Computer-Assisted Preoperative Simulations and 3D Printed Surgical Guides Enable Safe and Less-Invasive Mandibular Segmental Resection: Tailor-Made Mandibular Resection
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Inclusion Criteria
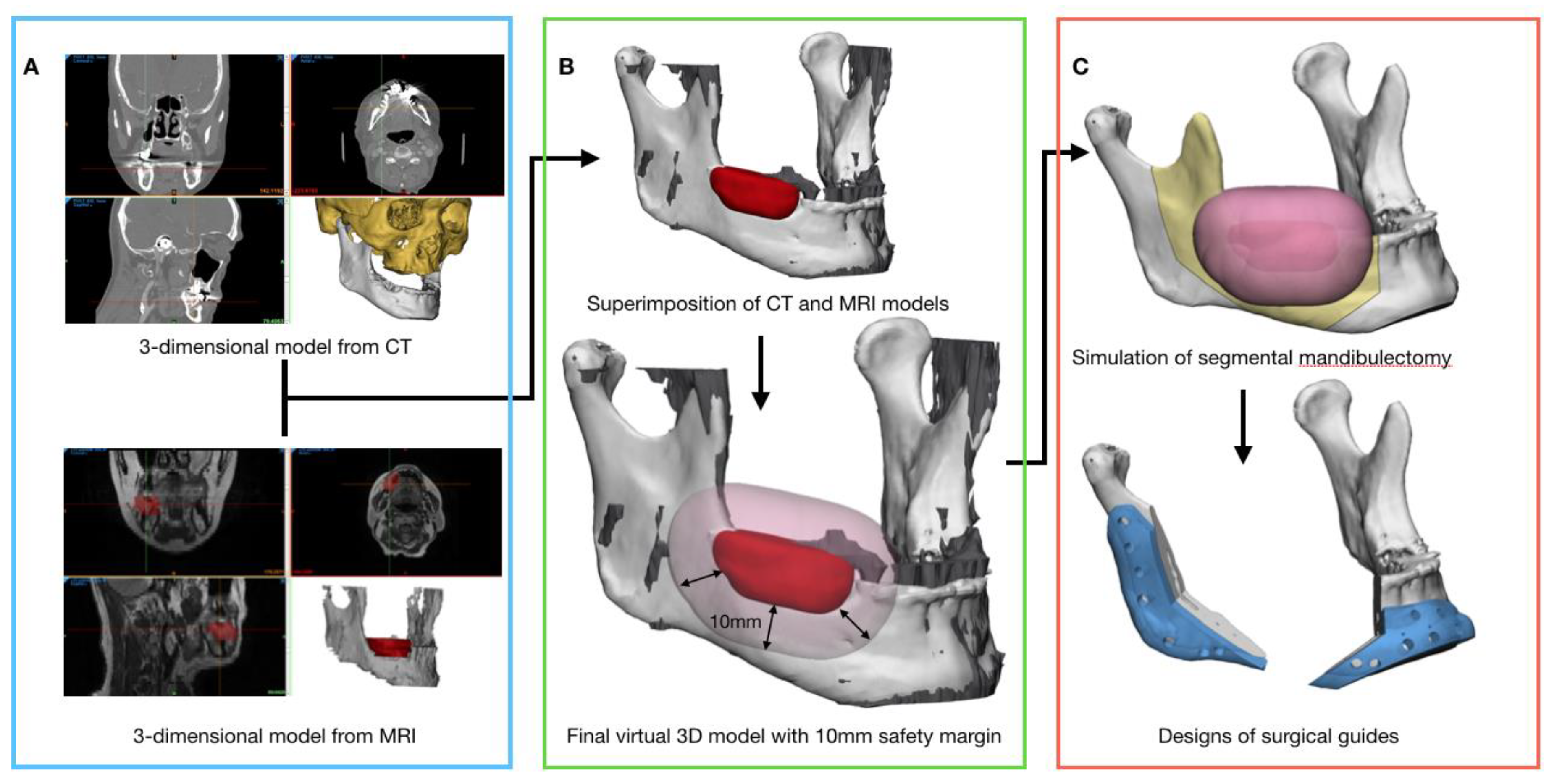
2.2. Data Acquisition and Three-Dimensional Virtual Models Preparation for Preoperative Surgical Simulation
2.3. Preoperative Surgical Simulation and Fabrication of the Surgical Guides
2.4. Evaluation Methods of the Surgical Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miles, B.A.; Goldstein, D.P.; Gilbert, R.W.; Gullane, P.J. Mandible reconstruction. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 317–322. [Google Scholar] [CrossRef]
- Cohen, A.; Laviv, A.; Berman, P.; Nashef, R.; Abu-Tair, J. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 661–666. [Google Scholar] [CrossRef] [PubMed]
- López-Arcas, J.M.; Arias, J.; Del Castillo, J.L.; Burgueño, M.; Navarro, I.; Morán, M.J.; Chamorro, M.; Martorell, V. The fibula osteomyocutaneous flap for mandible reconstruction: A 15-year experience. J. Oral Maxillofac. Surg. 2010, 68, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Weijs, W.L.; Coppen, C.; Schreurs, R.; Vreeken, R.D.; Verhulst, A.C.; Merkx, M.A.; Bergé, S.J.; Maal, T.J. Accuracy of virtually 3D planned resection templates in mandibular reconstruction. J. Cranio-Maxillofac. Surg. 2016, 44, 1828–1832. [Google Scholar] [CrossRef]
- Coppen, C.; Weijs, W.; Bergé, S.J.; Maal, T.J. Oromandibular reconstruction using 3D planned triple template method. J. Oral Maxillofac. Surg. 2013, 71, e243–e247. [Google Scholar] [CrossRef] [PubMed]
- Funayama, A.; Kojima, T.; Yoshizawa, M.; Mikami, T.; Kanemaru, S.; Niimi, K.; Oda, Y.; Kato, Y.; Kobayashi, T. A simple technique for repositioning of the mandible by a surgical guide prepared using a three-dimensional model after segmental mandibulectomy. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Serrano, C.; van den Brink, H.; Pineau, J.; Prognon, P.; Martelli, N. Benefits of 3D printing applications in jaw reconstruction: A systematic review and meta-analysis. J. Cranio-Maxillofac. Surg. 2019, 47, 1387–1397. [Google Scholar] [CrossRef]
- Kwon, T.-G. Accuracy and reliability of three-dimensional computer-assisted planning for orthognathic surgery. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 14. [Google Scholar] [CrossRef]
- Kraeima, J.; Dorgelo, B.; Gulbitti, H.; Steenbakkers, R.; Schepman, K.; Roodenburg, J.; Spijkervet, F.; Schepers, R.; Witjes, M. Multi-modality 3D mandibular resection planning in head and neck cancer using CT and MRI data fusion: A clinical series. Oral Oncol. 2018, 81, 22–28. [Google Scholar] [CrossRef]
- El-Hafez, Y.G.A.; Chen, C.-C.; Ng, S.-H.; Lin, C.-Y.; Wang, H.-M.; Chan, S.-C.; Chen, I.-H.; Huan, S.-F.; Kang, C.-J.; Lee, L.-Y. Comparison of PET/CT and MRI for the detection of bone marrow invasion in patients with squamous cell carcinoma of the oral cavity. Oral Oncol. 2011, 47, 288–295. [Google Scholar] [CrossRef]
- Loeffelbein, D.J.; Souvatzoglou, M.; Wankerl, V.; Martinez-Möller, A.; Dinges, J.; Schwaiger, M.; Beer, A.J. PET-MRI fusion in head-and-neck oncology: Current status and implications for hybrid PET/MRI. J. Oral Maxillofac. Surg. 2012, 70, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, S.K.; Isaacs, D.L.; Creager, A.; Shockley, W.; Weissler, M.; Armao, D. CT detection of mandibular invasion by squamous cell carcinoma of the oral cavity. Am. J. Roentgenol. 2001, 177, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Van Cann, E.; Koole, R.; Oyen, W.; de Rooy, J.; de Wilde, P.; Slootweg, P.; Schipper, M.; Merkx, M.; Stoelinga, P. Assessment of mandibular invasion of squamous cell carcinoma by various modes of imaging: Constructing a diagnostic algorithm. Int. J. Oral Maxillofac. Surg. 2008, 37, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, T.; Shiga, T.; Shirato, H.; Tsukamoto, E.; Tsuchiya, K.; Kato, T.; Ohmori, K.; Yamazaki, A.; Aoyama, H.; Hashimoto, S. Image fusion between 18FDG-PET and MRI/CT for radiotherapy planning of oropharyngeal and nasopharyngeal carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1051–1057. [Google Scholar] [CrossRef]
- Dai, J.; Wang, X.; Dong, Y.; Yu, H.; Yang, D.; Shen, G. Two-and Three-Dimensional Models for the Visualization of Jaw Tumors Based on CT–MRI Image Fusion. J. Craniofacial Surg. 2012, 23, 502–508. [Google Scholar] [CrossRef]
- Lee, J.-W.; Choi, B.-J.; Lee, D.-W.; Kwon, Y.-D. Double-barrelled vascularised fibular free flap using computer-assisted preoperative planning and a surgical template for accurate reconstruction of a segmental mandibular defect. Br. J. Oral Maxillofacial Surg. 2016, 54, 102–103. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.-L.; Maté-Sánchez, J.-E.; Delgado-Ruiz, R.; Ramírez-Fernández, M.-P. Calculation of bone graft volume using 3D reconstruction system. Med. Oral. Patol. Oral. Cir. Bucal. 2011, 16, e260–e264. [Google Scholar] [CrossRef][Green Version]
- Cappare, P.; Sannino, G.; Minoli, M.; Montemezzi, P.; Ferrini, F. Conventional versus digital impressions for full arch screw-retained maxillary rehabilitations: A randomized clinical trial. Int. J. Environ. Res. Public Health 2019, 16, 829. [Google Scholar] [CrossRef]
- Cattoni, F.; Teté, G.; Calloni, A.M.; Manazza, F.; Gastaldi, G.; Capparè, P. Milled versus moulded mock-ups based on the superimposition of 3D meshes from digital oral impressions: A comparative in vitro study in the aesthetic area. BMC Oral Health 2019, 19, 230. [Google Scholar] [CrossRef]
- Gherlone, E.; Capparé, P.; Vinci, R.; Ferrini, F.; Gastaldi, G.; Crespi, R. Conventional Versus Digital Impressions for “All-on-Four” Restorations. Int. J. Oral Maxillofac. Implant. 2016, 31, 324–330. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Ferrini, F.; Crespi, R.; Gastaldi, G.; Capparé, P. Digital impressions for fabrication of definitive “all-on-four” restorations. Implant. Dent. 2015, 24, 125–129. [Google Scholar] [CrossRef] [PubMed]

| Patient No. | Sex | Age (Years) | Diagnosis | Method of Reconstruction | |
|---|---|---|---|---|---|
| Microvascular Free Flap | Fixation Method | ||||
| 1 | M | 37 | Ameloblastoma | FFF | Miniplates |
| 2 | F | 52 | SCC | FFF | Miniplates |
| 3 | F | 56 | ORN | FFF | R-plate |
| 4 | M | 63 | ORN | FFF | Miniplates |
| 5 | F | 44 | Ameloblastoma | DCIA | R-plate |
| 6 | M | 56 | SCC | FFF | Miniplates |
| 7 | M | 62 | SCC | DCIA | Miniplates |
| 8 | M | 78 | Osteosarcoma | FFF | R-plate |
| 9 | M | 31 | Ameloblastoma | FFF | R-plate |
| 10 | M | 79 | SCC | FFF | R-plate |
| 11 | F | 74 | SCC | FFF | R-plate |
| 12 | F | 75 | SCC | FFF | Miniplates |
| 13 | M | 60 | SCC | FFF | R-plate |
| 14 | M | 70 | SCC | FFF | R-plate |
| 15 | M | 65 | SCC | FFF | R-plate |
| Total n = 15 | 10 men 5 women | Mean (SD) 60.13 (14.55) | |||
| Patient No. | Conventional Resection Simulation | Tailor-Made Resection Simulation | Tailor-Made Resection Simulation Vs. Post-OP | No. of Teeth Saved | Mandibular Border Defect Classification | Disease-Free Bone Margins | Recurrence/Metastasis (Follow-Up Period, Months) | |
|---|---|---|---|---|---|---|---|---|
| Primary | Final | |||||||
| 1 |  |  | 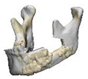 | 1 (#33) | SAM | AM | yes | no (34) |
| 2 |  |  |  | 0 | SA | A | yes | no (35) |
| 3 |  |  |  | 3 (#41, 42, 43) | MAS | MA | yes | no (17) |
| 4 |  |  | 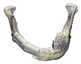 | 0 | MASS | MA | yes | no (23) |
| 5 |  |  | 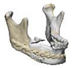 | 1 (#43) | MAS | Preservation of the whole mandibular border | yes | no (29) |
| 6 |  |  | 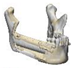 | 0 | MA | MA | yes | no (31) |
| 7 |  |  | 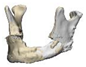 | 0 | AM | AM | yes | no (25) |
| 8 |  |  | 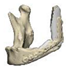 | 0 | MPC | PC | yes | no (29) |
| 9 |  |  | 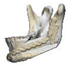 | 1 (#35) | AM | AM | yes | no (35) |
| 10 | 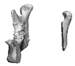 | 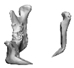 | 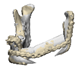 | 1 (#33) | SAM | AM | yes | no (29) |
| 11 | 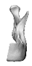 |  |  | 0 | CPMAS | CP | Yes (soft tissue involvement) | distant metastasis, lung (16) |
| 12 |  | 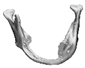 | 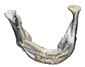 | 0 | MASS | Preservation of the mandibular border | yes | no (23) |
| 13 | 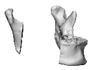 | 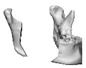 | 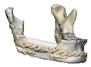 | 0 | MA | A | yes | no (27) |
| 14 |  |  | 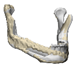 | 0 | MA | A | yes | no (29) |
| 15 |  |  |  | 1 (#33) | SAM | AM | yes | no (21) |
| Conventional Resection | Tailor-made Resection | Difference | p-Value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Volume (mm3) | 49,468.66 (14007.96) | 52,610.01 (13755.33) | −3141.35 (1355.14) | <0.001 |
| Surface (mm2) | 20,927.38 (4471.70) | 22,356.49 (4185.73) | −1419.10 (554.67) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, B.-Y.; Lee, J.-Y.; Jung, J.; Ohe, J.-Y.; Eun, Y.-G.; Lee, Y.; Lee, J.-W. Computer-Assisted Preoperative Simulations and 3D Printed Surgical Guides Enable Safe and Less-Invasive Mandibular Segmental Resection: Tailor-Made Mandibular Resection. Appl. Sci. 2020, 10, 1325. https://doi.org/10.3390/app10041325
Hwang B-Y, Lee J-Y, Jung J, Ohe J-Y, Eun Y-G, Lee Y, Lee J-W. Computer-Assisted Preoperative Simulations and 3D Printed Surgical Guides Enable Safe and Less-Invasive Mandibular Segmental Resection: Tailor-Made Mandibular Resection. Applied Sciences. 2020; 10(4):1325. https://doi.org/10.3390/app10041325
Chicago/Turabian StyleHwang, Bo-Yeon, Jae-Yeol Lee, Junho Jung, Joo-Young Ohe, Young-Gyu Eun, YoungChan Lee, and Jung-Woo Lee. 2020. "Computer-Assisted Preoperative Simulations and 3D Printed Surgical Guides Enable Safe and Less-Invasive Mandibular Segmental Resection: Tailor-Made Mandibular Resection" Applied Sciences 10, no. 4: 1325. https://doi.org/10.3390/app10041325
APA StyleHwang, B.-Y., Lee, J.-Y., Jung, J., Ohe, J.-Y., Eun, Y.-G., Lee, Y., & Lee, J.-W. (2020). Computer-Assisted Preoperative Simulations and 3D Printed Surgical Guides Enable Safe and Less-Invasive Mandibular Segmental Resection: Tailor-Made Mandibular Resection. Applied Sciences, 10(4), 1325. https://doi.org/10.3390/app10041325






