The Deep Circumflex Iliac Artery Flap for Mandibular Reconstruction and Donor Site Reconstruction with a Patient-Specific Implant: A Case Report
Abstract
1. Introduction
2. Case Presentation
2.1. Computer-Assisted Surgical Planning
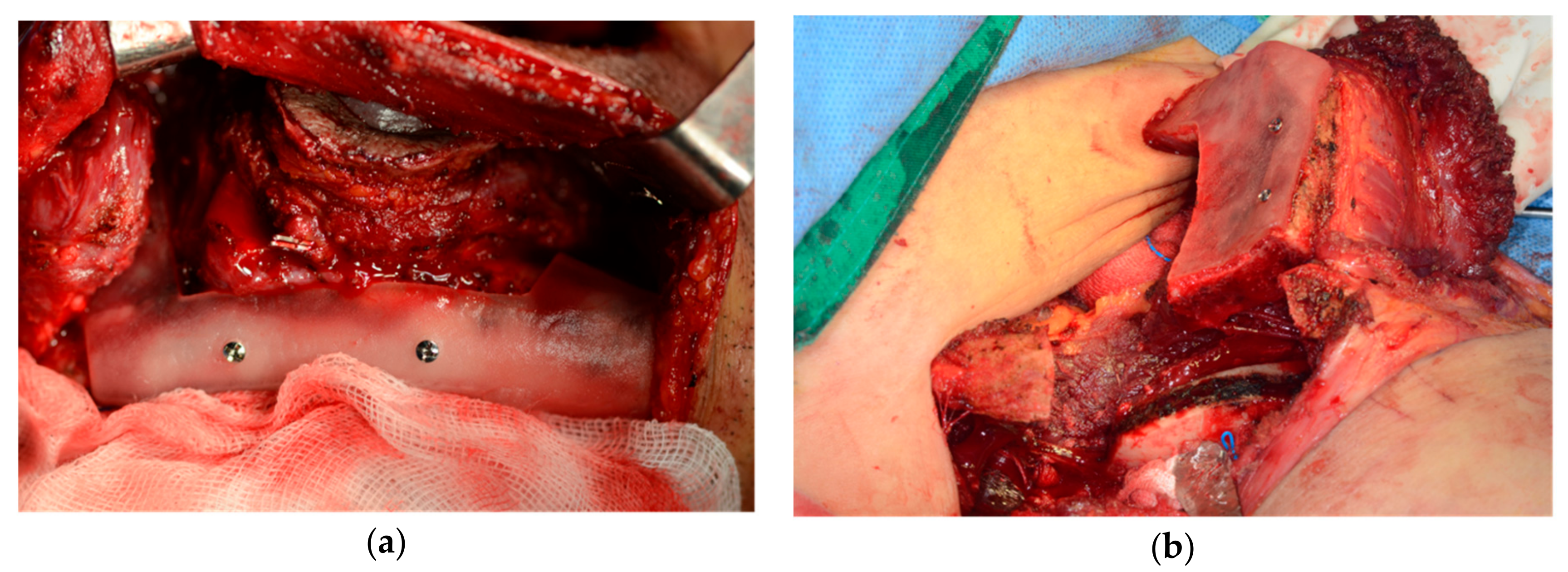
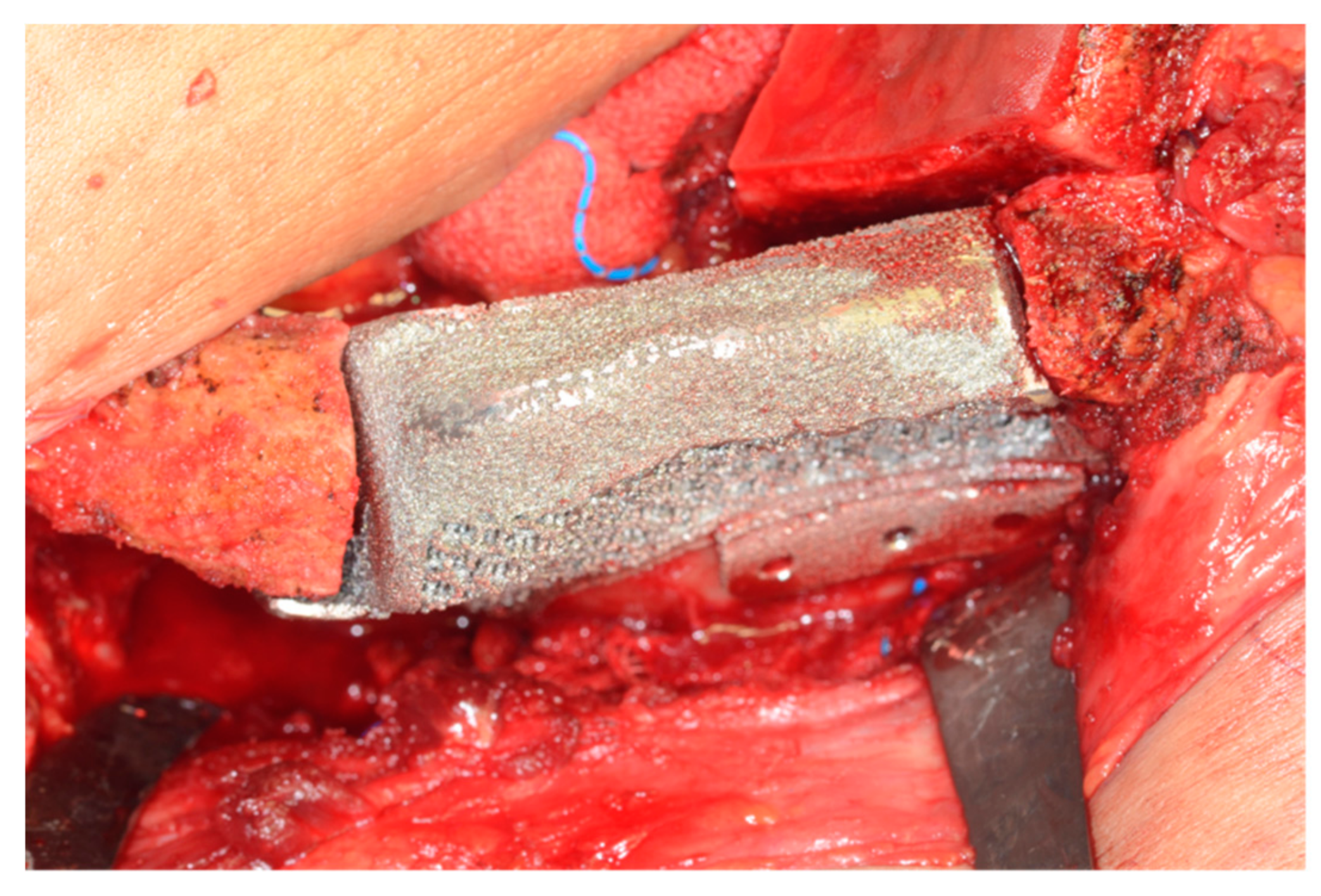
2.2. Surgical Technique
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Urken, M.L.; Vickery, C.; Weinberg, H.; Buchbinder, D.; Lawson, W.; Biller, H.F. The internal oblique-iliac crest osseomyocutaneous free flap in oromandibular reconstruction. Report of 20 cases. Arch. Otolaryngol. Head Neck Surg. 1989, 115, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Schmid, A.; Bodem, J.; Krisam, J.; Hoffmann, J.; Mertens, C. Donor site morbidity and quality of life after microvascular head and neck reconstruction with free fibula and deep-circumflex iliac artery flaps. J. Cranio Maxillofac. Surg. 2017, 45, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Toro, C. Iliac Flap Versus Fibula Flap in Mandibular Reconstruction. J. Craniofac. Surg. 2012, 23, 774–779. [Google Scholar] [CrossRef]
- Moon, S.-Y. Monocortical Deep Circumflex Iliac Artery Flap in Jaw Reconstruction. J Craniofac. Surg 2015, 26, 1294–1298. [Google Scholar] [CrossRef]
- Pagano, S.; Moretti, M.; Marsili, R.; Ricci, A.; Barraco, G.; Cianetti, S. Evaluation of the Accuracy of Four Digital Methods by Linear and Volumetric Analysis of Dental Impressions. Materials 2019, 12, 1958. [Google Scholar] [CrossRef] [PubMed]
- Disa, J.J.; Cordeiro, P.G. Mandible reconstruction with microvascular surgery. Semin. Surg. Oncol. 2000, 19, 226–234. [Google Scholar] [CrossRef]
- Schusterman, M.A.; Reece, G.P.; Kroll, S.S.; Weldon, M.E. Use of the AO plate for immediate mandibular reconstruction in cancer patients. Plast. Reconstr. Surg. 1991, 88, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, P.G.; Hidalgo, D.A. Soft tissue coverage of mandibular reconstruction plates. Head Neck 1994, 16, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, P.G.; Hidalgo, D.A. Conceptual considerations in mandibular reconstruction. Clin. Plast. Surg. 1995, 22, 61–69. [Google Scholar] [PubMed]
- Cordeiro, P.G.; Disa, J.J.; Hidalgo, D.A.; Hu, Q.Y. Reconstruction of the mandible with osseous free flaps: A 10-year experience with 150 consecutive patients. Plast. Reconstr. Surg. 1999, 104, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.G.; Townsend, P.; Corlett, R. Superiority of the deep circumflex iliac vessels as the supply for free groin flaps: Experimental work. Plast. Reconstr. Surg. 1979, 64, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.; Mayou, B.J. A new vascularized bone graft transferred by microvascular anastomosis as a free flap. Br. J. Surg. 1979, 66, 787–788. [Google Scholar] [CrossRef]
- Urken, M.L.; Vickery, C.; Weinberg, H.; Buchbinder, D.; Biller, H.F. The Internal Oblique-Iliac Crest Osseomyocutaneous Microvascular Free Flap in Head and Neck Reconstruction. J. Reconstr. Microsurg. 1989, 5, 203–214. [Google Scholar] [CrossRef]
- Boyd, J.B.; Rosen, I.; Rotstein, L.; Freeman, J.; Gullane, P.; Manktelow, R.; Zuker, R. The iliac crest and the radial forearm flap in vascularized oromandibular reconstruction. Am. J. Surg. 1990, 159, 301–308. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, J.-Y.; Hur, H.; Nam, W. Herniation after deep circumflex iliac artery flap: Two cases of rare complication. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 10. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.F.; Peng, X.; Samman, N. Donor-site morbidity of free fibula and DCIA flaps. J. Oral Maxillofac. Surg. 2013, 71, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Lloyd, C.J.; Paley, M.D.; Penfold, C.N. Repair of the deep circumflex iliac artery free flap donor site with Protack (titanium spiral tacks) and Prolene (polypropylene) mesh. Br. J. Oral Maxillofac. Surg. 2007, 45, 596–597. [Google Scholar] [CrossRef] [PubMed]
- Halsnad, S.M.; Dhariwal, D.K.; Bocca, A.P.; Evans, P.L.; Hodder, S.C. Titanium plate reconstruction of the osseous defect after harvest of a composite free flap using the deep circumflex iliac artery. Br. J. Oral Maxillofac. Surg. 2004, 42, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.L.; Kwon, J.S.; Woo, S.H.; Choi, Y.J. Simultaneous Bimaxillary Surgery and Mandibular Reconstruction with a 3-Dimensional Printed Titanium Implant Fabricated by Electron Beam Melting: A Preliminary Mechanical Testing of the Printed Mandible. J. Oral Maxillofac. Surg. 2016, 74, 1501.e1–1501.e15. [Google Scholar] [CrossRef] [PubMed]
- Keller, W.; Brägger, U.; Mombelli, A. Peri-implant microflora of implants with cemented and screw retained suprastructures. Clin. Oral Implant. Res. 1998, 9, 209–217. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Moon, S.Y. The Deep Circumflex Iliac Artery Flap for Mandibular Reconstruction and Donor Site Reconstruction with a Patient-Specific Implant: A Case Report. Appl. Sci. 2020, 10, 1587. https://doi.org/10.3390/app10051587
Kim HJ, Moon SY. The Deep Circumflex Iliac Artery Flap for Mandibular Reconstruction and Donor Site Reconstruction with a Patient-Specific Implant: A Case Report. Applied Sciences. 2020; 10(5):1587. https://doi.org/10.3390/app10051587
Chicago/Turabian StyleKim, Hyo Joon, and Seong Yong Moon. 2020. "The Deep Circumflex Iliac Artery Flap for Mandibular Reconstruction and Donor Site Reconstruction with a Patient-Specific Implant: A Case Report" Applied Sciences 10, no. 5: 1587. https://doi.org/10.3390/app10051587
APA StyleKim, H. J., & Moon, S. Y. (2020). The Deep Circumflex Iliac Artery Flap for Mandibular Reconstruction and Donor Site Reconstruction with a Patient-Specific Implant: A Case Report. Applied Sciences, 10(5), 1587. https://doi.org/10.3390/app10051587




