Abstract
This study developed a promising approach (low-temperature plasma polymerization with allylamine) to modify the titanium (Ti) surface, which helps the damaged tissue to heal faster. The Ti surface was first cleaned by argon (Ar) plasma, and then the functional amino-groups were coated on the Ti surface via plasma polymerization. The topography characteristics, wettability, and optimal plasma modification parameters were investigated through atomic force spectroscopy, secondary ion mass spectroscopy, and response surface methodology (RSM). Analytical results showed that the formation of a porous surface was found on the Ar plasma-modified Ti surfaces after Ar plasma modification with different parameters. The Ar plasma modification is an effective approach to remove surface contaminants and generate a porous topography on the Ti surface. As the Ti with Ar plasma modification was at 100 W and 190 m Torr for 12 min, the surface exhibited the maximum hydrophilic performance. In the allylamine plasma modifications, the contact angle values of the allylamine plasma-modified Ti surfaces varied between 70.15° and 88.26° in the designed parameters. The maximum concentration of amino-groups (31.58 nmole/cm2) can be obtained from the plasma-polymerized sample at 80 W and 150 mTorr for 22 min. Moreover, the cell response also demonstrated that the allylamine plasma-modified Ti sample with an optimal modification parameter (80 W, 22 min, and 150 mTorr) possessed great potential to increase cell adhesion ability. Thus, the optimal parameters of the low-temperature plasma polymerization with allylamine can be harvested using the RSM design. These data could provide new scientific information in the surface modification of Ti implant.
1. Introduction
Dental implants are designed to restore partial function of a replaced tooth. Generally, only the surface of the implant is in contact with the host tissue, so its material is vital in determining the biological properties [1,2,3,4,5,6]. Thus, improving the surface-modification technique of implants, which can enhance the interface healing between the implant and the bone, is always the key point in dental implant development. In 1969, Dr. Brånemark designed a titanium (Ti) metal cylinder with an optical chamber inside and screwed it into a rabbit’s thighbone. Several months later, it was found that the Ti cylinder was fused to the bone. This phenomenon was named as osseointegration [7]. With rapid development in biomedical research such as cell isolation, molecular biology, cell biology, and biochemistry, cellular and tissue engineering has been recognized as a new field [8]. To better understand the mechanisms of the regulation of bone, more research on long-term observations with osteoblasts and appropriate precursor cells as well as implants made with proactive biomaterial is desired [9]. New technological developments including new medical materials, products, and surgical techniques have greatly promoted clinical technologies. As a new product, dental implants, especially the surface part made with polymeric material, have to be biocompatible with the physiological environment. A previous research indicated that discharge plasma has the potential to be used to sterilize Ti dental implants [10]. Moreover, plasma modification techniques have also been applied to alter Ti surface properties (e.g., topography, chemical composition, and microstructure) to improve its biocompatibility and hemocompatibility [5,11,12,13].
Currently, various polymeric surface modification techniques are widely used in biomaterial research to produce thin polymer coatings [14,15]. It was found that the surface modification technique that deposits a thin plasma-polymerized layer (such as amino-groups) on the material surface can potentially enhance the biocompatibility and became a crucial approach [16,17]. Moreover, different bioactive biomolecules such as growth factors, peptides, proteins, and enzymes can be bonded by amino-groups on the material surface to facilitate cell adhesion and proliferation, and thus the surface biocompatibility of the material can be significantly promoted [18,19,20]. Though studies focusing on the application of plasma techniques were broadly performed, limited information between the plasma polymerization parameters and surface properties is available. As a result, the present study was to develop a promising plasma modification approach (low-temperature plasma polymerization with allylamine) to fabricate a functional coating (amino-groups) on the surface of Ti and investigate its surface characteristics and optimal plasma modification parameters.
2. Materials and Methods
2.1. Preparation of the Investigated Samples
The grade II Ti plates (99.7% purity, 10 mm in diameter, 2 mm in thickness) were ground by SiC metallographic papers (600–2000 grits), and then were polished with a SiO2 solution (particle size of 0.03 mm). The polished samples were immersed in a solution of acetone for 5 min and cleaned with distilled water for 20 min. Hereafter, the samples were modified with argon (Ar) plasma, then coated with allylamine through plasma polymerization in the same reactor. The Ar-plasma cleaning procedure was executed at 60, 80, 100, 120, and 140 W. The Ar flow rate was kept at 20 sccm. To understand the outcome of different modification parameters, the plasma was modified at 2, 7, 12, 17, and 22 min, and the working pressure was set in serial values (100, 145, 190, 225, and 280 mTorr). The plasma-modified parameters including radio frequency (RF) power (W), working pressure (mTorr), and modification duration (min) are listed in Table 1. After the Ar plasma cleaning, the samples were immediately modified by plasma polymerization with allylamine. A concentration of 5% allylamine was used as the process reagent. The optimal operating conditions of plasma polymerization deposition were studied using the response surface methodology (RSM, Softthome design expert software, Taipei, Taiwan). The plasma-polymerized parameters such as RF power, working pressure, and plasma deposition time are listed in Table 2.

Table 1.
Ar plasma modification parameters by the RSM.

Table 2.
Allylamine plasma modification parameters by the RSM.
2.2. Surface Properties Analysis
The superficial and cross-sectional characteristics of the modified samples were observed using a JEOL JSM-6500F scanning electron microscope (SEM; Tokyo, Japan) with an operating voltage of 15 kV and a JEOL JSM-2100 transmission electron microscope (TEM; Tokyo, Japan) with an operating voltage of 200 kV, respectively. The Vecco Nanoscope III D5000 atomic force microscope (AFM, Plainview, NY, USA) with a scanning probe of Si was also employed to examine the surface characteristics. The sample was scanned with a high-resolution of 512 scans at 1 Hz scanning rate under the tapping mode. The elemental compositions of the surfaces were detected by secondary ion mass spectroscopy (SIMS; CAMECA IMS-4f, Gennevilliers Cedex, France). In the SIMS experiment, the primary Ar ion beam was pulsed to obtain good mass resolution. The impact energy of 3 keV and ion etching rate of 0.05 nm·s−1 were used to gain the depth profiling information in the etching area of 2 mm × 2 mm. The wettability of the investigated samples with different parameters (Table 1 and Table 2) was simulated and analyzed through the RSM. Input the vital factors (power, time, and pressure) for characterizing the interactions, and then visualize the response surface from all angles with 2D graphs and rotatable 3D plots. Finally, the optimal operating parameter can be gained from the RSM analysis results.
2.3. Biocompatibility Evaluation
The polished Ti, Ar plasma-modified Ti, and allylamine plasma-modified Ti samples were subjected to cell culture in order to evaluate the adhesion and proliferation behaviors of the osteoblast-like cells (MG-63). Before conducting the biocompatibility experiments, the tested samples were sterilized in a sterilizer with ethylene oxide (3M 8XL, Saint Paul, MN, USA). Afterward, the tested samples were moved to the 24-well polystyrene plate. The Dulbecco’s modified Eagle’s medium (Gibco, Waltham, MA, USA) contained streptomycin (100 mg/mL), penicillin (100 units/mL), and fetal bovine serum (10%) was adopted as the culture medium. A density of 1 × 104 per 100 μL cell suspension was pipetted to each well. Hereafter, the tested samples were incubated at 37 °C for 8 h, one day, three days, and five days under a humidified atmosphere of 5% CO2 and 95% air, respectively. Finally, the MG-63 cells on the tested samples were visualized via the JEOL JSM-6500F SEM at various magnifications to evaluate their adhesion morphology features.
3. Results and Discussion
3.1. Characteristics of the Ar Plasma-Modified Ti Samples
Figure 1 illustrates the SEM micrographs of the polished Ti sample and Ar plasma-modified Ti sample (100 W, 12 min, and 190 mTorr). Obviously, the surface of the polished Ti sample showed some remaining polishing tracks (Figure 1a). After the Ar plasma modification, the sample revealed a flat surface with an ion bombarded feature as shown in Figure 1b. From a higher magnification observation by AFM (Figure 2), there were pores on the surface (as indicated by arrows), which indicated that Ar plasma modification is an effective method to generate porous topography on the Ti surface. Similar topography characteristics could also be found in other Ar plasma-modified Ti surfaces with different parameters (Table 1). The topography became slightly rougher as the plasma modification duration and power increased. This result was also similar to a previous study that demonstrated that under an Ar atmosphere with plasma spray, graded porous Ti coatings were deposited [21]. The SIMS spectra obtained from the Ti surface modified with Ar plasma (100 W, 12 min, and 190 mTorr) and without Ar plasma are shown in Figure 3. The native Ti oxide layer of ~13 nm was formed on the Ti surface without Ar plasma modification (Figure 3a). After Ar plasma modification, it was found that Ar plasma modification can remove residues possibly left from other procedures (Figure 3b), thus effectively producing purer and expected surfaces compared to materials prepared by other traditional methods [22]. Under different designed plasma parameters, similar SIMS spectrum features could also be detected on the modified Ti surfaces. The formation of a clean and porous surface can be attributed to when all the Ar atoms are ionized, most of the Ar ions react with the Ti material through ion bombardment by Ar plasma.
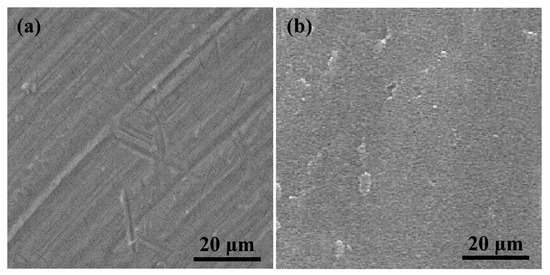
Figure 1.
SEM micrographs of (a) the polished Ti sample and (b) Ar plasma-modified Ti sample (100 W, 12 min, and 190 mTorr).
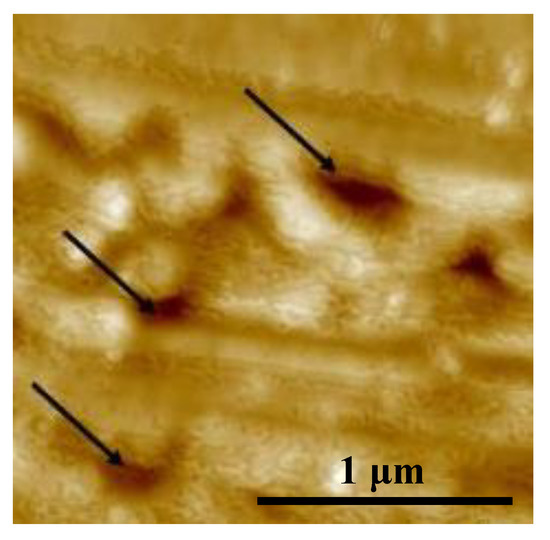
Figure 2.
AFM image of the Ar plasma-modified Ti sample (100 W, 12 min, and 190 mTorr).
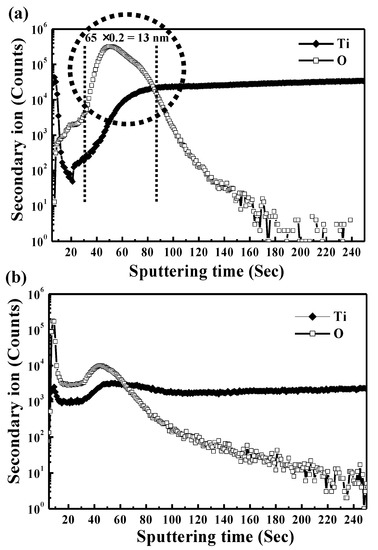
Figure 3.
SIMS spectra obtained from the Ti surface modified with (a) Ar plasma (100 W, 12 min, and 190 mTorr) and (b) without Ar plasma.
Figure 4 presents the RSM analysis result (contact angle) of the Ar plasma-modified Ti sample. As the Ti surface was modified at power 100 W, time 12 min, and chamber pressure 190 mTorr, the minimum contact angle of 14.50° was achieved, and the surface had the maximum hydrophilic property. However, it was found that the contact angle of the Ti surfaces is not proportional to the setting factors of power, time, and pressure in Ar plasma cleaning modification, according to the RSM analysis results. The Ar plasma cleaning process can obtain surfaces with a lower contact angle. The feature reveals that the wettability property of biomaterial surfaces can be improved by a controlled degree of surface cleaning. In a previous study, the contribution of polar interactions to the solid–liquid interface interaction energy increased with the increase in the glassy phase content of the oxide, resulting in a decrease in the measured contact angle [23]. Wettability should be considered when choosing dental implant biomaterials. Cell and tissue adhesion can be affected by the wettability performance of implants after implantation [24,25].
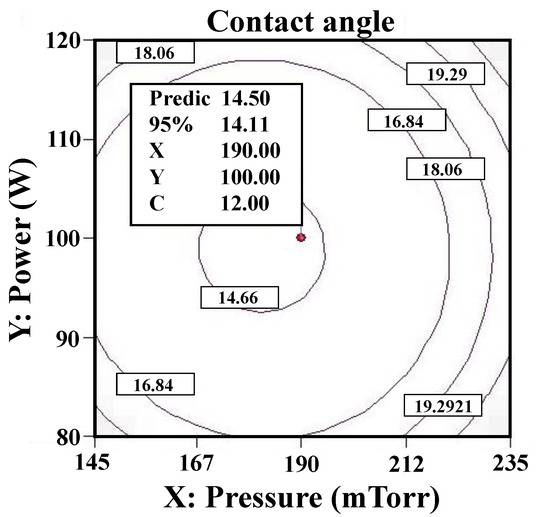
Figure 4.
The RSM analysis result (contact angle) of the Ar plasma-modified Ti sample (100 W, 12 min, and 190 mTorr).
3.2. Properties of the Allylamine Plasma-Modified Ti Samples
Figure 5 displays the RSM analysis results (contact angle) of the allylamine plasma-modified Ti sample. When the Ti was plasma-polymerized at power 80 W, deposition time 22 min, and chamber pressure 150 mTorr, the minimum contact angle of 70.15° was reached. Based on the RSM analysis results, it was found that the power, time, and pressure factors were proportional to the contact angle of the allylamine deposition surfaces during the allylamine plasma modification while deposition time had more of an influence than power and pressure within the parameters designed in this study. The contact angle values of the allylamine plasma-modified Ti surfaces varied between 70.15° and 88.26° in the designed parameters. Figure 6 shows the AFM image (1 μm × 1 μm) of the allylamine plasma-modified sample at 80 W and 150 mTorr for 22 min. It presented an island-like surface with amino-group deposition. From a higher magnification of the cross-sectional view by TEM (Figure 7), there was a formation of an amino-group layer with a thickness of ~21.4 nm on the Ti surface after this optimal parameter modification. Evidently, the RSM analysis results indicated that the allylamine plasma-modified Ti surfaces exhibited surface hydrophilicity, because their contact angle values were smaller than 90° [26,27]. The amino-groups as a functional surface is a high-energy one, and hence could be fully wetted by most liquids [28]. The wettability reflects the surface binding energy of the Ti interface between the bone cells. A surface with a hydrophilic property could facilitate cell adhesion, proliferation, and bone tissue healing [29]. Afterward, it promoted osseointegration of the interface between the implants and bone tissue [6].
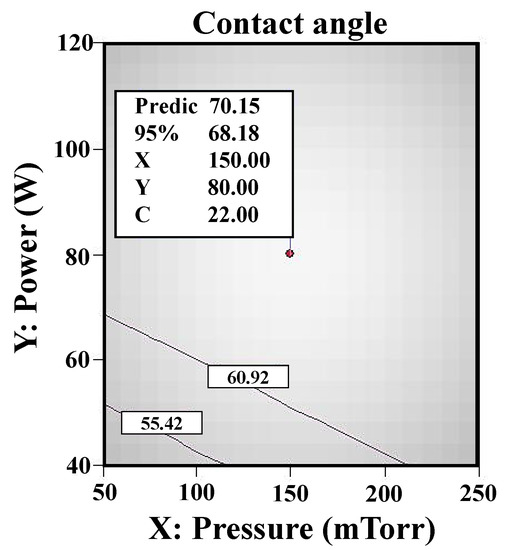
Figure 5.
The RSM analysis results (contact angle) of the allylamine plasma-modified Ti sample (80 W, 22 min, and 150 mTorr).
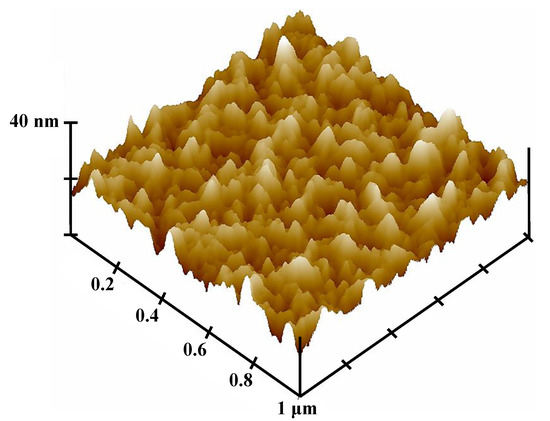
Figure 6.
AFM image of the allylamine plasma-modified sample (80 W, 22 min, and 150 mTorr).
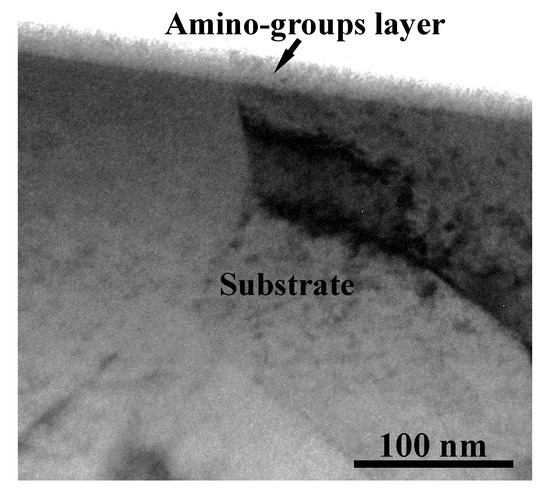
Figure 7.
A cross-sectional TEM micrograph of the allylamine plasma-modified sample (80 W, 22 min, and 150 mTorr).
3.3. Amino-Groups Bonding of the Allylamine Plasma-Modified Ti Samples
Figure 8 illustrates the RSM analysis results (concentration of amino-groups) of the allylamine plasma-modified Ti sample. The maximum concentration of amino-groups (31.58 nmole/cm2) can be obtained from the plasma-polymerized sample at power 80 W, deposition time 22 min, and chamber pressure 150 mTorr. According to the RSM analysis results, it was also discovered that the concentration of amino-groups deposited on the Ti surface is evidently a direct correlation to power and time during allylamine plasma modification. However, the deposition time can be a more vital effector compared with power. In addition, it also showed that the deposition with amino-groups was negatively correlated with pressure, which indicated that the flow rate of allylamine per second could not increase the bonding of amino-groups efficiently. This feature could be attributed to the fact that there is no way to induce allylamine to perform ionization efficiently due to the lack of vacuum degree in the chamber. This situation was similar to a previous study on hydrogen plasma modification [30]. When the hydrogen pressure was increased from 2.0 Torr to 14.0 Torr, the measured hydrogen dissociation rate was reduced from 0.83% to 0.14% at some discharge conditions. Accordingly, the parameter of pressure played an important role in the effect of the concentration of amino-groups on the Ti surface using the RSM.
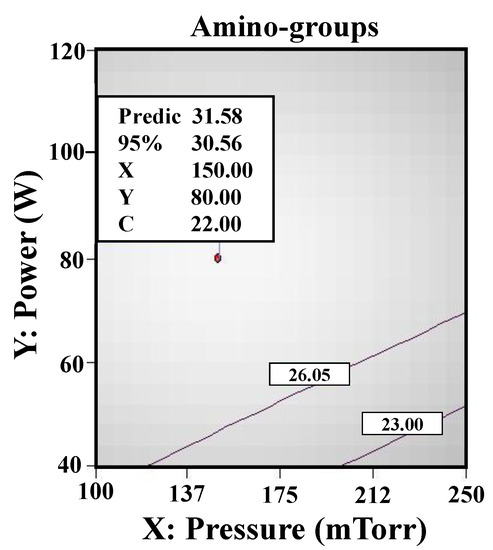
Figure 8.
The RSM analysis results (concentration of amino-groups) of the allylamine plasma-modified Ti sample (80 W, 22 min, and 150 mTorr).
3.4. Cellular Responses to the Investigated Samples
Figure 9 depicts the SEM micrographs of MG-63 cells on the polished Ti, Ar plasma-modified Ti (100 W, 12 min, and 190 mTorr), and allylamine plasma-modified Ti (80 W, 22 min, and 150 mTorr) samples after culturing for one day. As shown in Figure 9a, the MG-63 cells exhibited an island-like shape and adhered flat to the polished Ti sample. A similar cell adhesion response could also be seen on the Ar plasma-modified Ti (100 W, 12 min, and 190 mTorr) sample (Figure 9b). When increasing the incubation time, it was found that the MG-63 cells almost completely adhered to the polished Ti and Ar plasma-modified Ti surfaces. However, it was clearly seen that the MG-63 cells presented a superior adhesion response with numerous filopodia (as indicated by arrows) on the surface of the allylamine plasma-modified Ti (80 W, 22 min, and 150 mTorr) sample after culturing for one day (Figure 9c). Compared with the polished Ti and Ar plasma-modified Ti surfaces, the filopodia of cells not only adhered flat, but also tightly grabbed the amino-groups deposited surface. This cell response feature could also be observed on the allylamine plasma-modified Ti sample with a longer incubation time. Accordingly, the biocompatibility results prove that the allylamine plasma-modified Ti sample with an optimal modification parameter (80 W, 22 min, and 150 mTorr) has great potential to promote cell adhesion ability.
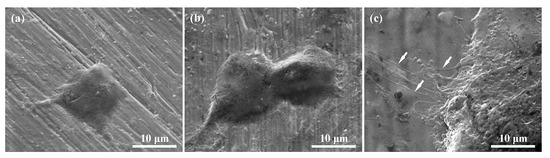
Figure 9.
SEM micrographs of the MG-63 cells on the investigated samples after culturing for one day: (a) Polished Ti, (b) Ar plasma-modified Ti (100 W, 12 min, and 190 mTorr), and (c) allylamine plasma-modified Ti (80 W, 22 min, and 150 mTorr).
Surface modification of the implant materials played a crucial factor in controlling bone cells. Osteoblast alignment is vital for the realization of anisotropic bone tissue microstructure. Some of the latest studies have focused on nanogrooved topography fabricated by a laser irradiation approach to control osteoblast alignment [31,32]. The results demonstrated the abnormal arrangement of bone matrix orthogonal to osteoblast arrangement through the nanogrooved surface. Moreover, Matsugaki et al. [33] also indicated that anisotropic dendrogenesis of osteocytes could be controlled with selective patterning of extracellular proteins. These findings provide new strategies to realize the ideal microstructure of bone tissue as well as provide molecular mechanisms for texture formation in biological systems. Furthermore, it is well known that biomaterials with amino-group coatings can enhance surface biocompatibility through cross-linking with bioactive materials such as proteins and growth factors, etc. [16,17]. Our research team has also demonstrated that the amino-groups can be uniformly deposited on a Ti surface to bind with albumin using the promising low-temperature plasma polymerization with allylamine approach [34]. It was found that the presence of reactive functional groups on the Ti surface significantly affects the reaction between the cell and bone tissue [35,36]. The formation of this feature can be attributed to the rapid absorption of biomolecules, mainly after the Ti implant surface is exposed to blood. Immobilized biomolecules can attract cells of host tissues to produce their own extracellular matrix and actively reshape the microniche. Moreover, bone cells on the Ti implant surfaces will secrete non-collagen proteins, forming organic minerals to form biomineralization. Through this interaction process, absorbed bioactive materials (such as growth factors or proteins) are induced on the Ti implant surface to facilitate bone healing and osseointegration [36,37]. Therefore, the plasma polymerization with allylamine approach can be potentially used to fabricate a functional surface layer on Ti implants to cross-link with bioactive biomolecules for improving biocompatibility properties.
4. Conclusions
In this study, we investigated the optimal modification parameters of low-temperature plasma polymerization with allylamine using the RSM. Both Ar and allylamine plasma-modified Ti samples were elucidated. Based on the RSM analysis results, the contact angle values of the modified Ti surfaces varied between 14.50° and 88.26° in the various designed parameters of Ar plasma and allylamine plasma modifications. The contact angle of the Ti surfaces was not proportional to the setting factors of power, time, and pressure in Ar plasma cleaning modification. In contrast, the power, time, and pressure factors were proportional to the contact angle of the allylamine plasma-modified surfaces. The concentration of amino-group deposition reduced when the applied pressure was increased. Hence, the pressure parameter played a crucial role in the effect of the concentration of amino-groups on the Ti surface via the RSM.
Author Contributions
Investigation and Writing—original draft, M.-S.H.; Software, C.-Y.W.; Supervision, K.-L.O. and K.E.; Project administration, B.-H.H.; Data curation, T.-H.C.; Validation, Y.-C.C.; Writing—review & editing, H.-Y.L. and C.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest in this work.
References
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices—Regulations, in vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef]
- Caplin, J.D.; Garcia, A.J. Implantable antimicrobial biomaterials for local drug delivery in bone infection models. Acta Biomater. 2019, 93, 2–11. [Google Scholar] [CrossRef]
- Gough, M.J.; Baird, J.R.; Bell, R.B. Implantable biomaterials to provide local immunotherapy following surgical resection. Oncotarget 2018, 9, 37612–37613. [Google Scholar] [CrossRef]
- Paterlini, T.T.; Nogueira, L.F.B.; Tovani, C.B.; Cruz, M.A.E.; Derradi, R.; Ramos, A.P. The role played by modified bioinspired surfaces in interfacial properties of biomaterials. Biophys. Rev. 2017, 9, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.J.; Chou, H.H.; Ou, K.L.; Sugiatno, E.; Ruslin, M.; Waris, R.A.; Huang, C.F.; Liu, C.M.; Peng, P.W. Evaluation of Surface Characteristics and Hemocompatibility on the Oxygen Plasma-Modified Biomedical Titanium. Metals 2018, 8, 513. [Google Scholar] [CrossRef]
- Hou, P.J.; Ou, K.L.; Wang, C.C.; Huang, C.F.; Ruslin, M.; Sugiatno, E.; Yang, T.S.; Chou, H.H. Hybrid micro/nanostructural surface offering improved stress distribution and enhanced osseointegration properties of the biomedical titanium implant. J. Mech. Behav. Biomed. 2018, 79, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Albertini, M.; Fernandez-Yague, M.; Lazaro, P.; Herrero-Climent, M.; Rios-Santos, J.V.; Bullon, P.; Gil, F.J. Advances in surfaces and osseointegration in implantology. Biomimetic surfaces. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e316–e325. [Google Scholar] [CrossRef]
- Ou, K.L.; Hosseinkhani, H. Development of 3D in vitro technology for medical applications. Int. J. Mol. Sci. 2014, 15, 17938–17962. [Google Scholar] [CrossRef]
- Dee, K.C.; Bizios, R. Mini-review: Proactive biomaterials and bone tissue engineering. Biotechnol. Bioeng. 1996, 50, 438–442. [Google Scholar] [CrossRef]
- Annunziata, M.; Canullo, L.; Donnarumma, G.; Caputo, P.; Nastri, L.; Guida, L. Bacterial inactivation/sterilization by argon plasma treatment on contaminated titanium implant surfaces: In vitro study. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e118–e121. [Google Scholar] [CrossRef]
- Gottlicher, M.; Rohnke, M.; Kunz, A.; Thomas, J.; Henning, R.A.; Leichtweiss, T.; Gemming, T.; Janek, J. Anodization of titanium in radio frequency oxygen discharge—Microstructure, kinetics & transport mechanism. Solid State Ion. 2016, 290, 130–139. [Google Scholar]
- Hung, W.C.; Chang, F.M.; Yang, T.S.; Ou, K.L.; Lin, C.T.; Peng, P.W. Oxygen-implanted induced formation of oxide layer enhances blood compatibility On titanium for biomedical applications. Mat. Sci. Eng. C-Mater. 2016, 68, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Cheng, H.C.; Huang, C.F.; Lin, C.T.; Lee, S.Y.; Chen, C.S.; Ou, K.L. Enhancement of biocompatibility on bioactive titanium surface by low-temperature plasma treatment. Jpn. J. Appl. Phys. 2005, 44, 8590–8598. [Google Scholar] [CrossRef]
- Ramiasa, M.N.; Cavallaro, A.A.; Mierczynska, A.; Christo, S.N.; Gleadle, J.M.; Hayball, J.D.; Vasilev, K. Plasma polymerised polyoxazoline thin films for biomedical applications. Chem. Commun. 2015, 51, 4279–4282. [Google Scholar] [CrossRef]
- Gentile, P.; Ghione, C.; Tonda-Turo, C.; Kalaskar, D.M. Peptide functionalisation of nanocomposite polymer for bone tissue engineering using plasma surface polymerization. RSC Adv. 2015, 5, 80039–80047. [Google Scholar] [CrossRef]
- Hamerli, P.; Weigel, T.; Groth, T.; Paul, D. Surface properties of and cell adhesion onto allylamine-plasma-coated polyethylenterephtalat membranes. Biomaterials 2003, 24, 3989–3999. [Google Scholar] [CrossRef]
- Crespin, M.; Moreau, N.; Masereel, B.; Feron, O.; Gallez, B.; Borght, T.V.; Michiels, C.; Lucas, S. Surface properties and cell adhesion onto allylamine-plasma and amine-plasma coated glass coverslips. J. Mater. Sci.-Mater. Med. 2011, 22, 671–682. [Google Scholar] [CrossRef]
- Wang, Z.M.; Wang, Z.F.; Lu, W.W.; Zhen, W.X.; Yang, D.Z.; Peng, S.L. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. Npg Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Walschus, U.; Hoene, A.; Patrzyk, M.; Lucke, S.; Finke, B.; Polak, M.; Lukowski, G.; Bader, R.; Zietz, C.; Podbielski, A.; et al. A Cell-Adhesive Plasma Polymerized Allylamine Coating Reduces the In Vivo Inflammatory Response Induced by Ti6Al4V Modified with Plasma Immersion Ion Implantation of Copper. J. Funct. Biomater. 2017, 8, 30. [Google Scholar] [CrossRef]
- Kunz, F.; Rebl, H.; Quade, A.; Matschegewski, C.; Finke, B.; Nebe, J.B. Osteoblasts with impaired spreading capacity benefit from the positive charges of plasma polymerised allylamine. Eur. Cell Mater. 2015, 29, 177–188. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Tian, J.M.; Tian, J.T.; Chen, Z.Q.; Deng, X.J.; Zhang, D.H. Preparation of graded porous titanium coatings on titanium implant materials by plasma spraying. J. Biomed. Mater. Res. 2000, 52, 333–337. [Google Scholar] [CrossRef]
- Cools, P.; de Geyter, N.; Vanderleyden, E.; Dubruel, P.; Morent, R. Surface Analysis of Titanium Cleaning and Activation Processes: Non-thermal Plasma Versus Other Techniques. Plasma Chem. Plasma P 2014, 34, 917–932. [Google Scholar] [CrossRef]
- Pereira, M.M.; Kurnia, K.A.; Sousa, F.L.; Silva, N.J.O.; Lopes-da-Silva, J.A.; Coutinhoa, J.A.P.; Freire, M.G. Contact angles and wettability of ionic liquids on polar and non-polar surfaces. Phys. Chem. Chem. Phys. 2015, 17, 31653–31661. [Google Scholar] [CrossRef] [PubMed]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Mekayarajjananonth, T.; Winkler, S. Contact angle measurement on dental implant biomaterials. J. Oral Implantol. 1999, 25, 230–236. [Google Scholar] [CrossRef]
- Lee, F.P.; Wang, D.J.; Chen, L.K.; Kung, C.M.; Wu, Y.C.; Ou, K.L.; Yu, C.H. Antibacterial nanostructured composite films for biomedical applications: Microstructural characteristics, biocompatibility, and antibacterial mechanisms. Biofouling 2013, 29, 295–305. [Google Scholar] [CrossRef]
- Shen, J.W.; Chen, Y.; Yang, G.L.; Wang, X.X.; He, F.M.; Wang, H.M. Effects of storage medium and UV photofunctionalization on time-related changes of titanium surface characteristics and biocompatibility. J. Biomed. Mater. Res. B 2016, 104, 932–940. [Google Scholar] [CrossRef]
- Bech, L.; Meylheuc, T.; Lepoittevin, B.; Roger, P. Chemical surface modification of poly(ethylene terephthalate) fibers by aminolysis and grafting of carbohydrates. J. Polym. Sci. Pol. Chem. 2007, 45, 2172–2183. [Google Scholar] [CrossRef]
- Wu, W.F.; Ou, K.L.; Chou, C.P.; Wu, C.C. Effects of nitrogen plasma treatment on tantalum diffusion barriers in copper metallization. J. Electrochem. Soc. 2003, 150, G83–G89. [Google Scholar] [CrossRef]
- Wang, W.G.; Xu, Y.; Yang, X.F.; Wang, W.C.; Zhu, A.M. Determination of atomic hydrogen in non-thermal hydrogen plasmas by means of molecular beam threshold ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1159–1166. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Matsugaki, A.; Kawahara, K.; Ninomiya, T.; Sawada, H.; Nakano, T. Unique arrangement of bone matrix orthogonal to osteoblast alignment controlled by Tspan11-mediated focal adhesion assembly. Biomaterials 2019, 209, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Matsugaki, A.; Aramoto, G.; Ninomiya, T.; Sawada, H.; Hata, S.; Nakano, T. Abnormal arrangement of a collagen/apatite extracellular matrix orthogonal to osteoblast alignment is constructed by a nanoscale periodic surface structure. Biomaterials 2015, 37, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Matsugaki, A.; Yamazaki, D.; Nakano, T. Selective patterning of netrin-1 as a novel guiding cue for anisotropic dendrogenesis in osteocytes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110391. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Liu, C.M.; Ou, K.L.; Chiang, H.J.; Sugiatno, E.; Wu, C.H.; Yen, H.J.; Chou, H.H. Nanostructured titanium dioxide layer combined with reactive functional groups as a promising biofunctional surface for biomedical applications. Ceram. Int. 2019, 45, 9712–9718. [Google Scholar] [CrossRef]
- Haimov, H.; Yosupov, N.; Pinchasov, G.; Juodzbalys, G. Bone Morphogenetic Protein Coating on Titanium Implant Surface: A Systematic Review. J. Oral Maxillofac. Res. 2017, 8, e1. [Google Scholar] [CrossRef]
- Biao, M.N.; Chen, Y.M.; Xiong, S.B.; Wu, B.Y.; Yang, B.C. Synergistic effects of fibronectin and bone morphogenetic protein on the bioactivity of titanium metal. J. Biomed. Mater. Res. A 2017, 105, 2485–2498. [Google Scholar] [CrossRef]
- Kammerer, P.W.; Schiegnitz, E.; Palarie, V.; Dau, M.; Frerich, B.; Al-Nawas, B. Influence of platelet-derived growth factor on osseous remodeling properties of a variable-thread tapered dental implant in vivo. Clin. Oral Implants Res. 2017, 28, 201–206. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).