Permeability Evaluation of Clay-quartz Mixtures Based on Low-Field NMR and Fractal Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Theoretical Basis
2.1.1. NMR Theory
2.1.2. Fractal Theory
2.2. Materials
2.3. Sample Preparation
2.4. Methods
3. Results
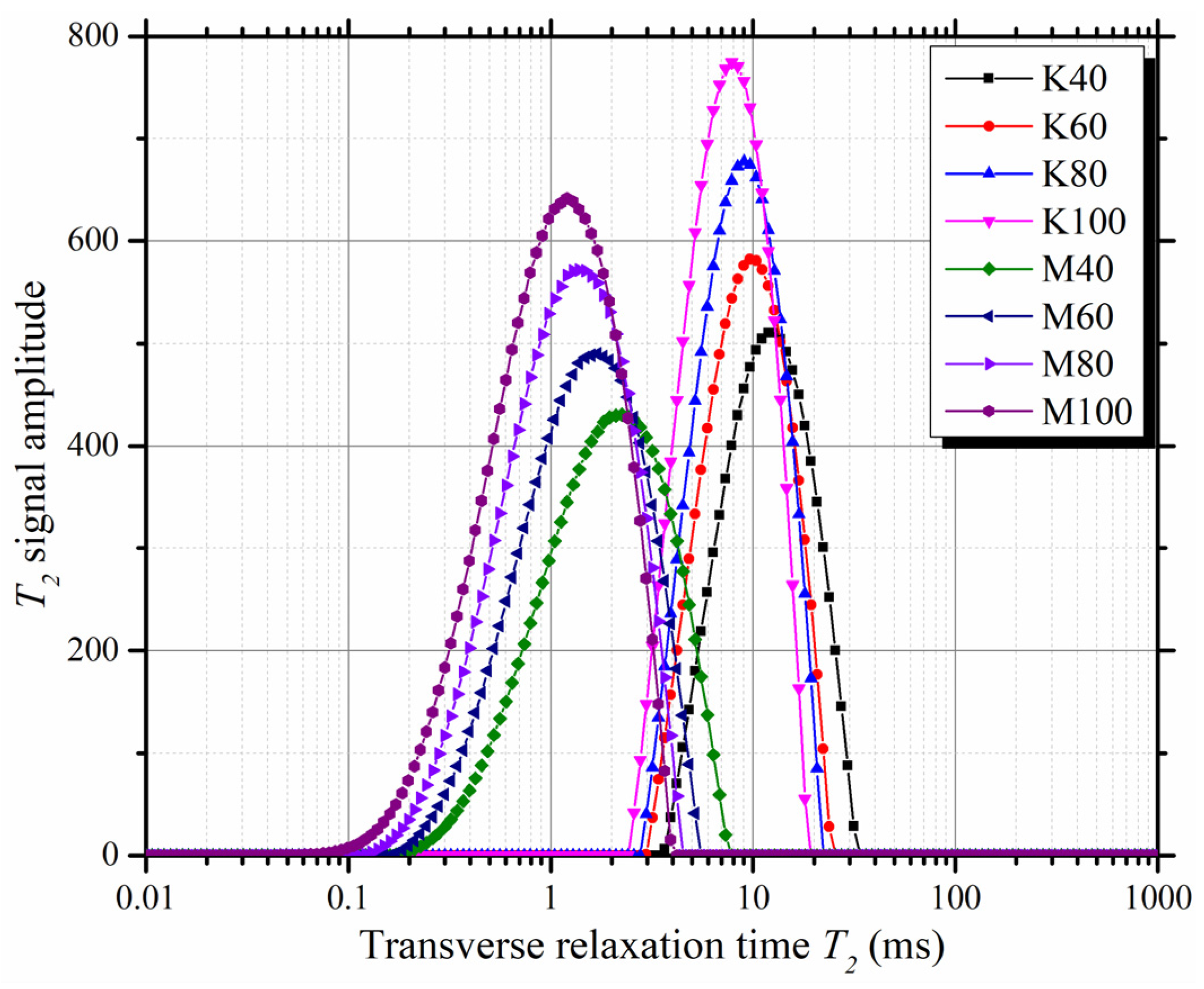
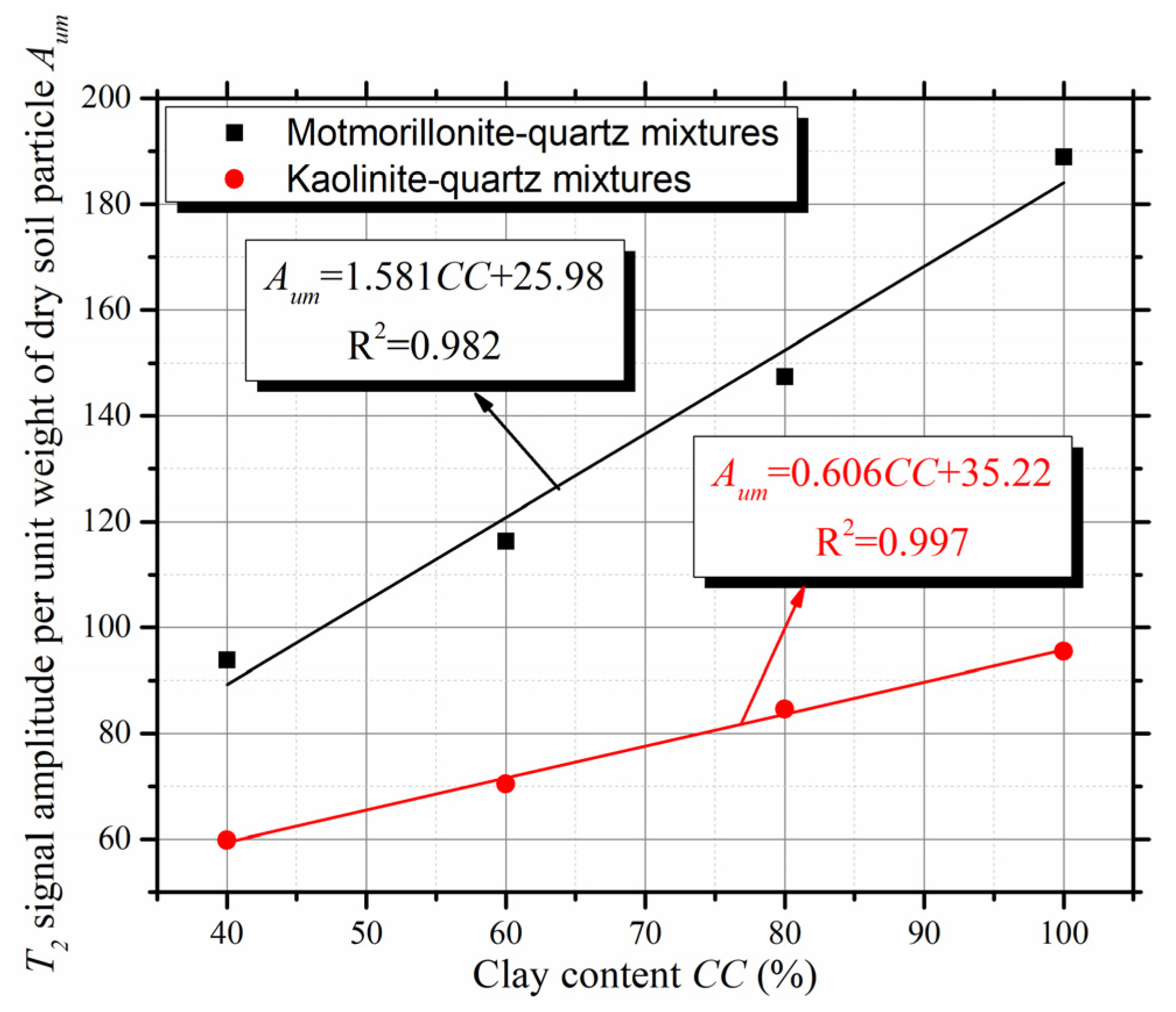
3.1. T2 Spectra of Saturated Mixtures
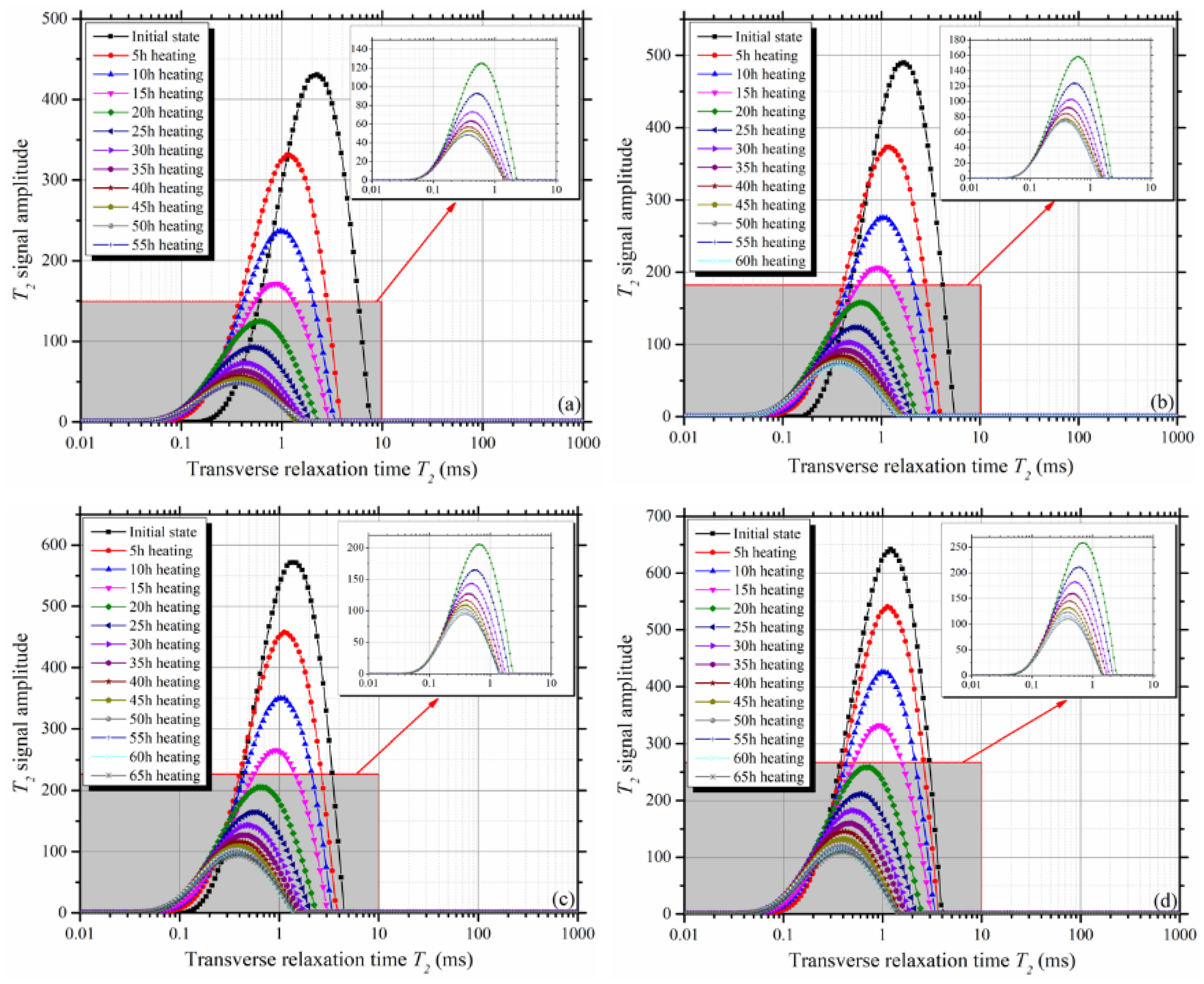
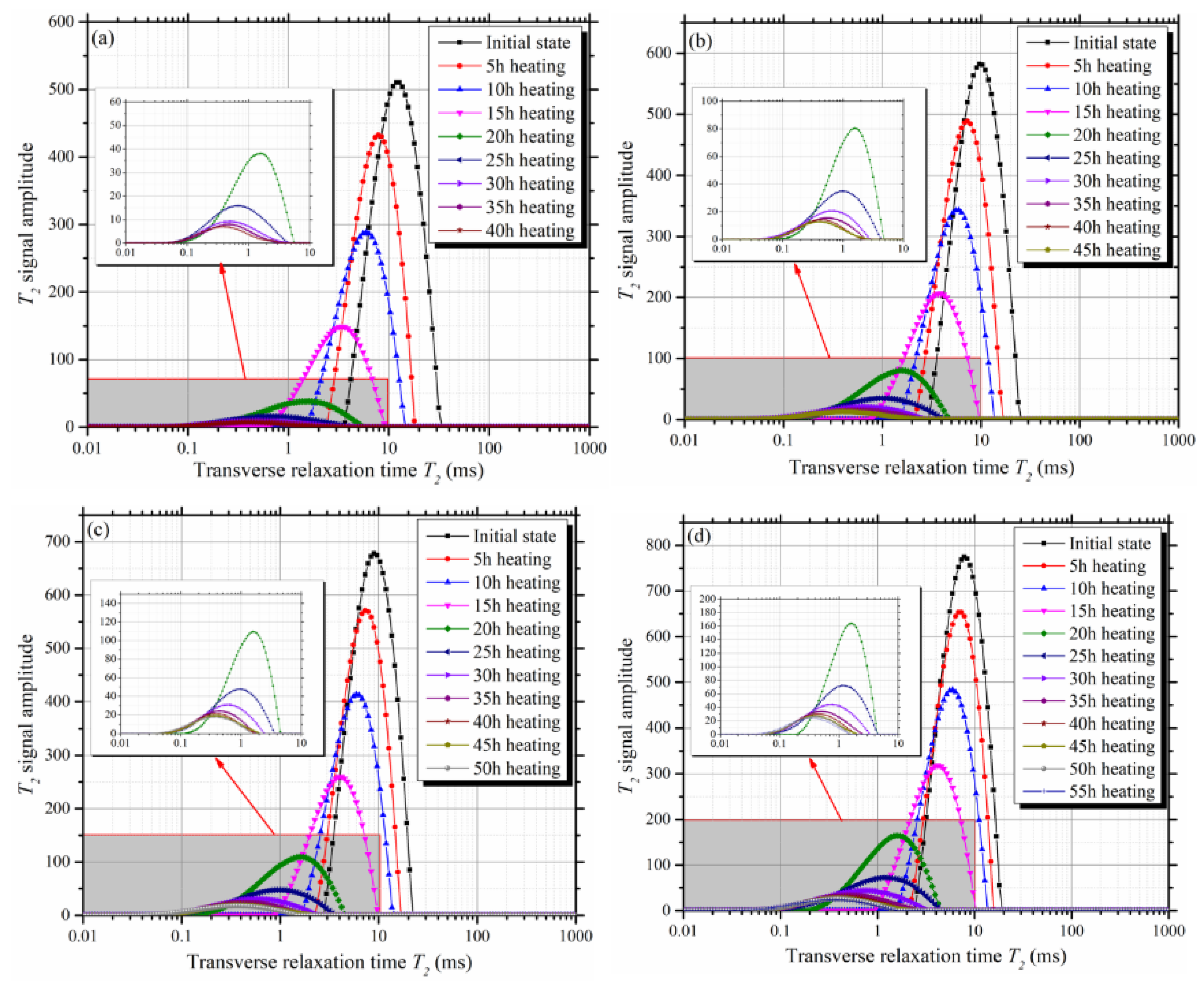
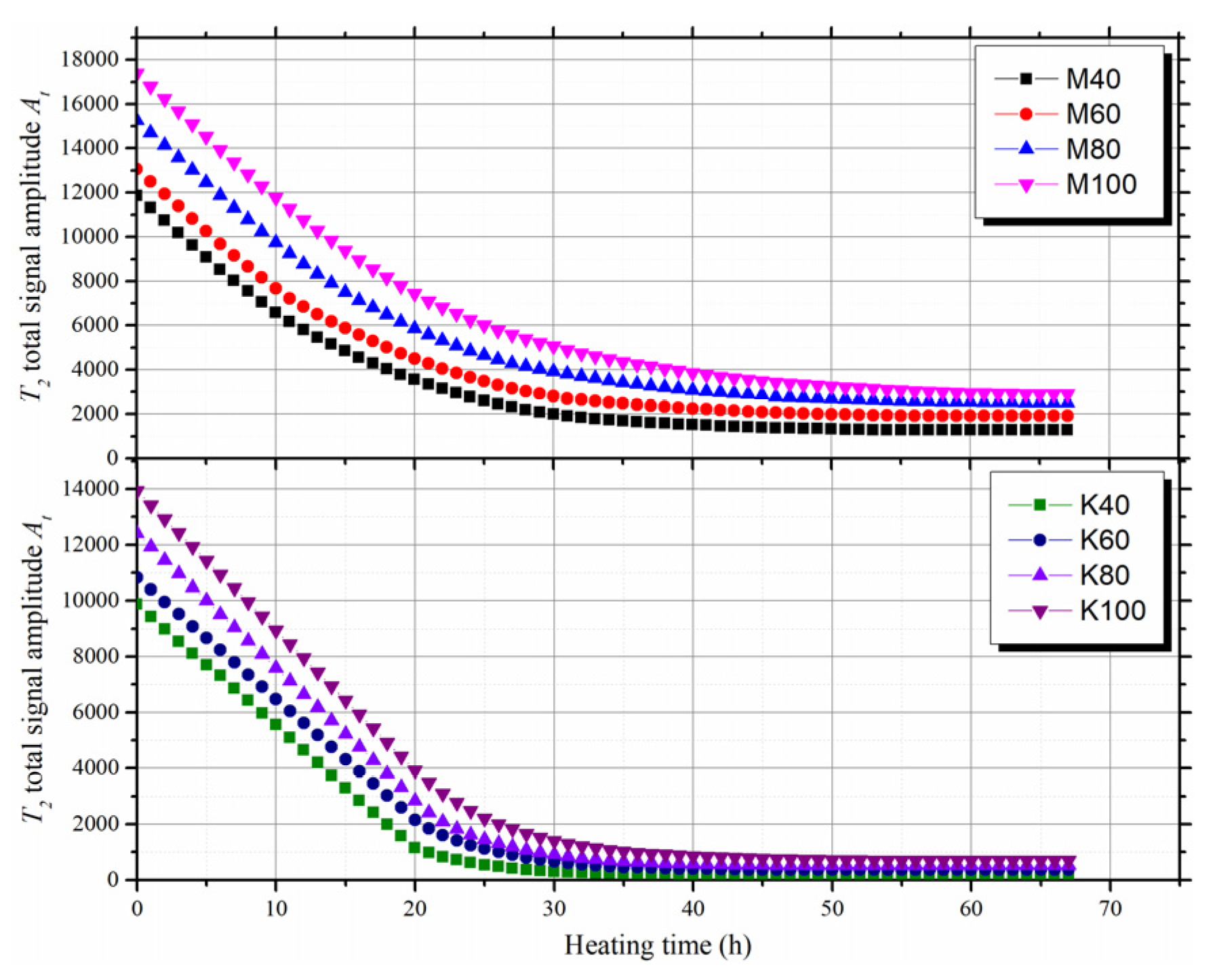
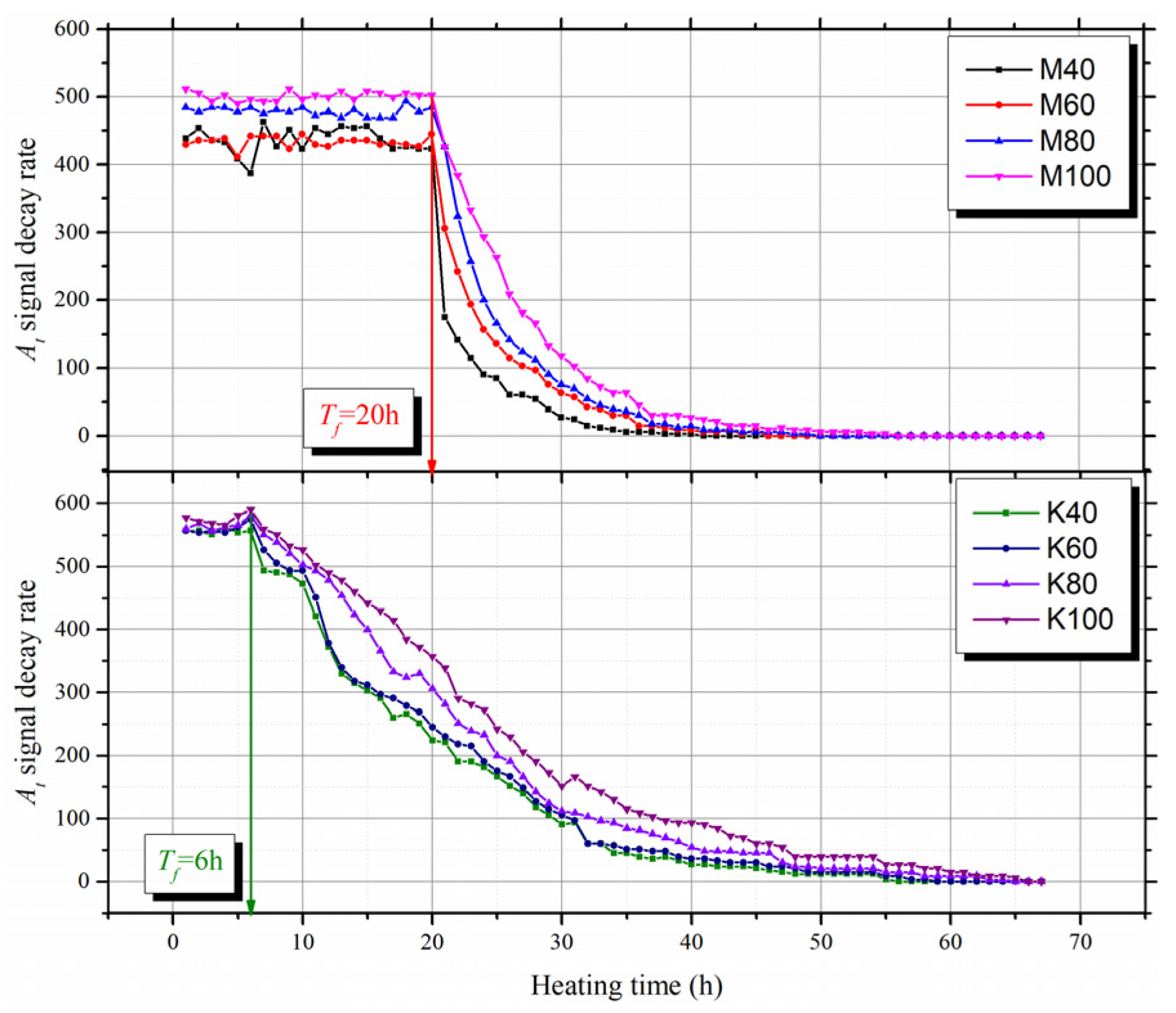
3.2. Variation of T2 Spectra During Evaporation
4. Discussion
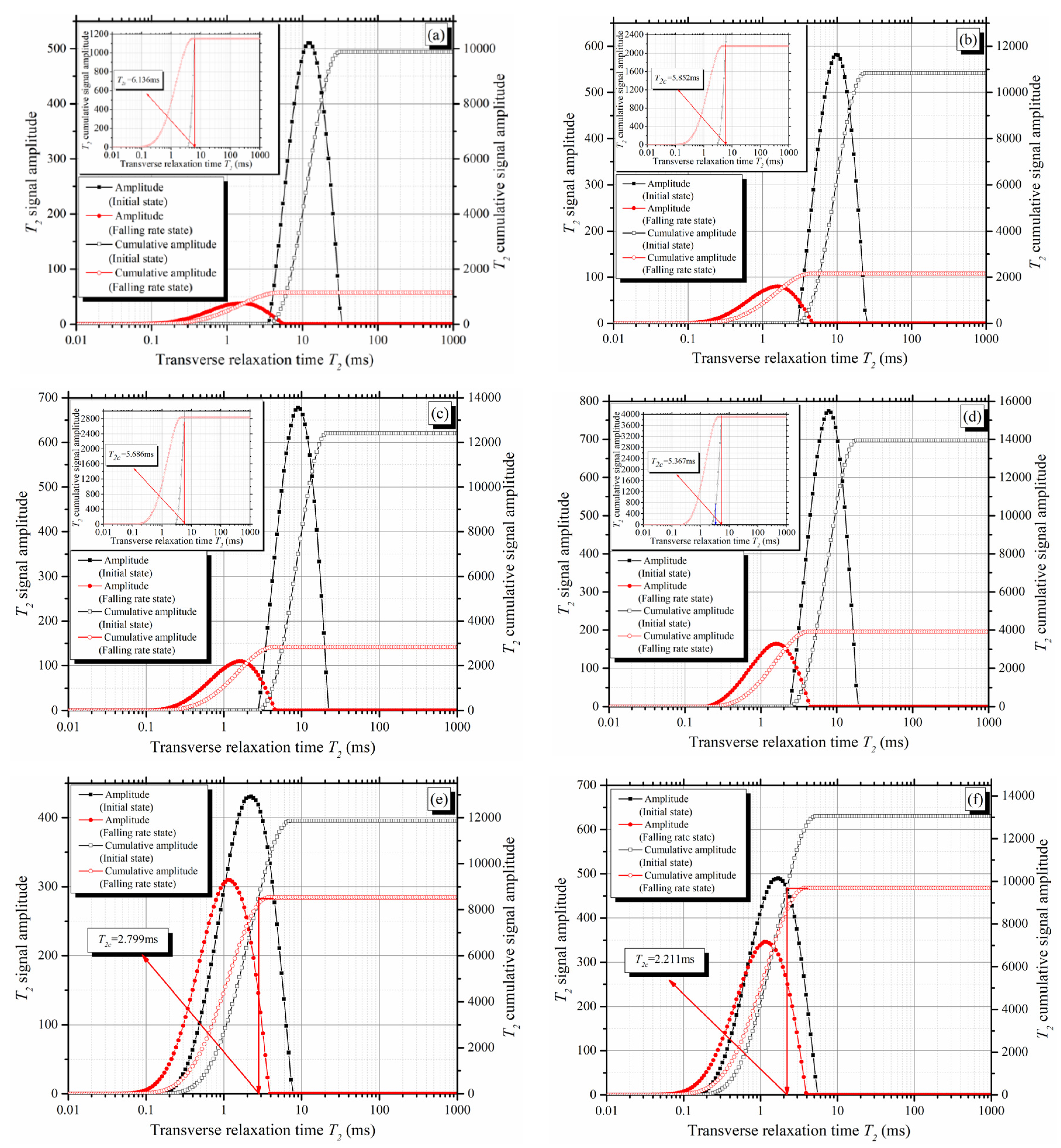
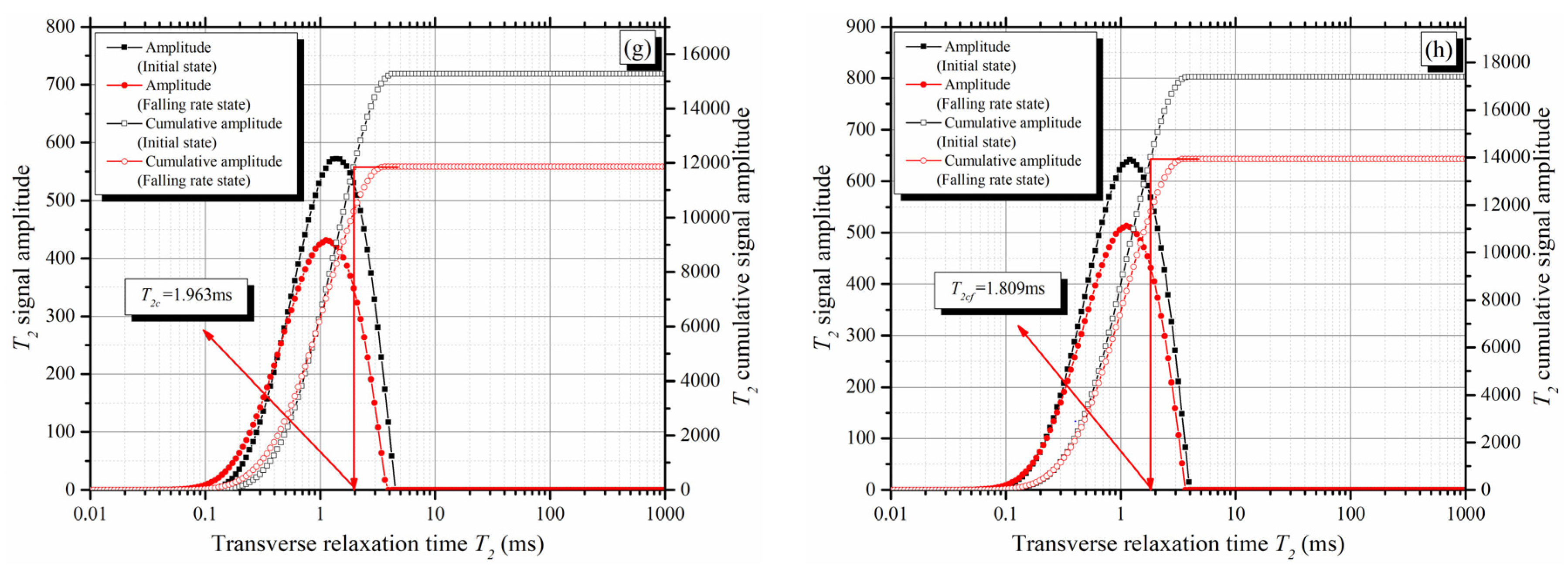
4.1. Novel Method for Determining the T2 Cut-Off
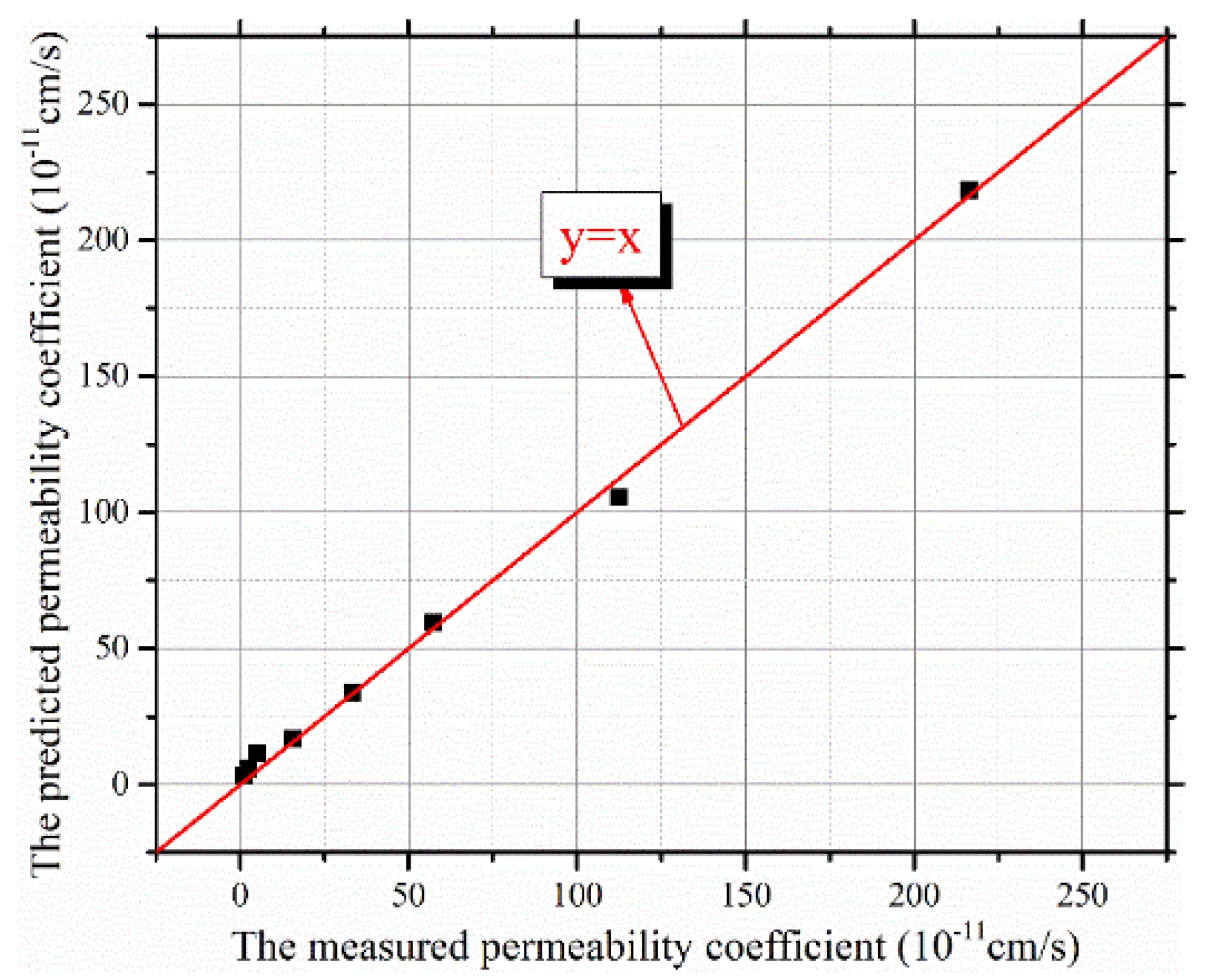
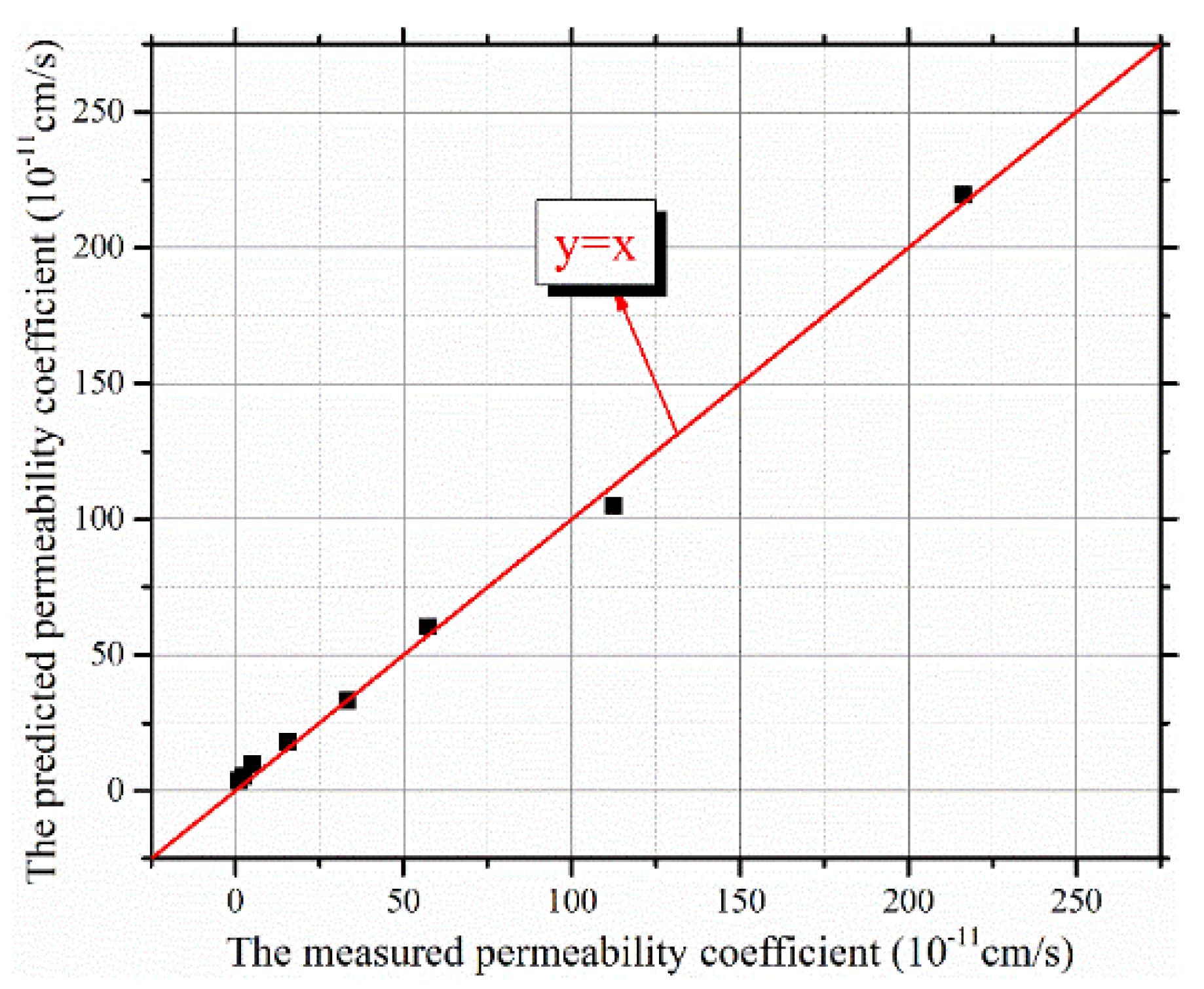
4.2. TC Permeability Coefficient Prediction Model
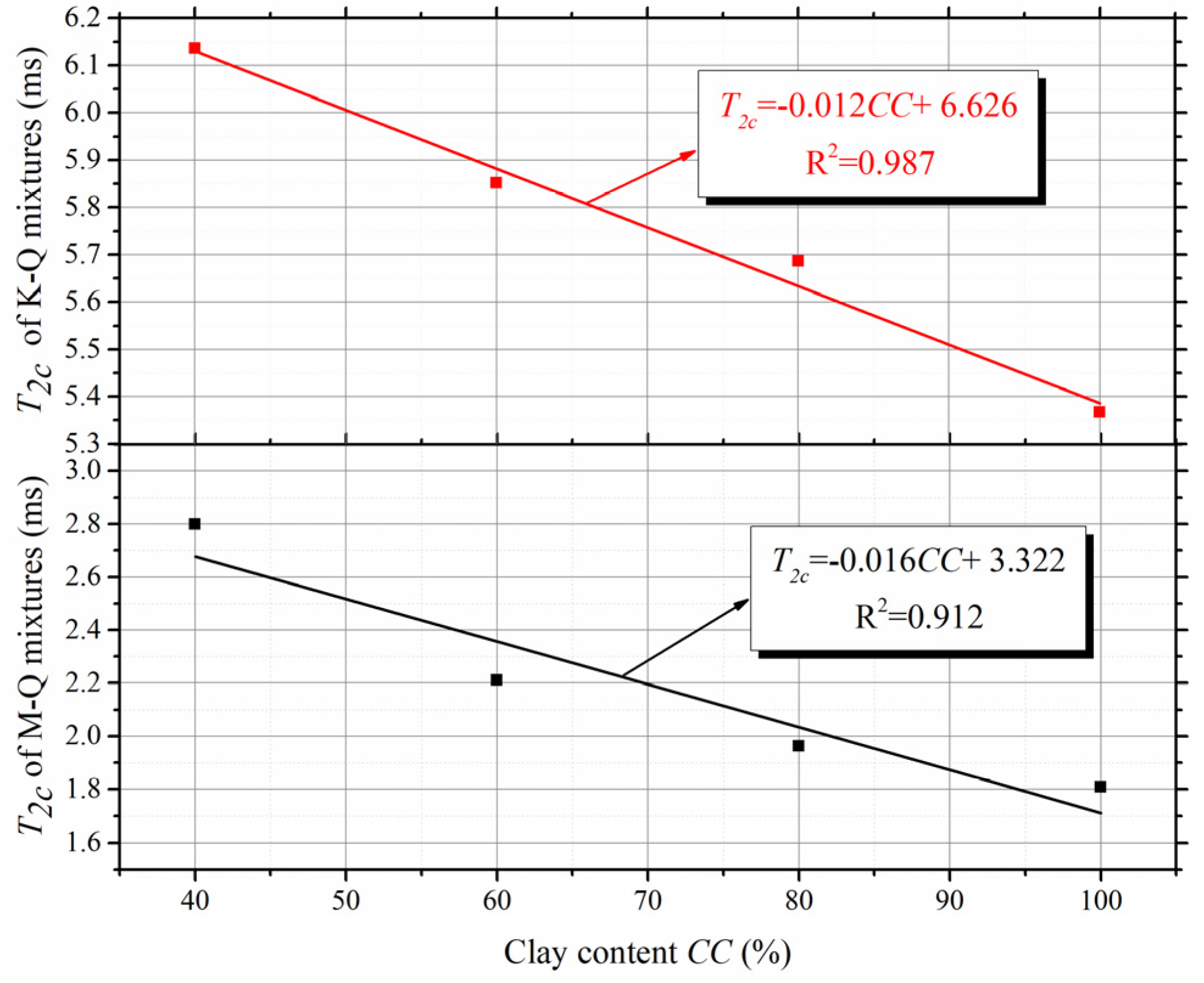
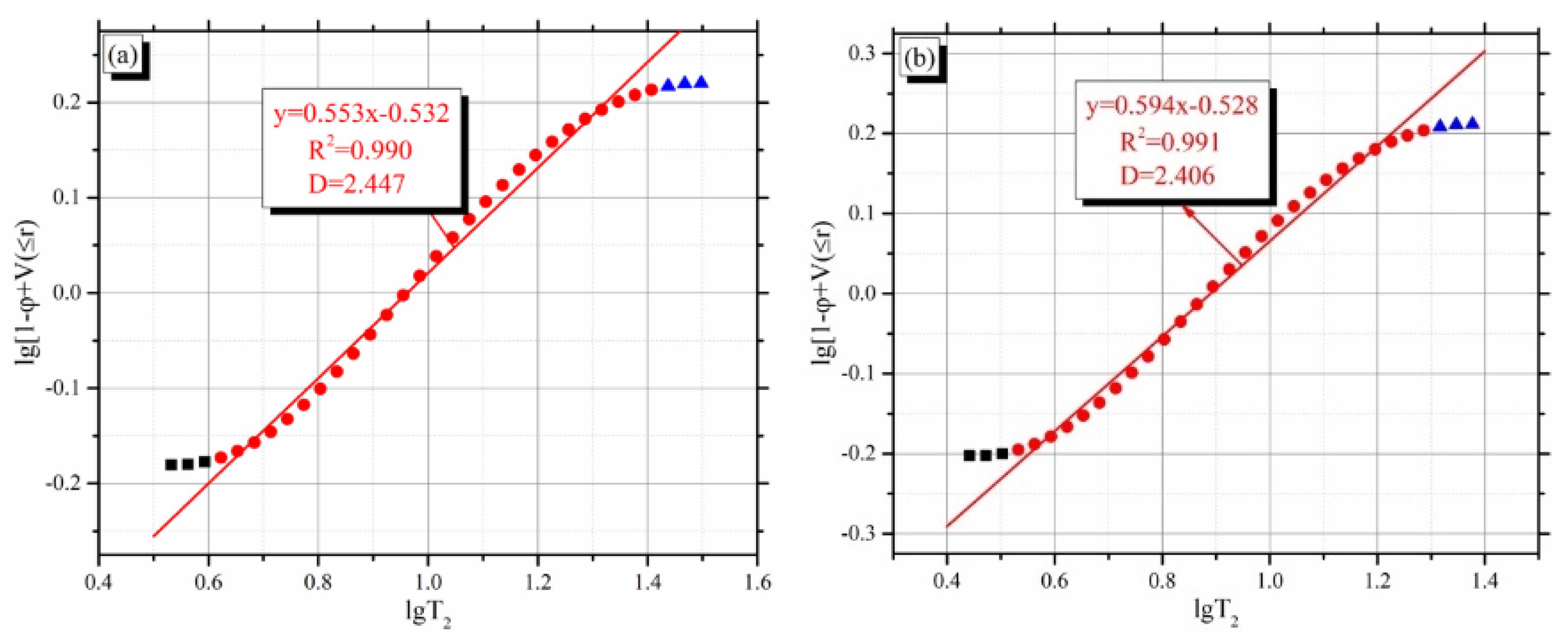
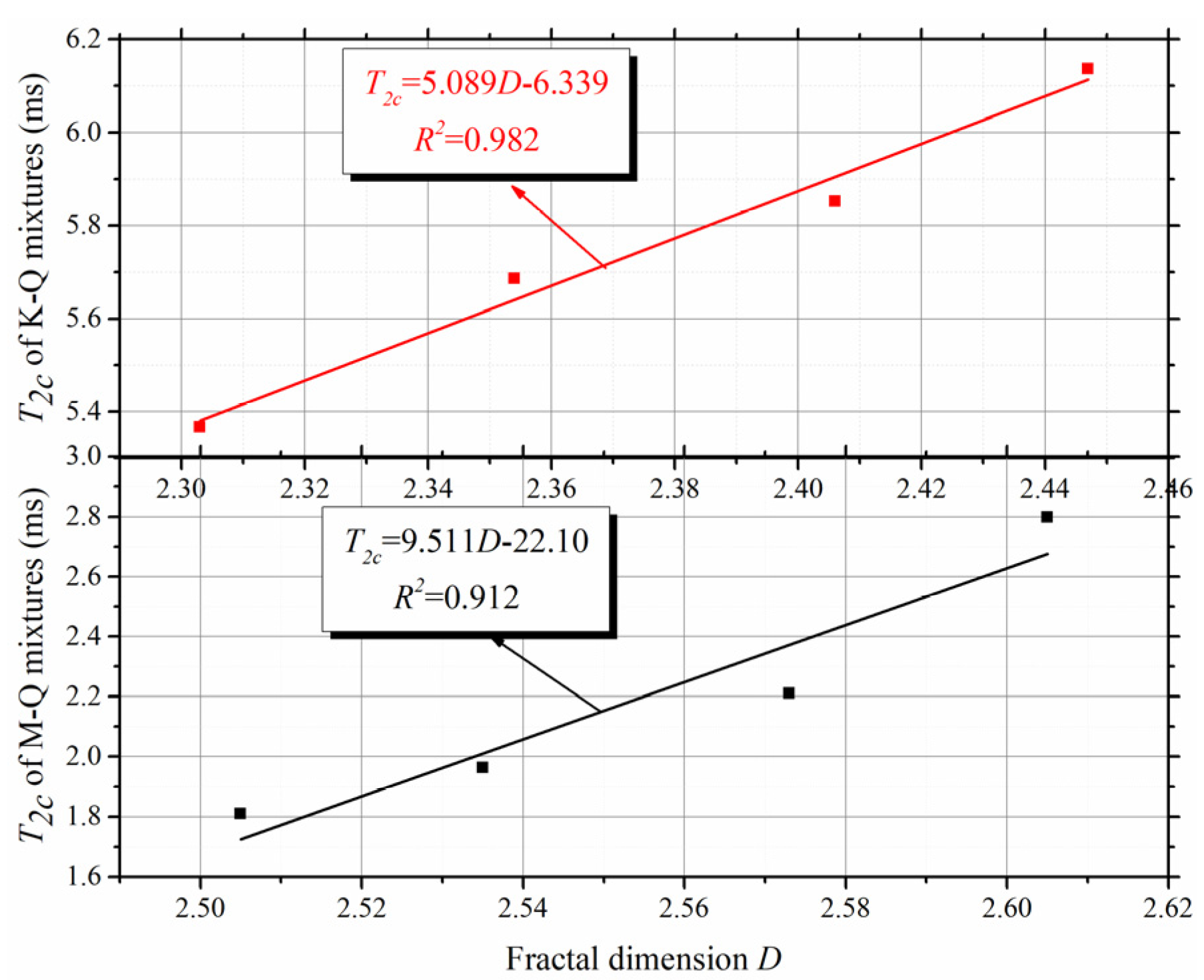
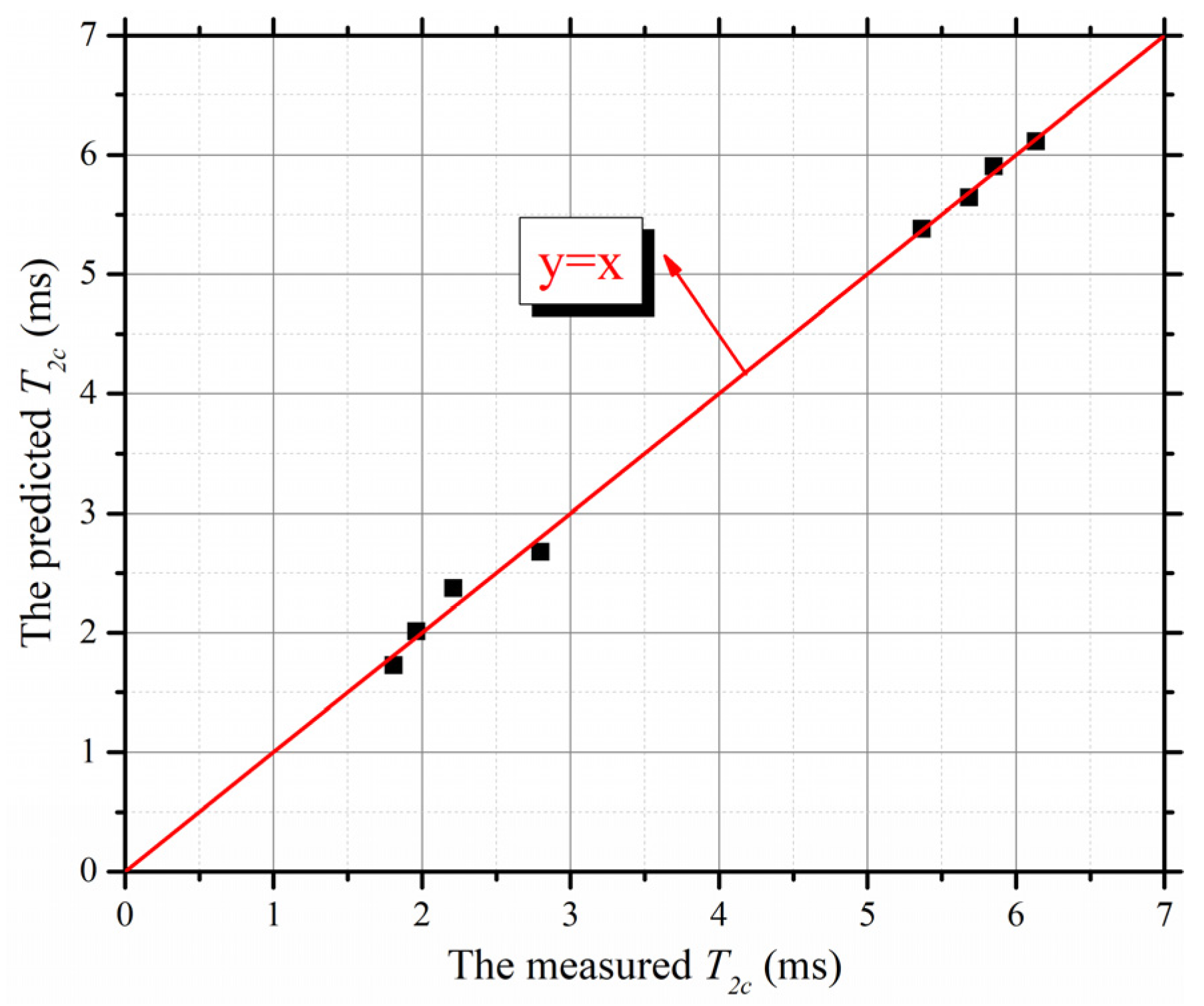
4.3. Determination Method of T2c Based on Fractal Analysis
5. Conclusions
- The T2 spectra of M-Q mixtures and K-Q mixtures are quite different. The T2p of M-Q mixtures and K-Q mixtures ranged from 1.20 to 2.25 ms and 7.84 to 11.89 ms, respectively. The T2p can be used to determine the kind of clay mineral in the clay-quartz mixture;
- During evaporation, all the T2 spectra of clay-quartz mixtures shifted to the left. This indicated that shrinkage of the mixture volume led to a decrease of the total pore radius;
- According to the decay rate of the T2 total signal amplitude, the evaporation process can be divided into two stages: the constant rate stage and the falling rate stage. The T2 cut-offs can be determined from the T2 spectra of the mixtures at the initial saturated state and the beginning of the falling rate stage;
- A prediction model for permeability coefficients of clay-quartz mixtures based on the T-C model was established. The relationship between T2 cut-offs and fractal dimensions of T2 spectra of saturated mixtures was also proposed. Based on these, a simplified method for predicting permeability coefficients of clay-quartz mixtures by using only the T2 spectrum of saturated samples without centrifugal and evaporation tests was established. Compared with the traditional method, which is effort- and time-consuming, the new prediction method proposed in this study can determine the permeability coefficients of clay-quartz mixtures rapidly, nondestructively, and accurately.
Author Contributions
Funding
Conflicts of Interest
References
- Regadio, M.; Ruiz, A.I.; Rodriguez-Rastrero, M.; Cuevas, J. Containment and attenuating layers: An affordable strategy that preserves soil and water from landfill pollution. Waste Manag. 2015, 46, 408–419. [Google Scholar] [CrossRef]
- Al Rashid, Q.A.; Abuel-Naga, H.M.; Leong, E.C.; Lu, Y.; Al Abadi, H. Experimental-artificial intelligence approach for characterizing electrical resistivity of partially saturated clay liners. Appl. Clay Sci. 2018, 156, 1–10. [Google Scholar] [CrossRef]
- Karunaratne, G.P.; Chew, S.H.; Lee, S.L.; Sinha, A.N. Bentonite:Kaolinite clay liner. Geosynth. Int. 2001, 8, 113–133. [Google Scholar] [CrossRef]
- Zhao, Q.; Choo, H.; Bhatt, A.; Burns, S.E.; Bate, B. Review of the fundamental geochemical and physical behaviors of organoclays in barrier applications. Appl. Clay Sci. 2017, 142, 2–20. [Google Scholar] [CrossRef]
- Dolinar, B. Predicting the hydraulic conductivity of saturated clays using plasticity-value correlations. Appl. Clay Sci. 2009, 45, 90–94. [Google Scholar] [CrossRef]
- De Camillis, M.; Di Emidio, G.; Bezuijen, A.; Verastegui Flores, D.; Van Stappen, J.; Cnudde, V. Effect of wet-dry cycles on polymer treated bentonite in seawater: Swelling ability, hydraulic conductivity and crack analysis. Appl. Clay Sci. 2017, 142, 52–59. [Google Scholar] [CrossRef]
- Lubelli, B.; de Winter, D.A.M.; Post, J.A.; van Hees, R.P.J.; Drury, M.R. Cryo-FIB–SEM and MIP study of porosity and pore size distribution of bentonite and kaolin at different moisture contents. Appl. Clay Sci. 2013, 80–81, 358–365. [Google Scholar] [CrossRef]
- Thom, R.; Sivakumar, R.; Sivakumar, V.; Murray, E.J.; Mackinnon, P. Pore size distribution of unsaturated compacted kaolin: The initial states and final states following saturation. Geotechnique 2007, 57, 469–474. [Google Scholar] [CrossRef]
- Deng, Y.F.; Wu, Z.L.; Cui, Y.J.; Liu, S.Y.; Wang, Q. Sand fraction effect on hydro-mechanical behavior of sand-clay mixture. Appl. Clay Sci. 2017, 135, 355–361. [Google Scholar] [CrossRef]
- Tiwari, B.; Ajmera, B. A new correlation relating the shear strength of reconstituted soil to the proportions of clay minerals and plasticity characteristics. Appl. Clay Sci. 2011, 53, 48–57. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Tan, Y.; Zhu, F.; He, D.J.; Zhu, J.H. Shrinkage property of bentonite-sand mixtures as influenced by sand content and water salinity. Constr. Build. Mater. 2019, 224, 78–88. [Google Scholar] [CrossRef]
- Watabe, Y.; Yamada, K.; Saitoh, K. Hydraulic conductivity and compressibility of mixtures of Nagoya clay with sand or bentonite. Geotechnique 2011, 61, 211–219. [Google Scholar] [CrossRef]
- Behroozmand, A.A.; Keating, K.; Auken, E. A review of the principles and applications of the NMR technique for near-surface characterization. Surv. Geophys. 2014, 36, 27–85. [Google Scholar] [CrossRef]
- Li, J.J.; Yin, J.X.; Zhang, Y.N.; Lu, S.F.; Wang, W.M.; Li, J.B.; Chen, F.W.; Meng, Y.L. A comparison of experimental methods for describing shale pore features—A case study in the Bohai Bay Basin of Eastern China. Int. J. Coal Geol. 2015, 152, 39–49. [Google Scholar] [CrossRef]
- Lyu, C.H.; Ning, Z.F.; Wang, Q.; Chen, M.Q. Application of NMR T2 to pore size distribution and movable fluid distribution in tight sandstones. Energy Fuels 2018, 32, 1395–1405. [Google Scholar] [CrossRef]
- Yao, Y.B.; Liu, D.M.; Che, Y.; Tang, D.Z.; Tang, S.H.; Huang, W.H. Petrophysical characterization of coals by low-field nuclear magnetic resonance (NMR). Fuel 2010, 89, 1371–1380. [Google Scholar] [CrossRef]
- Liu, M.; Xie, R.H.; Li, C.X.; Gao, L. A new method for determining tight sandstone permeability based on the characteristic parameters of the NMR T2 distribution. Appl. Magn. Reson. 2017, 48, 1009–1029. [Google Scholar] [CrossRef]
- Tan, M.J.; Mao, K.Y.; Song, X.D.; Yang, X.; Xu, J.J. NMR petrophysical interpretation method of gas shale based on core NMR experiment. J. Pet. Sci. Eng. 2015, 136, 100–111. [Google Scholar] [CrossRef]
- Yao, Y.B.; Liu, D.M.; Liu, J.G.; Xie, S.B. Assessing the water migration and permeability of large intact bituminous and anthracite coals using NMR relaxation spectrometry. Transp. Porous Med. 2015, 107, 527–542. [Google Scholar] [CrossRef]
- Ji, X.F.; Song, D.Y.; Zhao, H.T.; Li, Y.B.; He, K.K. Experimental analysis of pore and permeability characteristics of coal by low-field NMR. Appl. Sci. 2018, 8, 1374. [Google Scholar] [CrossRef]
- Coates, G.R.; Xiao, L.; Prammer, M.G. NMR Logging: Principles and Applications; Gulf Publishing Company: Houston, TX, USA, 1999. [Google Scholar]
- Kenyon, W.; Day, P.; Straley, C.; Willemsen, J. A three-part study of NMR longitudinal relaxation properties of water-saturated sandstones. SPE Form. Eval. 1988, 3, 622–636. [Google Scholar] [CrossRef]
- Timur, A. An investigation of permeability, porosity, & residual water saturation relationships for sandstone reservoirs. Log. Anal. 1968, 9, 10. [Google Scholar]
- Li, A.; Ding, W.L.; Wang, R.Y.; He, J.H.; Wang, X.H.; Sun, Y.X.; Gu, Y.; Jiao, N.L. Petrophysical characterization of shale reservoir based on nuclear magnetic resonance (NMR) experiment: A case study of Lower Cambrian Qiongzhusi Formation in Eastern Yunnan Province, South China. J. Nat. Gas Sci. Eng. 2017, 37, 29–38. [Google Scholar] [CrossRef]
- Xiao, L.; Li, J.R.; Mao, Z.Q.; Lu, J.; Yu, H.Y.; Guo, H.P.; Li, G.R. A method to determine nuclear magnetic resonance (NMR) T2 cutoff based on normal distribution simulation in tight sandstone reservoirs. Fuel 2018, 225, 472–482. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Lebedev, M.; Al-Yaseri, A.; Yu, H.Y.; Xu, X.M.; Iglauer, S. Characterization of nanoscale rockmechanical properties and microstructures of a Chinese sub-bituminous coal. J. Nat. Gas Sci. Eng. 2018, 52, 106–116. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Y.B.; Liu, D.M.; Zheng, S.J.; Sun, G.X.; Chang, Y.H. Shale pore size classification: An NMR fluid typing method. Mar. Pet. Geol. 2018, 96, 591–601. [Google Scholar] [CrossRef]
- Testamanti, M.N.; Rezaee, R. Determination of NMR T2 cut-off for clay bound water in shales: A case study of Carynginia Formation, Perth basin, Western Australia. J. Pet. Sci. Eng. 2017, 149, 497–503. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Rezaee, R.; Verrall, M.; Hu, S.Y.; Zou, J.; Testmanti, N. Pore characterization and clay bound water assessment in shale with a combination of NMR and low-pressure nitrogen gas adsorption. Int. J. Coal Geol. 2018, 194, 11–21. [Google Scholar] [CrossRef]
- Tang, C.S.; Shi, B.; Liu, C.; Gao, L.; Inyang, H.I. Experimental investigation of the desiccation cracking behavior of soil layers during drying. J. Mater. Civ. Eng. 2011, 23, 873–878. [Google Scholar] [CrossRef]
- Tang, C.S.; Shi, B.; Liu, C.; Suo, W.B.; Gao, L. Experimental characterization of shrinkage and desiccation cracking in thin clay layer. Appl. Clay Sci. 2011, 52, 69–77. [Google Scholar] [CrossRef]
- Ge, X.M.; Fan, Y.R.; Zhu, X.J.; Chen, Y.G.; Li, R.Z. Determination of nuclear magnetic resonance T2 cutoff value based on multifractal theory—An application in sandstone with complex pore structure. Geophysics 2015, 80, D11–D21. [Google Scholar] [CrossRef]
- Ouyang, Z.Q.; Liu, D.M.; Cai, Y.D.; Yao, Y.B. Fractal analysis on heterogeneity of pore–fractures in middle–high rank coals with NMR. Energy Fuels 2016, 30, 5449–5458. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Rezaee, R. Fractal analysis of the pore structure for clay bound water and potential gas storage in shales based on NMR and N2 gas adsorption. J. Pet. Sci. Eng. 2019, 177, 756–765. [Google Scholar] [CrossRef]
- Zheng, S.J.; Yao, Y.B.; Liu, D.M.; Cai, Y.D.; Liu, Y. Characterizations of full-scale pore size distribution, porosity and permeability of coals: A novel methodology by nuclear magnetic resonance and fractal analysis theory. Int. J. Coal Geol. 2018, 196, 148–158. [Google Scholar] [CrossRef]
- Zheng, S.J.; Yao, Y.B.; Liu, D.M.; Cai, Y.D.; Liu, Y.; Li, X.W. Nuclear magnetic resonance T2 cutoffs of coals: A novel method by multifractal analysis theory. Fuel 2019, 241, 715–724. [Google Scholar] [CrossRef]
- Zhou, L.; Kang, Z.H. Fractal characterization of pores in shales using NMR: A case study from the Lower Cambrian Niutitang Formation in the Middle Yangtze Platform, Southwest China. J. Nat. Gas Sci. Eng. 2016, 35, 860–872. [Google Scholar] [CrossRef]
- Filgueira, R.R.; Fournier, L.L.; Cerisola, C.I.; Gelati, P.; Garcia, M.G. Particle-size distribution in soils: A critical study of the fractal model validation. Geoderma 2006, 134, 327–334. [Google Scholar] [CrossRef]
- Lipiec, J.; Hatano, R.; Slowinska-Jurkiewicz, A. The fractal dimension of pore distribution patterns in variously-compacted soil. Soil Tillage Res. 1998, 47, 61–66. [Google Scholar] [CrossRef]
- Peng, G.; Xiang, N.; Lv, S.Q.; Zhang, G.Q. Fractal characterization of soil particle-size distribution under different land-use patterns in the Yellow River Delta Wetland in China. J. Soils Sediments 2014, 14, 1116–1122. [Google Scholar] [CrossRef]
- Xu, Y.F.; Sun, D.A. A fractal model for soil pores and its application to determination of water permeability. Physica A 2002, 316, 56–64. [Google Scholar] [CrossRef]
- Jaeger, F.; Rudolph-Mohr, N.; Lang, F.; Schaumann, G.E. Effects of soil solution’s constituents on proton NMR relaxometry of soil samples. Soil Sci. Soc. Am. J. 2008, 72, 1694–1707. [Google Scholar] [CrossRef]
- Meyer, M.; Buchmann, C.; Schaumann, G.E. Determination of quantitative pore-size distribution of soils with 1H NMR relaxometry. Eur. J. Soil Sci. 2018, 69, 393–406. [Google Scholar] [CrossRef]
- Tian, H.H.; Wei, C.F.; Wei, H.Z.; Zhou, J.Z. Freezing and thawing characteristics of frozen soils: Bound water content and hysteresis phenomenon. Cold Reg. Sci. Technol. 2014, 103, 74–81. [Google Scholar] [CrossRef]
- Tao, G.L.; Chen, Y.; Kong, L.W.; Xiao, H.L.; Chen, Q.S.; Xia, Y.X. A simple fractal-based model for soil-water characteristic curves incorporating effects of initial void ratios. Energies 2018, 11, 1419. [Google Scholar] [CrossRef]
- Tao, G.L.; Chen, Y.; Xiao, H.L.; Chen, Q.S.; Wan, J. Determining soil-water characteristic curves from mercury intrusion porosimeter test data using fractal theory. Energies 2019, 12, 752. [Google Scholar] [CrossRef]
- Zhang, J.R.; Tao, G.L.; Huang, L.; Yuan, L. Porosity models for determining the pore-size distribution of rocks and soils and their applications. Chin. Sci. Bull. 2010, 55, 3960–3970. [Google Scholar] [CrossRef]
- Tao, G.L.; Zhang, J.R. Two categories of fractal models of rock and soil expressing volume and size-distribution of pores and grains. Chin. Sci. Bull. 2009, 54, 4458–4467. [Google Scholar] [CrossRef]
- Carr, H.Y.; Purcell, E.M. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modifed spin-echo method for measuring nuclear relaxation times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Franzoni, M.B.; Levstein, P.R. Manifestations of the absence of spin diffusion in multipulse NMR experiments on diluted dipolar solids. Phys. Rev. B 2005, 72, 235410. [Google Scholar] [CrossRef]
- Petrova, M.V.; Doktorov, A.B.; Lukzen, N.N. CPMG echo amplitudes with arbitrary refocusing angle: Explicit expressions, asymptotic behavior, approximations. J. Magn. Reson. 2011, 212, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, F.; Bowe, S.; As, H.V.; Schaumann, G.E. Evaluation of 1H NMR relaxometry for the assessment of pore size distribution in soil samples. Eur. J. Soil Sci. 2009, 60, 1052–1064. [Google Scholar] [CrossRef]
- Kong, L.W.; Sayem, H.M.; Tian, H.H. Influence of drying-wetting cycles on soil-water characteristic curve of undisturbed granite residual soils and microstructure mechanism by nuclear magnetic resonance (NMR) spin-spin relaxation time (T2) relaxometry. Can. Geotech. J. 2018, 55, 208–216. [Google Scholar] [CrossRef]
- Stingaciu, L.R.; Weihermüller, L.; Haber-Pohlmeier, S.; Stapf, S.; Vereecken, H.; Pohlmeier, A. Determination of pore size distribution and hydraulic properties using nuclear magnetic resonance relaxometry: A comparative study of laboratory methods. Water Resour. Res. 2010, 46, W11510. [Google Scholar] [CrossRef]
- Song, Y.Q.; Venkataramanan, L.; Hurlimann, M.D.; Flaum, M.; Frulla, P.; Straley, C. T1-T2 correlation spectra obtained using a fast two-dimensional laplace inversion. J. Magn. Reson. 2002, 154, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Dines, K.A.; Lytle, R.J. Computerized geophysical tomography. Proc. IEEE 1979, 67, 1065–1073. [Google Scholar] [CrossRef]
- Keating, K.; Falzone, S. Relating nuclear magnetic resonance relaxation time distributions to void-size distributions for unconsolidated sand packs. Geophysics 2013, 78, D461–D472. [Google Scholar] [CrossRef]
- Prammer, M.; Drack, E.; Bouton, J.; Gardner, J.; Coates, G.; Chandler, R.; Miller, M. Measurements of clay-bound water and total porosity by magnetic resonance logging. Log. Anal. 1996, 37, 22. [Google Scholar]




| Property. | Materials | ||
|---|---|---|---|
| Quartz | Kaolinite | Montmorillonite | |
| Atterberg limits | |||
| Plastic limit (%) | − | 32.00 | 61.34 |
| Liquid limit (%) | − | 68.99 | 178.65 |
| Plasticity index | − | 36.99 | 117.31 |
| Grain size distribution | |||
| Clay (%;<0.005 mm) | 1.63 | 70.23 | 84.10 |
| Silt (%; 0.005–0.075 mm) | 4.15 | 29.77 | 15.90 |
| Sand (%; >0.075 mm) | 94.22 | 0 | 0 |
| Chemical composition | |||
| SiO2 (%) | 98.85 | 57.82 | 66.63 |
| Al2O3 (%) | 0.57 | 35.24 | 16.07 |
| K2O (%) | − | 3.89 | − |
| Fe2O3 (%) | 0.08 | 1.86 | 6.50 |
| CaO (%) | 0.18 | − | 3.99 |
| MgO (%) | − | 0.37 | 5.32 |
| Specific gravity | 2.68 | 2.76 | 2.61 |
| Classification | − | CH | CH |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Sha, A.; Wang, W. Permeability Evaluation of Clay-quartz Mixtures Based on Low-Field NMR and Fractal Analysis. Appl. Sci. 2020, 10, 1585. https://doi.org/10.3390/app10051585
Lu Z, Sha A, Wang W. Permeability Evaluation of Clay-quartz Mixtures Based on Low-Field NMR and Fractal Analysis. Applied Sciences. 2020; 10(5):1585. https://doi.org/10.3390/app10051585
Chicago/Turabian StyleLu, Zhen, Aimin Sha, and Wentong Wang. 2020. "Permeability Evaluation of Clay-quartz Mixtures Based on Low-Field NMR and Fractal Analysis" Applied Sciences 10, no. 5: 1585. https://doi.org/10.3390/app10051585
APA StyleLu, Z., Sha, A., & Wang, W. (2020). Permeability Evaluation of Clay-quartz Mixtures Based on Low-Field NMR and Fractal Analysis. Applied Sciences, 10(5), 1585. https://doi.org/10.3390/app10051585




