Modification of Interfacial Interactions in Ceramic-Polymer Nanocomposites by Grafting: Morphology and Properties for Powder Injection Molding and Additive Manufacturing
Abstract
1. Introduction
2. Materials and Methods
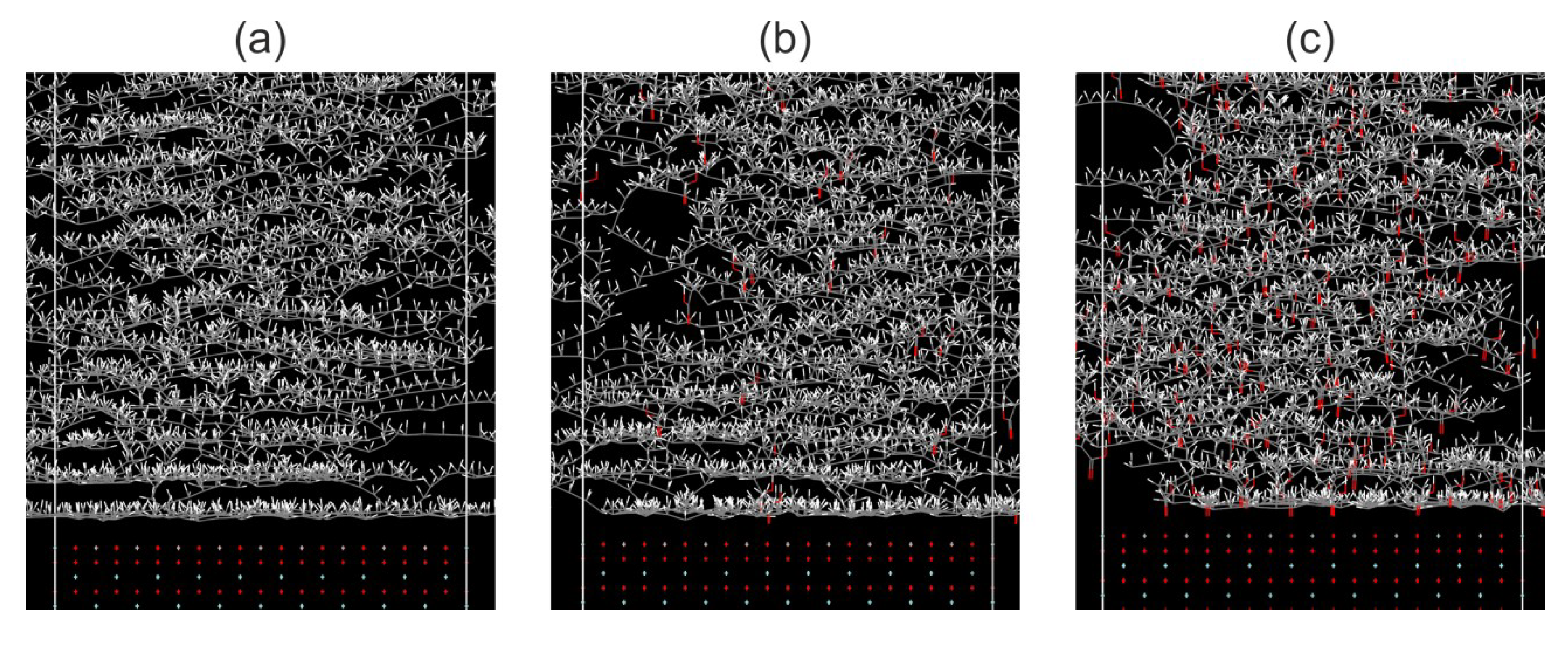
2.1. Molecular Dynamics Simulation
2.2. Materials
2.3. Preparation of the Compounds
2.4. Calculation of the Interfacial Tension by Contact Angle Measurements
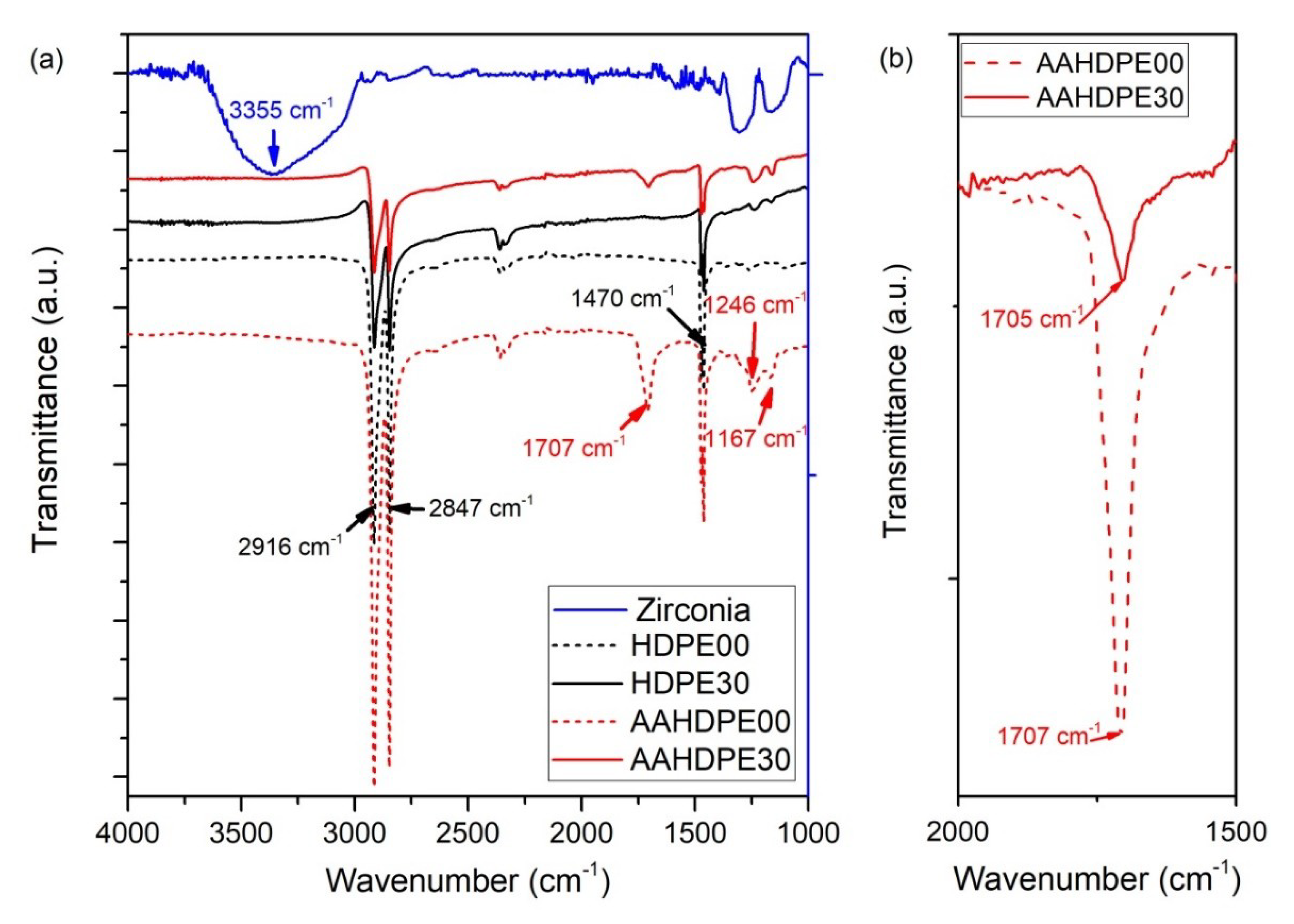
2.5. Attenuated Total Reflection Spectroscopy
2.6. Rheological Evaluation
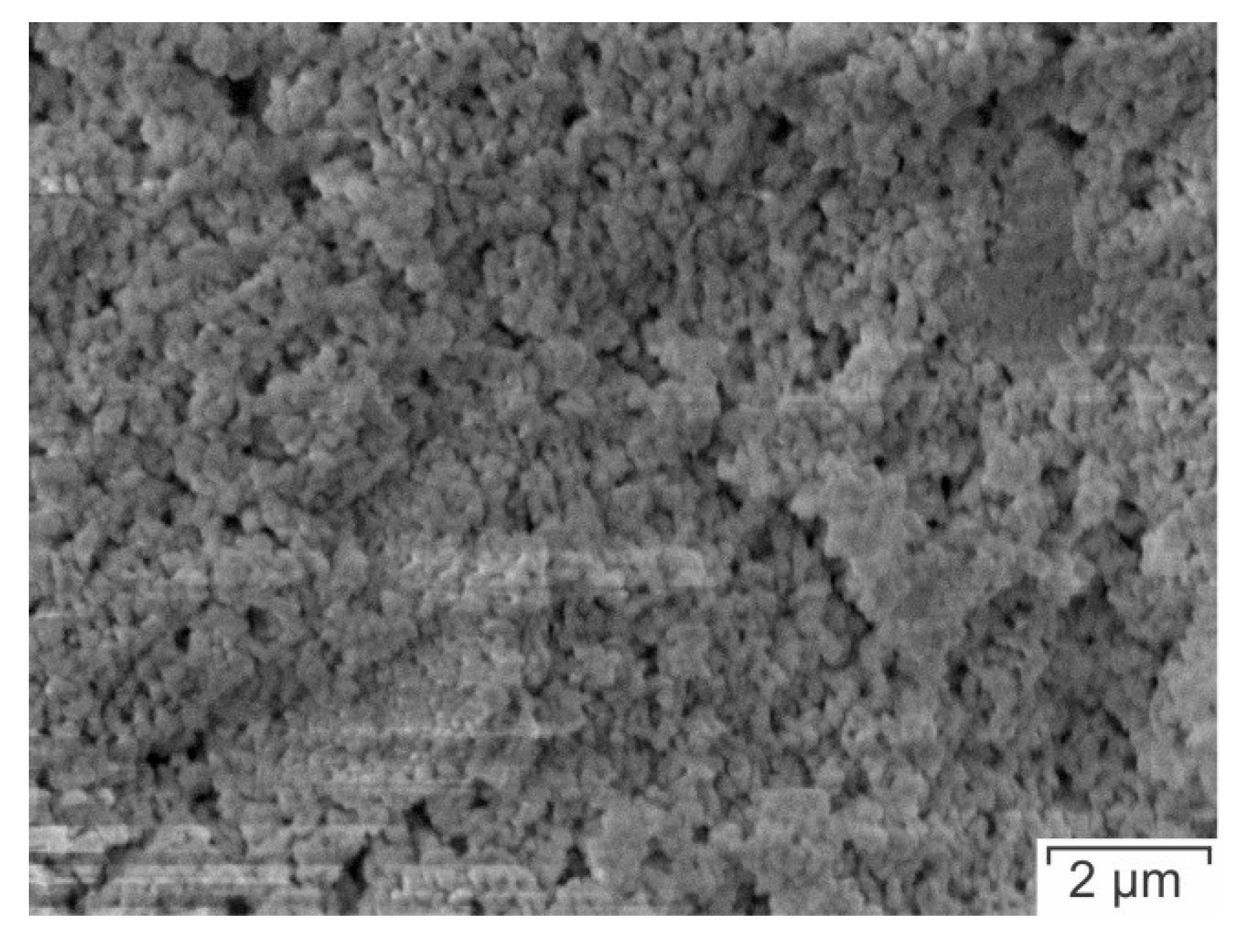
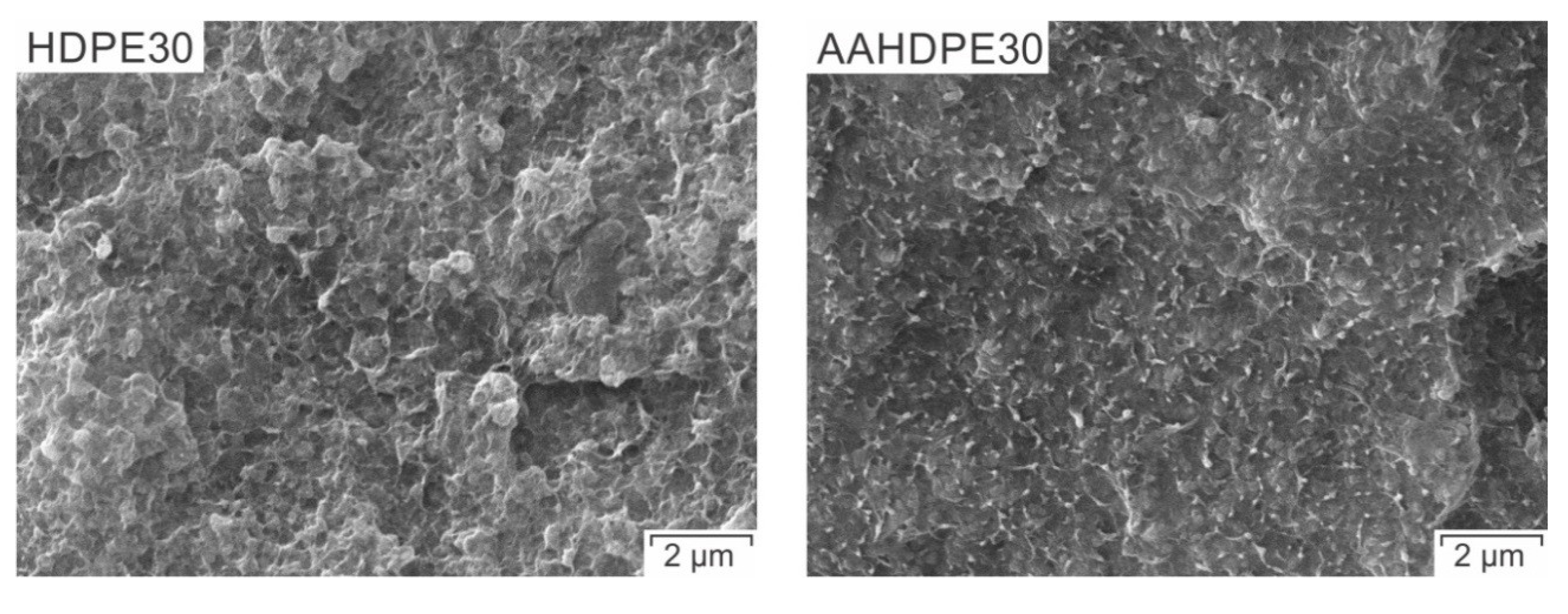
2.7. Morphology Analyses
2.8. Tensile Tests
2.9. Differential Scanning Calorimetry
3. Results and Discussion
3.1. MD Simulations
3.2. Calculation of the Interfacial Tension by Contact Angle Measurements
3.3. Attenuated Total Reflection Spectroscopy
3.4. Morphology
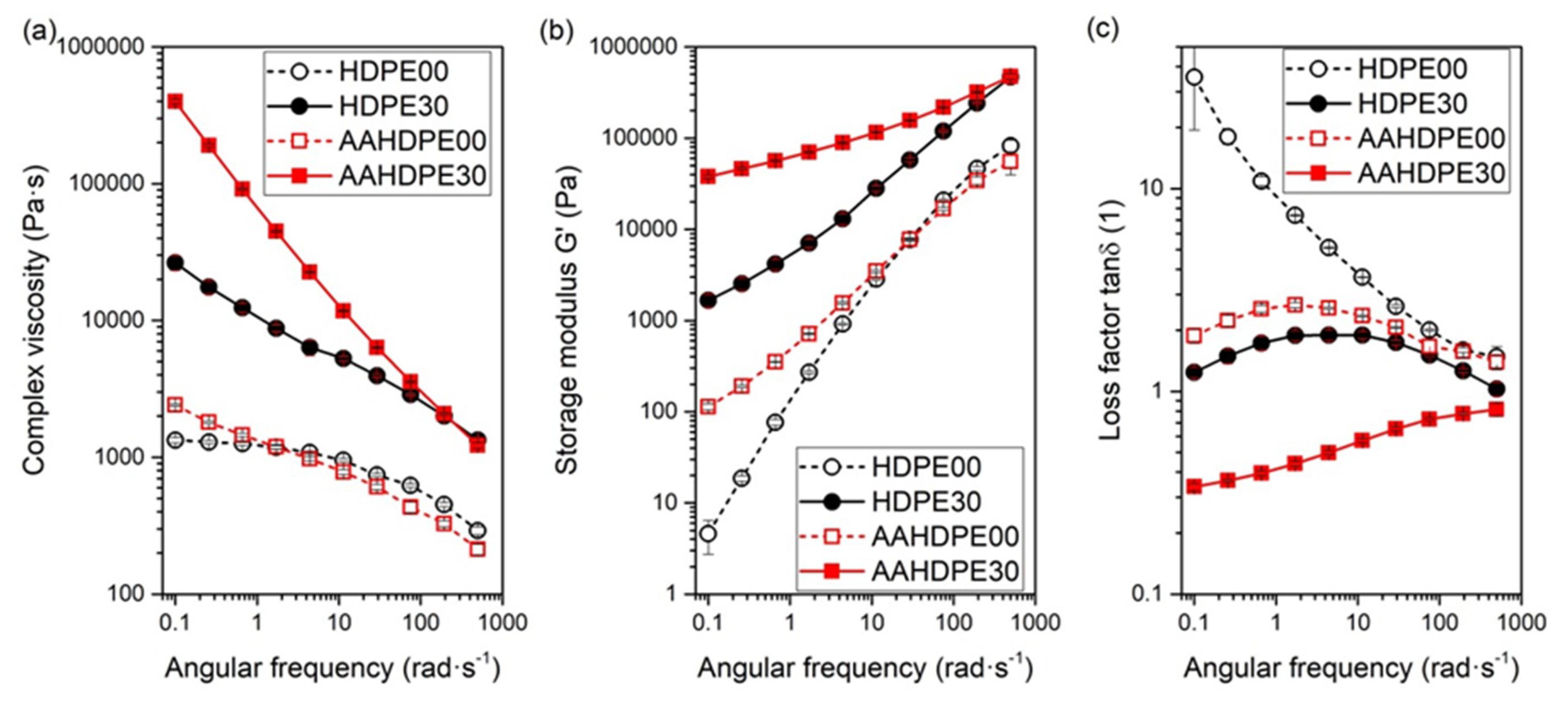
3.5. Viscoelastic Properties
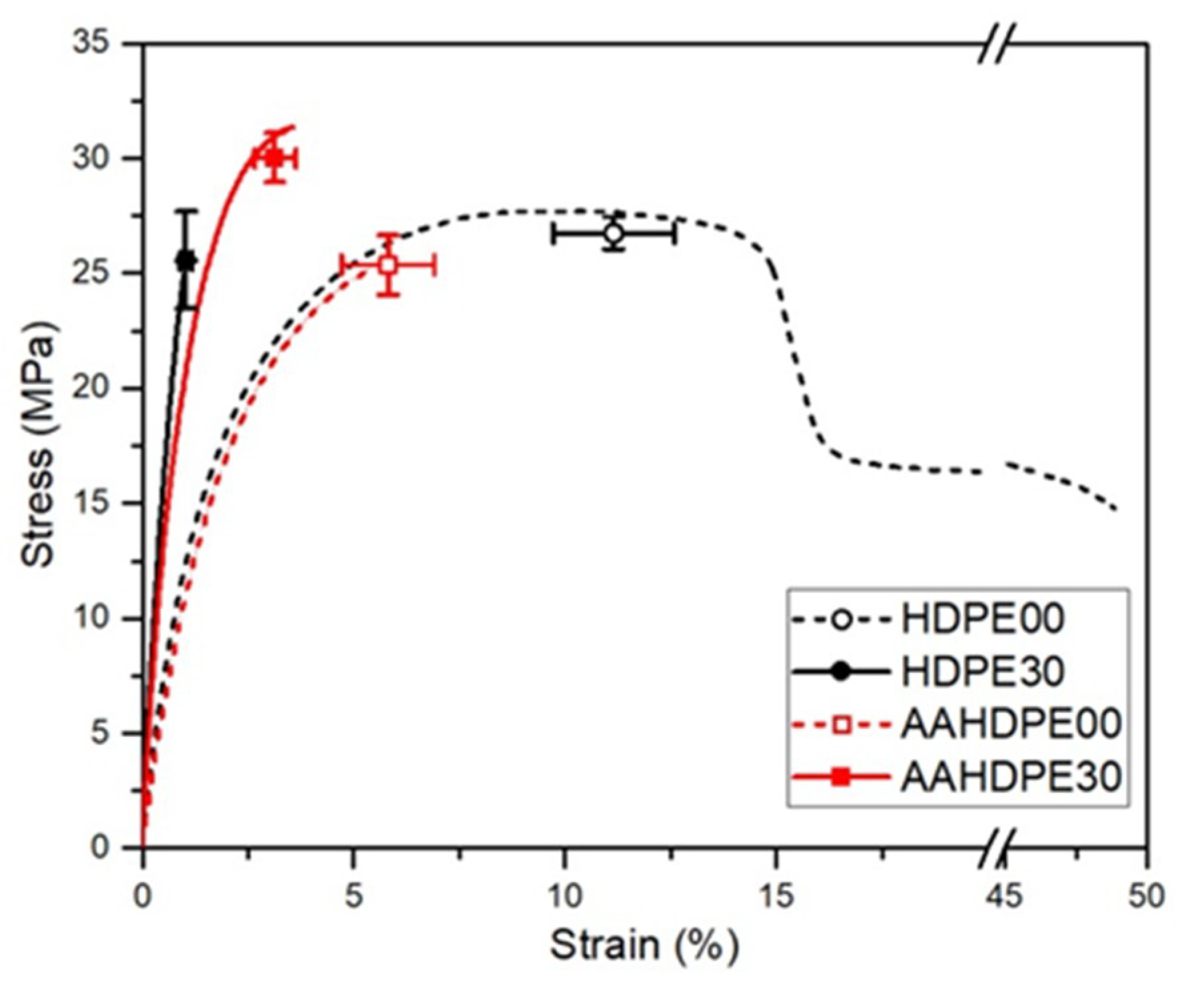
3.6. Tensile Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dziadek, M.; Stodolak-Zych, E.; Cholewa-Kowalska, K. Biodegradable ceramic-polymer composites for biomedical applications: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1175–1191. [Google Scholar] [CrossRef]
- Park, H.-J.; Kwak, S.-Y.; Kwak, S. Wear-Resistant Ultra High Molecular Weight Polyethylene/Zirconia Composites Prepared by in situ Ziegler-Natta Polymerization. Macromol. Chem. Phys. 2005, 206, 945–950. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Zhan, C.; Yu, G.; Lu, Y.; Wang, L.; Wujcik, E.; Wei, S. Conductive polymer nanocomposites: A critical review of modern advanced devices. J. Mater. Chem. C 2017, 5, 1569–1585. [Google Scholar] [CrossRef]
- Hippi, U.; Mattila, J.; Korhonen, M.; Seppälä, J.V. Compatibilization of polyethylene/aluminum hydroxide (PE/ATH) and polyethylene/magnesium hydroxide (PE/MH) composites with functionalized polyethylenes. Polymer 2003, 44, 1193–1201. [Google Scholar] [CrossRef]
- Wen, J.-x.; Zhu, T.-b.; Xie, Z.-p.; Cao, W.-b.; Liu, W. A strategy to obtain a high-density and high-strength zirconia ceramic via ceramic injection molding by the modification of oleic acid. Int. J. Miner. Metall. Mater. 2017, 24, 718–725. [Google Scholar] [CrossRef]
- Auscher, M.-C.; Fulchiron, R.; Fougerouse, N.; Périé, T.; Cassagnau, P. Zirconia based feedstocks: Influence of particle surface modification on the rheological properties. Ceram. Int. 2017, 43, 16950–16956. [Google Scholar] [CrossRef]
- Hanemann, T.; Heldele, R.; Mueller, T.; Hausselt, J. Influence of Stearic Acid Concentration on the Processing of ZrO2-Containing Feedstocks Suitable for Micropowder Injection Molding. Int. J. Appl. Ceram. Technol. 2011, 8, 865–872. [Google Scholar] [CrossRef]
- Lin, S.T.; German, R.M. Interaction between binder and powder in injection moulding of alumina. J. Mater. Sci. 1994, 29, 5207–5212. [Google Scholar] [CrossRef]
- McNulty, T.F.; Shanefield, D.J.; Danforth, S.C.; Safari, A. Dispersion of Lead Zirconate Titanate for Fused Deposition of Ceramics. J. Am. Ceram. Soc. 1999, 82, 1757–1760. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive Manufacturing of Metallic and Ceramic Components by the Material Extrusion of Highly-Filled Polymers: A Review and Future Perspectives: A Review and Future Perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Cano, S.; Gonzalez-Gutierrez, J.; Sapkota, J.; Spoerk, M.; Arbeiter, F.; Schuschnigg, S.; Holzer, C.; Kukla, C. Additive manufacturing of zirconia parts by fused filament fabrication and solvent debinding: Selection of binder formulation. Addit. Manuf. 2019, 26, 117–128. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Ruan, W.H. Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: A review. Mater. Sci. Technol. 2006, 22, 787–796. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Krentz, T.; Natarajan, B.; Neely, T.; Schadler, L.S. Polymer Nanocomposite Interfaces: The Hidden Lever for Optimizing Performance in Spherical Nanofilled Polymers. In Interface/Interphase in Polymer Nanocomposites; Netravali, A.N., Mittal, K.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–69. [Google Scholar]
- Gooneie, A.; Hufenus, R. Polymeric Solvation Shells around Nanotubes: Mesoscopic Simulation of Interfaces in Nanochannels. Macromolecules 2019, 52, 8803–8813. [Google Scholar] [CrossRef]
- Drelich, J.; Miller, J.D. Critical review of wetting and adhesion phenomena in the preparation of polymer-mineral composites. Miner. Metall. Process. 1995, 12, 197–204. [Google Scholar] [CrossRef]
- Jenni, M.; Schimmer, L.; Zauner, R.; Stampfl, J.; Morris, J. Quantitative study of powder binder separation of feedstocks. Powder Inject. Mould. Int. 2008, 2, 50–55. [Google Scholar]
- Mannschatz, A.; Höhn, S.; Moritz, T. Powder-binder separation in injection moulded green parts. J. Eur. Ceram. Soc. 2010, 30, 2827–2832. [Google Scholar] [CrossRef]
- Gorjan, L.; Reiff, L.; Liersch, A.; Clemens, F. Ethylene vinyl acetate as a binder for additive manufacturing of tricalcium phosphate bio-ceramics. Ceram. Int. 2018, 44, 15817–15823. [Google Scholar] [CrossRef]
- Fan, N.C.; Chen, Y.Y.; Wei, W.C.J.; Liu, B.H.; Wang, A.B.; Luo, R.C. Porous Al2O3 catalyst carrier by 3D additive manufacturing for syngas reforming. J. Ceram. Process. Res. 2017, 18, 676–682. [Google Scholar]
- Xu, Y.; Fang, Z.; Tong, L. On promoting intercalation and exfoliation of bentonite in high-density polyethylene by grafting acrylic acid. J. Appl. Polym. Sci. 2005, 96, 2429–2434. [Google Scholar] [CrossRef]
- Bula, K.; Jesionowski, T. Effect of Polyethylene Functionalization on Mechanical Properties and Morphology of PE/SiO2 Composites. Compos. Interfaces 2010, 17, 603–614. [Google Scholar] [CrossRef]
- Hoang, T.; Truc, T.A.; Thanh, D.T.M.; Chinh, N.T.; Tham, D.Q.; Trang, N.T.T.; Vu Giang, N.; Lam, V.D. Tensile, rheological properties, thermal stability, and morphology of ethylene vinyl acetate copolymer/silica nanocomposites using EVA-g-maleic anhydride. J. Compos. Mater. 2014, 48, 505–511. [Google Scholar] [CrossRef]
- Wang, Y.; Yeh, F.-C.; Lai, S.-M.; Chan, H.-C.; Shen, H.-F. Effectiveness of functionalized polyolefins as compatibilizers for polyethylene/wood flour composites. Polym. Eng. Sci. 2003, 43, 933–945. [Google Scholar] [CrossRef]
- Minkova, L.; Peneva, Y.; Tashev, E.; Filippi, S.; Pracella, M.; Magagnini, P. Thermal properties and microhardness of HDPE/clay nanocomposites compatibilized by different functionalized polyethylenes. Polym. Test. 2009, 28, 528–533. [Google Scholar] [CrossRef]
- Wongpanit, P.; Khanthsri, S.; Puengboonsri, S.; Manonukul, A. Effects of acrylic acid-grafted HDPE in HDPE-based binder on properties after injection and debinding in metal injection molding. Mater. Chem. Phys. 2014, 147, 238–246. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Vassiliou, A.; Pavlidou, E.; Karayannidis, G.P. Compatibilisation effect of PP-g-MA copolymer on iPP/SiO2 nanocomposites prepared by melt mixing. Eur. Polym. J. 2005, 41, 1965–1978. [Google Scholar] [CrossRef]
- Zhu, A.; Cai, A.; Yu, Z.; Zhou, W. Film characterization of poly(styrene-butylacrylate-acrylic acid)-silica nanocomposite. J. Colloid Interface Sci. 2008, 322, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Rahimi, M.; Müller-Plathe, F. Molecular Dynamics Simulation of a Silica Nanoparticle in Oligomeric Poly(methyl methacrylate): A Model System for Studying the Interphase Thickness in a Polymer–Nanocomposite via Different Properties. Macromolecules 2013, 46, 8680–8692. [Google Scholar] [CrossRef]
- Anastassiou, A.; Mavrantzas, V.G. Molecular Structure and Work of Adhesion of Poly(n -butyl acrylate) and Poly(n -butyl acrylate- co -acrylic acid) on α-Quartz, α-Ferric Oxide, and α-Ferrite from Detailed Molecular Dynamics Simulations. Macromolecules 2015, 48, 8262–8284. [Google Scholar] [CrossRef]
- Gooneie, A.; Gonzalez-Gutierrez, J.; Holzer, C. Atomistic Modelling of Confined Polypropylene Chains between Ferric Oxide Substrates at Melt Temperature. Polymers 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Gooneie, A.; Schuschnigg, S.; Holzer, C. A Review of Multiscale Computational Methods in Polymeric Materials. Polymers 2017, 9, 16. [Google Scholar] [CrossRef]
- Kisin, S.; Božović Vukić, J.; van der Varst, P.G.T.; With, G.d.; Koning, C.E. Estimating the Polymer−Metal Work of Adhesion from Molecular Dynamics Simulations. Chem. Mater. 2007, 19, 903–907. [Google Scholar] [CrossRef]
- Gooneie, A.; Holzer, C. Reinforced local heterogeneities in interfacial tension distribution in polymer blends by incorporating carbon nanotubes. Polymer 2017, 125, 90–101. [Google Scholar] [CrossRef]
- BYK-Chemie GmbH. Technical Data Sheet SCONA TPPE 2400 GAHD; BYK-Chemie GmbH: Wesel, Germany, 2017. [Google Scholar]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef]
- González-Martín, M.L.; Labajos-Broncano, L.; Jańczuk, B.; Bruque, J.M. Wettability and surface free energy of zirconia ceramics and their constituents. J. Mater. Sci. 1999, 34, 5923–5926. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte der Benetzungstheorie und ihre Anwendung auf die Untersuchung und Veränderung der Oberflächeneigenschaften von Polymeren. Farbe Lack 1971, 77, 997–1005. [Google Scholar]
- Spoerk, M.; Gonzalez-Gutierrez, J.; Lichal, C.; Cajner, H.; Berger, G.R.; Schuschnigg, S.; Cardon, L.; Holzer, C. Optimisation of the Adhesion of Polypropylene-Based Materials during Extrusion-Based Additive Manufacturing. Polymers 2018, 10, 490. [Google Scholar] [CrossRef]
- Berger, G.R.; Steffel, C.; Friesenbichler, W. A study on the role of wetting parameters on friction in injection moulding. IJMPT 2016, 52, 193. [Google Scholar] [CrossRef]
- Rueda, M.M.; Auscher, M.-C.; Fulchiron, R.; Périé, T.; Martin, G.; Sonntag, P.; Cassagnau, P. Rheology and applications of highly filled polymers: A review of current understanding. Prog. Polym. Sci. 2017, 66, 22–53. [Google Scholar] [CrossRef]
- Rabinowitsch, B. Über die Viskosität und Elastizität von Solen. Z. Für Phys. Chem. 1929, 145, 1–26. [Google Scholar] [CrossRef]
- Bagley, E.B. End Corrections in the Capillary Flow of Polyethylene. J. Appl. Phys. 1957, 28, 624–627. [Google Scholar] [CrossRef]
- Spoerk, M.; Sapkota, J.; Weingrill, G.; Fischinger, T.; Arbeiter, F.; Holzer, C. Shrinkage and Warpage Optimization of Expanded-Perlite-Filled Polypropylene Composites in Extrusion-Based Additive Manufacturing. Macromol. Mater. Eng. 2017, 12, 1700143. [Google Scholar] [CrossRef]
- Decker, C.; Zahouily, K. Surface modification of polyolefins by photografting of acrylic monomers. Macromol. Symp. 1998, 129, 99–108. [Google Scholar] [CrossRef]
- Cao, Z.; Lei, J.; Zhang, W. Structure and adhesion properties of acrylic acid grafted high-density polyethylene powders synthesized by a novel photografting method. J. Appl. Polym. Sci. 2008, 109, 2316–2320. [Google Scholar] [CrossRef]
- Wang, H.; Brown, H.R. UV grafting of methacrylic acid and acrylic acid on high-density polyethylene in different solvents and the wettability of grafted high-density polyethylene. II. Wettability. J. Polym. Sci. A Polym. Chem. 2004, 42, 263–270. [Google Scholar] [CrossRef]
- Reddy, C.M.; Weikart, C.M.; Yasuda, H.K. The Effect of Interfacial Tension on the Adhesion of Cathodic E-coat to Aluminum Alloys. J. Adhes. 1999, 71, 167–187. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Z.P.; Yang, X.F.; Wu, Y.; Jia, C.; Bo, T.; Wang, L. Surface Modification Mechanism of Stearic Acid to Zirconia Powders Induced by Ball Milling for Water-Based Injection Molding. J. Am. Ceram. Soc. 2011, 94, 1327–1330. [Google Scholar] [CrossRef]
- Merle-Méjean, T.; Barberis, P.; Othmane, S.B.; Nardou, F.; Quintard, P.E. Chemical forms of hydroxyls on/in Zirconia: An FT-IR study. J. Eur. Ceram. Soc. 1998, 18, 1579–1586. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts, 3rd ed.; John Wiley & Sons, Inc.: Chichester, UK, 2001; ISBN 0470093072. [Google Scholar]
- D’Amelia, R.P.; Gentile, S.; Nirode, W.F.; Huang, L. Quantitative Analysis of Copolymers and Blends of Polyvinyl Acetate (PVAc) Using Fourier Transform Infrared Spectroscopy (FTIR) and Elemental Analysis (EA). World J. Chem. Educ. 2016, 4, 25–31. [Google Scholar] [CrossRef]
- Nasreddine, V.; Halla, J.; Reven, L. Conformation of Adsorbed Random Copolymers: A Solid-State NMR and FTIR-PAS Study. Macromolecules 2001, 34, 7403–7410. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, S.; Kim, H.-J.; Yang, H.-S. Thermal properties of bio-flour-filled polyolefin composites with different compatibilizing agent type and content. Thermochim. Acta 2006, 451, 181–188. [Google Scholar] [CrossRef]
- Lai, S.-M.; Yeh, F.-C.; Wang, Y.; Chan, H.-C.; Shen, H.-F. Comparative study of maleated polyolefins as compatibilizers for polyethylene/wood flour composites. J. Appl. Polym. Sci. 2003, 87, 487–496. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Zhang, X.; Shen, J.; Chi, L.; Fuchs, H. A new approach for the fabrication of an alternating multilayer film of poly(4-vinylpyridine) and poly(acrylic acid) based on hydrogen bonding. Macromol. Rapid Commun. 1997, 18, 509–514. [Google Scholar] [CrossRef]
- Du, M.; Guo, B.; Lei, Y.; Liu, M.; Jia, D. Carboxylated butadiene–styrene rubber/halloysite nanotube nanocomposites: Interfacial interaction and performance. Polymer 2008, 49, 4871–4876. [Google Scholar] [CrossRef]
- Mutsuddy, B.C.; Ford, R.G. Ceramic Injection Moulding; Chapman & Hall: London, UK, 1995; ISBN 0412538105. [Google Scholar]
- Laghaei, M.; Sadeghi, M.; Ghalei, B.; Shahrooz, M. The role of compatibility between polymeric matrix and silane coupling agents on the performance of mixed matrix membranes: Polyethersulfone/MCM-41. J. Membr. Sci. 2016, 513, 20–32. [Google Scholar] [CrossRef]
- Lall, P.; Islam, S.; Dornala, K.; Suhling, J.; Shinde, D. Nano-Underfills and Potting Compounds for Fine-Pitch Electronics. In Nanopackaging; Morris, J.E., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 513–574. [Google Scholar]
- Venkataraman, N.; Rangarajan, S.; Matthewson, M.J.; Safari, A.; Danforth, S.C.; Yardimci, A.; Guceri, S.I. Mechanical and Rheological Properties of Feedstock Material for Fused Deposition of Ceramics and Metals (FDC and FDMet) and their Relationship to Process Performance. In Proceedings of the Solid Freeform Fabrication Symposium. Solid Freeform Fabrication Symposium, Austin, TX, USA, 9–11 August 1999. [Google Scholar]
- Malmberg, A.; Gabriel, C.; Steffl, T.; Münstedt, H.; Löfgren, B. Long-Chain Branching in Metallocene-Catalyzed Polyethylenes Investigated by Low Oscillatory Shear and Uniaxial Extensional Rheometry. Macromolecules 2002, 35, 1038–1048. [Google Scholar] [CrossRef]
- Liang, X.-K.; Luo, Z.; Yang, L.; Wei, J.-T.; Yuan, X.; Zheng, Q. Rheological properties and crystallization behaviors of long chain branched polyethylene prepared by melt branching reaction. J. Polym. Eng. 2018, 38, 7–17. [Google Scholar] [CrossRef]
- Brito, G.F.; Xin, J.; Zhang, P.; Mélo, T.J.A.; Zhang, J. Enhanced melt free radical grafting efficiency of polyethylene using a novel redox initiation method. RSC Adv. 2014, 4, 26425. [Google Scholar] [CrossRef]
- Ghosh, P.; Dev, D. Reactive processing of polyethylene: Effect of peroxide-induced graft copolymerization of some acrylic monomers on polymer structure melt rheology and relaxation behavior. Eur. Polym. J. 1998, 34, 1539–1547. [Google Scholar] [CrossRef]
- Sharif-Pakdaman, A.; Morshedian, J.; Jahani, Y. Influence of the silane grafting of polyethylene on the morphology, barrier, thermal, and rheological properties of high-density polyethylene/organoclay nanocomposites. J. Appl. Polym. Sci. 2012, 125, 305–313. [Google Scholar] [CrossRef]
- Küçük, I.; Gevgilili, H.; Kalyon, D.M. Effects of dispersion and deformation histories on rheology of semidilute and concentrated suspensions of multiwalled carbon nanotubes. J. Rheol. 2013, 57, 1491–1514. [Google Scholar] [CrossRef]
- Durmus, A.; Kasgoz, A.; Macosko, C.W. Linear low density polyethylene (LLDPE)/clay nanocomposites. Part I: Structural characterization and quantifying clay dispersion by melt rheology. Polymer 2007, 48, 4492–4502. [Google Scholar] [CrossRef]
- Hanemann, T. Nanoparticle surface polarity influence on the flow behavior of polymer matrix composites. Polym. Compos. 2013, 34, 1425–1432. [Google Scholar] [CrossRef]
- Shang, S.W.; Williams, J.W.; Söderholm, K.-J.M. Work of adhesion influence on the rheological properties of silica filled polymer composites. J. Mater. Sci. 1995, 30, 4323–4334. [Google Scholar] [CrossRef]
- Fisher, I.; Siegmann, A.; Narkis, M. The effect of interface characteristics on the morphology, rheology and thermal behavior of three-component polymer alloys. Polym. Compos. 2002, 23, 34–48. [Google Scholar] [CrossRef]
- Spoerk, M.; Savandaiah, C.; Arbeiter, F.; Sapkota, J.; Holzer, C. Optimization of mechanical properties of glass-spheres-filled polypropylene composites for extrusion-based additive manufacturing. Polym. Compos. 2017, 83, 768. [Google Scholar] [CrossRef]
- Zhang, Q.; Archer, L.A. Poly(ethylene oxide)/Silica Nanocomposites: Structure and Rheology. Langmuir 2002, 18, 10435–10442. [Google Scholar] [CrossRef]
- Carrot, C.; Majesté, J.-C.; Olalla, B.; Fulchiron, R. On the use of the model proposed by Leonov for the explanation of a secondary plateau of the loss modulus in heterogeneous polymer–filler systems with agglomerates. Rheol. Acta 2010, 49, 513–527. [Google Scholar] [CrossRef]
- McNulty, T.F.; Mohammadi, F.; Bandyopadhyay, A.; Shanefield, D.J.; Danforth, S.C.; Safari, A. Development of a binder formulation for fused deposition of ceramics. Rapid Prototyp. J. 1998, 4, 144–150. [Google Scholar] [CrossRef]
- Enneti, R.K.; Onbattuvelli, V.P.; Atre, S.V. Powder Binder Formulation and Compound Manufacture in Metal Injection Molding (MIM). In Handbook of Metal Injection Molding; Heaney, D.F., Ed.; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Miltner, H.E.; Watzeels, N.; Gotzen, N.-A.; Goffin, A.-L.; Duquesne, E.; Benali, S.; Ruelle, B.; Peeterbroeck, S.; Dubois, P.; Goderis, B.; et al. The effect of nano-sized filler particles on the crystalline-amorphous interphase and thermal properties in polyester nanocomposites. Polymer 2012, 53, 1494–1506. [Google Scholar] [CrossRef]
- Gopakumar, T.G.; Lee, J.A.; Kontopoulou, M.; Parent, J.S. Influence of clay exfoliation on the physical properties of montmorillonite/polyethylene composites. Polymer 2002, 43, 5483–5491. [Google Scholar] [CrossRef]

| Polymer | Acrylic Acid Content (mol%) | |
|---|---|---|
| PE | PE | 0 |
| 5AAPE | AA-PE | 5 |
| 30AAPE | AA-PE | 30 |
| Polymer | Acrylic Acid Content (mol%) | Zirconia Content (vol%) | |
|---|---|---|---|
| HDPE00 | HDPE | 0 | 0 |
| HDPE30 | HDPE | 0 | 30 |
| AAHDPE00 | AA-HDPE | >5 | 0 |
| AAHDPE30 | AA-HDPE | >5 | 30 |
| Polymer | Ebinding (105 kcal·mol−1) |
|---|---|
| PE | −4.2997 |
| 5AAPE | −4.8202 |
| 30AAPE | −7.6500 |
| Contact Angle (°) | Surface Energy (mN/m) | ||||
|---|---|---|---|---|---|
| Material | |||||
| AAHDPE00 | 97 | 53 | 32.6 | 0.55 | 33.14 |
| HDPE00 | 99.3 | 54.2 | 31.9 | 0.34 | 32.24 |
| ZrO2 | 71.8 | 40.9 | 39.15 | 6.75 | 45.9 |
| Tetragonal Y2O3 stabilized ZrO2 | 74 | 47 | 35.93 | 6.67 | 42.6 |
| 3%Y2O3-ZrO2 | 66.4 | 40 | 39.61 | 9.14 | 48.75 |
| Interfacial Tension (mN/m) | |||
|---|---|---|---|
| Material | ZrO2 | Tetragonal Y2O3 Stabilized ZrO2 | 3%Y2O3-ZrO2 |
| AAHDPE00 | 3.74 | 3.46 | 5.53 |
| HDPE00 | 4.43 | 4.12 | 6.37 |
| HDPE00 | HDPE30 | AAHDPE00 | AAHDPE30 | |
|---|---|---|---|---|
| UTS (MPa) | 26.75 ± 0.7 | 25.56 ± 2.1 | 25.37 ± 1.3 | 30.01 ± 1.07 |
| εUTS (%) | 11.15 ± 1.43 | 1.03 ± 0.13 | 5.81 ± 1.1 | 3.13 ± 0.49 |
| ES (GPa) | 1.44 ± 0.05 | 3.41 ± 0.13 | 1.33 ± 0.15 | 2.64 ± 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano, S.; Gooneie, A.; Kukla, C.; Rieß, G.; Holzer, C.; Gonzalez-Gutierrez, J. Modification of Interfacial Interactions in Ceramic-Polymer Nanocomposites by Grafting: Morphology and Properties for Powder Injection Molding and Additive Manufacturing. Appl. Sci. 2020, 10, 1471. https://doi.org/10.3390/app10041471
Cano S, Gooneie A, Kukla C, Rieß G, Holzer C, Gonzalez-Gutierrez J. Modification of Interfacial Interactions in Ceramic-Polymer Nanocomposites by Grafting: Morphology and Properties for Powder Injection Molding and Additive Manufacturing. Applied Sciences. 2020; 10(4):1471. https://doi.org/10.3390/app10041471
Chicago/Turabian StyleCano, Santiago, Ali Gooneie, Christian Kukla, Gisbert Rieß, Clemens Holzer, and Joamin Gonzalez-Gutierrez. 2020. "Modification of Interfacial Interactions in Ceramic-Polymer Nanocomposites by Grafting: Morphology and Properties for Powder Injection Molding and Additive Manufacturing" Applied Sciences 10, no. 4: 1471. https://doi.org/10.3390/app10041471
APA StyleCano, S., Gooneie, A., Kukla, C., Rieß, G., Holzer, C., & Gonzalez-Gutierrez, J. (2020). Modification of Interfacial Interactions in Ceramic-Polymer Nanocomposites by Grafting: Morphology and Properties for Powder Injection Molding and Additive Manufacturing. Applied Sciences, 10(4), 1471. https://doi.org/10.3390/app10041471











