesion of red blood cells? Front. Physiol. 2018, 9, 914. [Google Scholar] [CrossRef]
- Ciana, A.; Achilli, C.; Gaur, A.; Minetti, G. Membrane remodelling and vesicle formation during ageing of human red blood cells. Cell. Physiol Biochem 2017, 42, 1127–1138. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Zimring, J.C.; Busch, M. Chronological storage age and metabolic age of stored red blood cells: Are they the same? Transfusion 2019. [Google Scholar] [CrossRef]
- Koch, C.G.; Duncan, A.I.; Figueroa, P.; Dai, L.; Sessler, D.I.; Frank, S.M.; Ness, P.M.; Mihaljevic, T.; Blackstone, E.H. Real age: Red blood cell aging during storage. Ann. Thorac. Surg. 2019, 107, 973–980. [Google Scholar] [CrossRef]
- Hess, J.R. Red cell storage. J. Proteomics. 2010, 73, 368–373. [Google Scholar] [CrossRef]
- Racek, J.; Herynkova, R.; Holecek, V.; Jerabek, Z.; Slama, V. Influence of antioxidants on the quality of stored blood. Vox Sang. 1997, 72, 16–19. [Google Scholar] [CrossRef]
- Dumaswala, U.J.; Wilson, M.J.; Wu, Y.L.; Wykle, J.; Zhuo, L.; Douglass, L.M.; Daleke, D.L. Glutathione loading prevents free radical injury in red blood cells after storage. Free Radic. Res. 2000, 33, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Suzuki, Y.; Tateishi, N.; Shiba, M.; Muraoka, M.; Tadokoro, K.; Maeda, N. Gamma-ray-irradiated red blood cells stored in mannitol-adenine-phosphate medium: Rheological evaluation and susceptibility to oxidative stress. Vox Sang. 2000, 79, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, O.; de Franceschi, L.; de Gironcoli, M.; Girelli, D.; Corrocher, R. Potassium loss and cellular dehydration of stored erythrocytes following incubation in autologous plasma: Role of the KCl cotransport system. Vox Sang. 1993, 65, 95–102. [Google Scholar] [CrossRef]
- Ciana, A.; Achilli, C.; Minetti, G. Spectrin and Other Membrane-Skeletal Components in Human Red Blood Cells of Different Age. Cell. Physiol. Biochem. 2017, 42, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Orbach, A.; Zelig, O.; Yedgar, S.; Barshtein, G. Biophysical and Biochemical Markers of Red Blood Cells Fragility. Transfus. Med. Hemother. 2017, 44, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Bosman, G.J.; Werre, J.M.; Willekens, F.L.; Novotny, V.M. Erythrocyte ageing in vivo and in vitro: Structural aspects and implications for transfusion. Transfus. Med. 2008, 18, 335–347. [Google Scholar] [CrossRef]
- Bosman, G.J.C.G.M.; Stappers, M.; Novotny, V.M.J. Changes in band 3 structure as determinants of erythrocyte integrity during storage and survival after transfusion. Blood Transfus. Italy 2010, 8, S48–S52. [Google Scholar] [CrossRef]
- Wolfe, L.C.; Byrne, A.M.; Lux, S.E. Molecular defect in the membrane skeleton of blood bank-stored red cells. Abnormal spectrin-protein 4.1-actin complex formation. J. Clin. Investig. 1986, 78, 1681–1686. [Google Scholar] [CrossRef]
- Arashiki, N.; Kimata, N.; Manno, S.; Mohandas, N.; Takakuwa, Y. Membrane peroxidation and methemoglobin formation are both necessary for band 3 clustering: Mechanistic insights into human erythrocyte senescence. Biochemistry 2013, 52, 5760–5769. [Google Scholar] [CrossRef]
- Pantaleo, A.; Giribaldi, G.; Mannu, F.; Arese, P.; Turrini, F. Naturally occurring anti-band 3 antibodies and red blood cell removal under physiological and pathological conditions. Autoimmun. Rev. 2008, 7, 457–462. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Janes, J.; Stapley, R.; Patel, R.P.; Gladwin, M.T.; Kim-Shapiro, D.B. Mechanism of faster NO scavenging by older stored red blood cells. Redox Biol. 2014, 2, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Almac, E.; Bezemer, R.; Hilarius-Stokman, P.M.; Goedhart, P.; de Korte, D.; Verhoeven, A.J.; Ince, C. Red blood cell storage increases hypoxia-induced nitric oxide bioavailability and methemoglobin formation in vitro and in vivo. Transfusion 2014, 54, 3178–3185. [Google Scholar] [CrossRef] [PubMed]
- Dzik, W. Fresh blood for everyone? Balancing availability and quality of stored RBCs. Transfus. Med. 2008, 18, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Dumaswala, U.J.; Zhuo, L.; Jacobsen, D.W.; Jain, S.K.; Sukalski, K.A. Protein and lipid oxidation of banked human erythrocytes: Role of glutathione. Free Radic. Biol. Med. 1999, 27, 1041–1049. [Google Scholar] [CrossRef]
- Whillier, S.; Raftos, J.E.; Sparrow, R.L.; Kuchel, P.W. The effects of long-term storage of human red blood cells on the glutathione synthesis rate and steady-state concentration. Transfusion 2011, 51, 1450–1459. [Google Scholar] [CrossRef]
- Bennett-Guerrero, E.; Veldman, T.H.; Doctor, A.; Telen, M.J.; Ortel, T.L.; Reid, T.S.; Mulherin, M.A.; Zhu, H.; Buck, R.D.; Califf, R.M.; et al. Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. USA 2007, 104, 17063–17068. [Google Scholar] [CrossRef]
- Bardyn, M.; Rappaz, B.; Jaferzadeh, K.; Crettaz, D.; Tissot, J.D.; Moon, I.; Turcatti, G.; Lion, N.; Prudent, M. Red blood cells ageing markers: A multi-parametric analysis. Blood Transfus. 2017, 15, 239–248. [Google Scholar] [CrossRef]
- Kozlova, E.; Chernysh, A.; Moroz, V.; Sergunova, V.; Gudkova, O.; Kuzovlev, A. Nanodefects of membranes cause destruction of packed red blood cells during long-term storage. Exp. Cell. Res. 2015. [Google Scholar] [CrossRef]
- Santacruz-Gomez, K.; Silva-Campa, E.; Alvarez-Garcia, S.; Mata-Haro, V.; Soto-Puebla, D.; Pedroza-Montero, M. An AFM approach of RBC micro and nanoscale topographic features during storage. Int. J. Med Health Biomed. Pharmac. Eng. 2014, 8, 449–452. [Google Scholar]
- Relevy, H.; Koshkaryev, A.; Manny, N.; Yedgar, S.; Barshtein, G. Blood banking-induced alteration of red blood cell flow properties. Transfusion 2008, 48, 136–146. [Google Scholar] [CrossRef]
- D’Alessandro, A.; D’Amici, G.M.; Vaglio, S.; Zolla, L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: From metabolism to proteomics. Haematologica 2012, 97, 107–115. [Google Scholar] [CrossRef] [PubMed]
- D’Amici, G.M.; Rinalducci, S.; Zolla, L. Proteomic analysis of RBC membrane protein degradation during blood storage. J. Proteome Res. 2007, 6, 3242–3255. [Google Scholar] [CrossRef] [PubMed]
- Gevi, F.; D’Alessandro, A.; Rinalducci, S.; Zolla, L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J. Proteom. 2012, 76, 168–180. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, M.S.; Jagger, J.; Duggan, M.; White, M.; Ellis, C.; Chin-Yee, I.H. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA-1 for 29 days: Implications for animal models of transfusion. Transfus. Med. 2000, 10, 291–303. [Google Scholar] [CrossRef]
- Bosman, G.J.; Lasonder, E.; Groenen-Dopp, Y.A.; Willekens, F.L.; Werre, J.M. The proteome of erythrocyte-derived microparticles from plasma: New clues for erythrocyte aging and vesiculation. J. Proteom. 2012, 76, 203–210. [Google Scholar] [CrossRef]
- Freitas Leal, J.K.; Preijers, F.; Brock, R.; Adjobo-Hermans, M.; Bosman, G. Red Blood Cell Homeostasis and Altered Vesicle Formation in Patients with Paroxysmal Nocturnal Hemoglobinuria. Front. Physiol. 2019, 10, 578. [Google Scholar] [CrossRef]
- Willekens, F.L.; Werre, J.M.; Groenen-Dopp, Y.A.; Roerdinkholder-Stoelwinder, B.; de Pauw, B.; Bosman, G.J. Erythrocyte vesiculation: A self-protective mechanism? Br. J. Haematol. 2008, 141, 549–556. [Google Scholar] [CrossRef]
- Lutz, H.U.; Bogdanova, A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front. Physiol. 2013, 4, 387. [Google Scholar] [CrossRef]
- Huisjes, R.; Bogdanova, A.; van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; van Wijk, R. Squeezing for Life—Properties of Red Blood Cell Deformability. Front. Physiol. 2018, 9, 656. [Google Scholar] [CrossRef]
- Aubron, C.; Nichol, A.; Cooper, D.J.; Bellomo, R. Age of red blood cells and transfusion in critically ill patients. Ann. Intensive. Care 2013, 3, 2. [Google Scholar] [CrossRef]
- Hoehn, R.S.; Jernigan, P.L.; Chang, A.L.; Edwards, M.J.; Pritts, T.A. Molecular mechanisms of erythrocyte aging. Biol. Chem. 2015, 396, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Garraud, O.; Tariket, S.; Sut, C.; Haddad, A.; Aloui, C.; Chakroun, T.; Laradi, S.; Cognasse, F. Transfusion as an Inflammation Hit: Knowns and Unknowns. Front. Immunol. 2016, 7, 534. [Google Scholar] [CrossRef] [PubMed]
- Sut, C.; Tariket, S.; Chou, M.L.; Garraud, O.; Laradi, S.; Hamzeh-Cognasse, H.; Seghatchian, J.; Burnouf, T.; Cognasse, F. Duration of red blood cell storage and inflammatory marker generation. Blood Transfus. 2017, 15, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Silliman, C.C.; Moore, E.E.; Kelher, M.R.; Khan, S.Y.; Gellar, L.; Elzi, D.J. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion 2011, 51, 2549–2554. [Google Scholar] [CrossRef]
- Peters, A.L.; Vervaart, M.A.; van Bruggen, R.; de Korte, D.; Nieuwland, R.; Kulik, W.; Vlaar, A.P. Non-polar lipids accumulate during storage of transfusion products and do not contribute to the onset of transfusion-related acute lung injury. Vox Sang. 2017, 112, 25–32. [Google Scholar] [CrossRef]
- Roussel, C.; Dussiot, M.; Marin, M.; Morel, A.; Ndour, P.A.; Duez, J.; Le Van Kim, C.; Hermine, O.; Colin, Y.; Buffet, P.A.; et al. Spherocytic shift of red blood cells during storage provides a quantitative whole cell-based marker of the storage lesion. Transfusion 2017, 57, 1007–1018. [Google Scholar] [CrossRef]
- Karam, O.; Tucci, M.; Toledano, B.J.; Robitaille, N.; Cousineau, J.; Thibault, L.; Lacroix, J.; Le Deist, F. Length of storage and in vitro immunomodulation induced by prestorage leukoreduced red blood cells. Transfusion 2009, 49, 2326–2334. [Google Scholar] [CrossRef]
- Levy, E.; David, M.; Barshtein, G.; Yedgar, S.; Livshits, L.; Ben Ishai, P.; Feldman, Y. Dielectric Response of Cytoplasmic Water and Its Connection to the Vitality of Human Red Blood Cells. II. The Influence of Storage. J. Phys. Chem. B 2017, 121, 5273–5278. [Google Scholar] [CrossRef]
- Petrovic, A.; Krauskopf, A.; Hassler, E.; Stollberger, R.; Scheurer, E. Time related changes of T1, T2, and T2 of human blood in vitro. Forensic Sci. Int. 2016, 262, 11–17. [Google Scholar] [CrossRef]
- Feldman, Y.; Puzenko, A.; Ishai, P.B.; Greenbaum, A.G. The dielectric response of interfacial water —From the ordered structures to the single hydrated shell. Colloids Polym. 2014, 29, 1923–1932. [Google Scholar]
- Ling, G.N.; Walton, C.L. What retains water in living cells? Science 1976, 191, 293–295. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).