Abstract
The main purpose of this study was to consider the impact of Vitex agnus-castus hydroalcoholic extract, containing phytoestrogenic compounds on growth indices, sex ratio and histology of gonads of female Zebrafish. Fish larvae (4-day-old after hatching) were nourished with investigational diets (0 (control group T0), 5 g (T1), 10 g (T2) and 15 g (T3) kg−1 food) from first active feeding for 90 days. The results showed that Condition Factor (CF), Food Conversion Rate (FCR), Body Weight Gain (BWG), and Specific Growth Rate (SGR) were affected meaningfully by hydroalcoholic extract of Vitex agnus-castus (p < 0.05). These factors were significantly higher in compare to T3 treatment (p < 0.05). With increasing concentration of the extract in the diet, the ratio of female to male increased Treatment of T3 resulted in 87.23% feminization. Overall, this study suggests the use of T3 treatment achieves the best reproduction performance and a higher percentage of females in zebrafish as a model for aquaculture species. According to the results, the lowest levels of glucose and cholesterol were observed in T3 treatment and showed a significant changes with the control and treatments (p < 0.05).
1. Introduction
The use of zebrafish (Danio rerio) is growing rapidly as a particular species in aquaculture research. It has mainly became popular as a model of vertebrate progress because zebrafish embryos grow quickly and are visible. It can be motivated to breed during the year, and growth from a fertilized egg to breeding stage takes only about 3–4 months in the laboratory [1,2,3,4,5]. Their short reproduction period (three months) makes them a model candidate for histology and genetic researches [6,7]. There is not much literature in cases of Vitex agnus-castus extract on the gonad morphology for zebrafish. Huet (2000) reported that zebrafish has ovary-like gonads during initial life-time and the bisexual difference happening through the development.
In recent years, the scientific consideration in phytoestrogens has grown significantly, especially in the medical field [8,9]. These plant combinations are comparable to animal estrogens [10], which are commonly used in aquaculture, and they also have excessive costs compared to plant extracts and their use requires expert labor. Additionally, synthetic hormones have the potential to gather in the aquatic environment [11]. The usage of herbal medicine is a new and effective approach in the aquaculture industry [12,13,14,15].
Vitex agnus-castus (Verbenaceae) is a phytoestrogen herb; therefore, it has medical usage in different countries. It is a small shrub native to European, Mediterranean, and Central Asian countries. Also, it has been long used traditionally for its medicinal properties for female reproduction conditions. Phytoestrogens are similar to 17 β-estradiol, in terms of the structure or properties and function [16,17]. Vitex, systematically known as Vitex agnus-castus, belongs to the Verbenaceae family [18]. All parts of this plant have a different kind of essence among which sabinene, cineol, sesquiterpene, and pinene. Essential oils are other components of this plant [19,20]. Vitex agnus-castus is of the main plants used in herbal medicine for regulating hormones and women’s hormonal sicknesses [21]. It is mainly used to treat breast maladies, menstrual irregularities and uterine bleeding [22]. Other properties discovered in Iranian traditional medicine for Vitex agnus-castus, include anti-inflammatory, nutritious, aphrodisiac, diuretic, appetizing, carminative, relaxing and anti-flatulence properties. This herb is one of phytoestrogen. Most of the phytoestrogens-based provisions are easily available on the market, and are suggested as legal and non-toxic anti-oestrogenic compounds (to counterbalance the side effects of anabolic steroids, mainly based on methoxyflurane [23], as sexual enhancing compounds based mainly on plant extracts such as Vitex agnus-castus (isoflavones). Vitex agnus-castus phytoestrogen contains isoflavones with estrogenic activity.
Britt et al., (2002) [24] indicated that phytoestrogens could play a main role in the reproductive process, like estrogens. There are numerous studies associated with subjects, involving the properties of varied hormones on reproduction physiology, morphological reproductive toxicology and development of the ovaries in Zebrafish [25,26,27]. In contrast, there are not numerous studies using the phytoestrogen in the gonad development and sex ratio of this species in the literature. This is the first report regarding the potential of Vitex agnus-castus as a feminization agent in cultured food fish. Therefore, this study was conducted to consider the effects of diverse levels of Vitex agnus-castus hydroalcoholic extract on the sex ratio, histology and growth indices of the gonad in zebrafish (Danio rerio).
2. Materials and Methods
Fish were nourished daily with Biomar Feed (Made in French) and Shironomid sp. Nauplii. Fish were conserved at a temperature of 25 ± 1 °C, 12 D:12 L photoperiod and pH 7.2 ± 0.2. The water was changed every day. For embryo production and egg, healthy fish without disease and overt abnormalities were used as parental fish. Spawning of zebrafish happened in the early morning when aquaria lights were switched on. On day 4 post-fertilization (pf) feeding of the hatched larvae was started with the paramecium, and then with freshwater rotifers (Brachionus calyciflorus). The number of fish per aquarium was adjusted to 35 individuals in each glass aquariums (15 cm × 20 cm × 40 cm with 20 L dechlorinated tap water). The research was done in 12 glass tank at 30 fish-1 tanks in triplicate. These aquariums were accidentally divided into four experimental groups. The aquariums were equipped with aerated system to preserve dissolved oxygen near saturation levels and were maintained at 25 ± 1 °C under a 12 L:12 D photoperiod during the experiment. The commercial pellet diet, mixed with the suitable extract concentration and gelatin, was mixed together in this diet. The pelleted diets were air-dried, ground and sieved to produce a proper crumble (0.5 mm). Then the feed was kept at 4 °C until feeding trial. The control diet was provided to add only water and gelatin [28]. From day 14 pf fish were fed with Biomar Feed (Made in France) containing varying levels of hydroalcoholic extract of Vitex agnus-castus fruit and leaves (at 0 (T0), 5 (T1), 10 (T2), 15 (T3) g−1 kg extract of diet) were prepared and fed to the fish twice daily at 7% of their body weight for 90 days. The physical and chemical properties of water were measured daily using a Water checker. Chemical and physical characteristics of water during the investigational period are shown in Table 1

Table 1.
Physical and chemical characteristics of water during the experimental period in this study.
2.1. Growth Performance
The body weight (WG, g) was considered as the increase in weight of zebrafish at the end of the experimental period, WG = Wf − Wi, where Wf and Wi are the final and preliminary body weights, respectively [29]. The specific growth rate (SGR, %−1 day) was measured according to the formula (lnWf−lnWi) × 100−1Δt; where Δt is the time interval (in days) between Wi and Wf measurements. The food conversion ratio (FCR) was assessed as food consumption-1 weight gain [29]. Fish mortality was considered every day.
2.2. Histology
The samples were taken on day 60 after hatching for the histological study of the gonad (three fish from all groups). The fishes were fixed as a whole Bouin’s fluid for 24 h. The fixed fishes mounted and dehydrated in paraffin blocks. Five-μm thick parts of fish blocks were cut, mounted on glass slides and stained with Eosin and Hematoxylin for histopathological analysis, using a bright field light microscope. For the analysis of the ovary cycle, the oocytes were divided into four phases according to the development stages and sizes of oocytes: Cortical alveolus, primary growth (cytoplasmic and yolk growth), mature oocyte and vitellogenin growth.
2.3. Preparation of Herbal Extracts and Experimental Diets
Four treatments including T0 (control), T1 (5 g), T2 (10 g) and T3 (15 g) prepared from the same basal diet to ensure unchanging composition. The basal diet comprised around 52.45% protein, 18.44% lipid, 2.1% fiber, 11.35% ash, 8.12% moisture and had 4598.56 (cal−1 g) energy on a dry matter base. Hydroalcoholic extract dissolved in demineralized water was mixed (at 5, 10 and 15 g kg−1 basal diet in the T5, T10, and T15 treatments. The diets were dried at room temperature (25 °C) and stored frozen (4 °C). The components of Vitex agnus-castus are shown in Table 2.

Table 2.
The nutrient composition of Vitex agnus-castus.
2.4. Herbal Extract
Vitex agnus-castus medicinal plant was collected from herbal medicine at Gorgan University in Iran and its distinctiveness was confirmed using monographs [30]. According to research done by other researchers, both the leaf and fruit of this plant contain phytoestrogenic compounds [31,32]. Extraction was done by the “maceration” method. First of all 50 g of the leave and fruit of Vitex agnus-castus in the dried form were weighted with a digital balance. Then, they were mashed and then added to 1500 cc of a solvent (half ethanol and half water) and were shaken by 90 cycle−1 min for 48 h until they got homogenous. The solutions were filtered with a strainer and put on a rotary evaporator (Heidolph WD 2000, Schwabach, Germany) to evaporate the solvent. Finally, the pure extracts were preserved in sterile vials in the refrigerator to be used in microbial tests.
2.5. Sex Ratio
At the end of the experimental period, the number of males and females was accounted. There were irregular sex ratios in the treatment groups compared to controls. The number of females was higher in the groups that received the Vitex agnus-castus extract diets. The control group had close to the typical gender ratio.
At the end of the experiment period, fish feeding was stopped for 24 h. The fish were washed twice with sterile phosphate buffer (PBS, pH = 7.2) and then centrifuged for 10 min at −4 °C and at about 3000 g to measure biochemical indices. The supernatant was collected and then centrifuged at about 3 g for 5 min and kept at −20 °C until the experiments were done. The digestive tracts of the fish were separated and washed with phosphate buffer (PBS) to measure digestive enzymes and then homogenized with physiological serum and supernatant was extracted through centrifugation for 10 min at about 3000 g at 4 °C [33,34].
The levels of total protein, cholesterol and glucose were measured using laboratory kits (Pars Test Co., Tehran, Iran) and spectrophotometer with a wavelength of 546 nm. The digestive enzymes (lipase, amylase, and protease) were measured according to the method, presented by Bernfeld (1951) [34] and Worthington (1991) [35]. Amylase unit activity was considered according to the weight (mg) of maltose released for 10 min at 30 °C. The activity of the protease unit was expressed based on the amount of tyrosine released during the 15-min period under test conditions. The activity of the unit of lipase was based on 0.20 NaOH required to neutralize the released fatty acids during 18 h of incubation at pH 6.6 and 30 °C. The activity of these enzymes was designed based on enzyme unit per mg protein (U mg−1 protein).
2.6. Measuring the Chemical Composition of the Fish Body
Two fishes were randomly sampled from each experimental vessel to determine the chemical composition of the carcass at the end of the experiment period. Chemical analysis of carcass composition was done according to AOAC standard method. The amount of carcass proteins using the soxhlet method, Kjeldahl and fat by soluble fat in ether, moisture through sample assignment at 105 °C and sample weighing after cooling and measuring the ash by burning the sample at a temperature 550 °C for 6 h, and the sample weighing was calculated after cooling.
2.7. Statistical Analysis
The randomized design with 3 replicates was used in the present study. Data were tested for the homogeneity of variance and analyzed using one-way ANOVA, followed by the comparison of means by Duncan’s multiple range test (α = 0.05). The statistical analysis was carried out using SPSS Version 22. The data are shown as the mean ± standard deviation.
3. Results
3.1. Growth Indices
The obtained results shown that body specific growth rate (SGR), weight gain (BWG), food conversion rate (FCR) and condition factor (CF) were affected significantly by Vitex agnus-castus hydroalcoholic extract (p < 0.05) (Table 3). Regarding the results of a mean comparison of these factors, it was observed that these factors were significantly (p < 0.05) higher at T3 treatment. Also, the maximum survival rate observed in the treatment of T3, but there was no significant difference (p > 0.05) among treatments (Table 3).

Table 3.
Growth indices of Danio rerio feed with different levels of Vitex agnus-castus for 90 days.
3.2. Gonad Histology
An almost identical number of females and males were detected in the control treatment (Table 4). Nevertheless, the number of females was significantly higher than the number of males in all extract-treated groups with the best sex reversal in the T3 treatment, indicating that feminization is a dose-dependent process. The oocyte development was divided into four stages (primary growth, cortical alveolus, vitellogenin, and mature oocyte). Oocyte diameters were observed to vary between 0.54–0.75 mm, that highest was in T3 (Table 4). Histological and morphological investigations of the gonads revealed no intersex fish in all groups.

Table 4.
Reproductive effects of Vitex agnus-castus extract on broodstock of zebrafish.
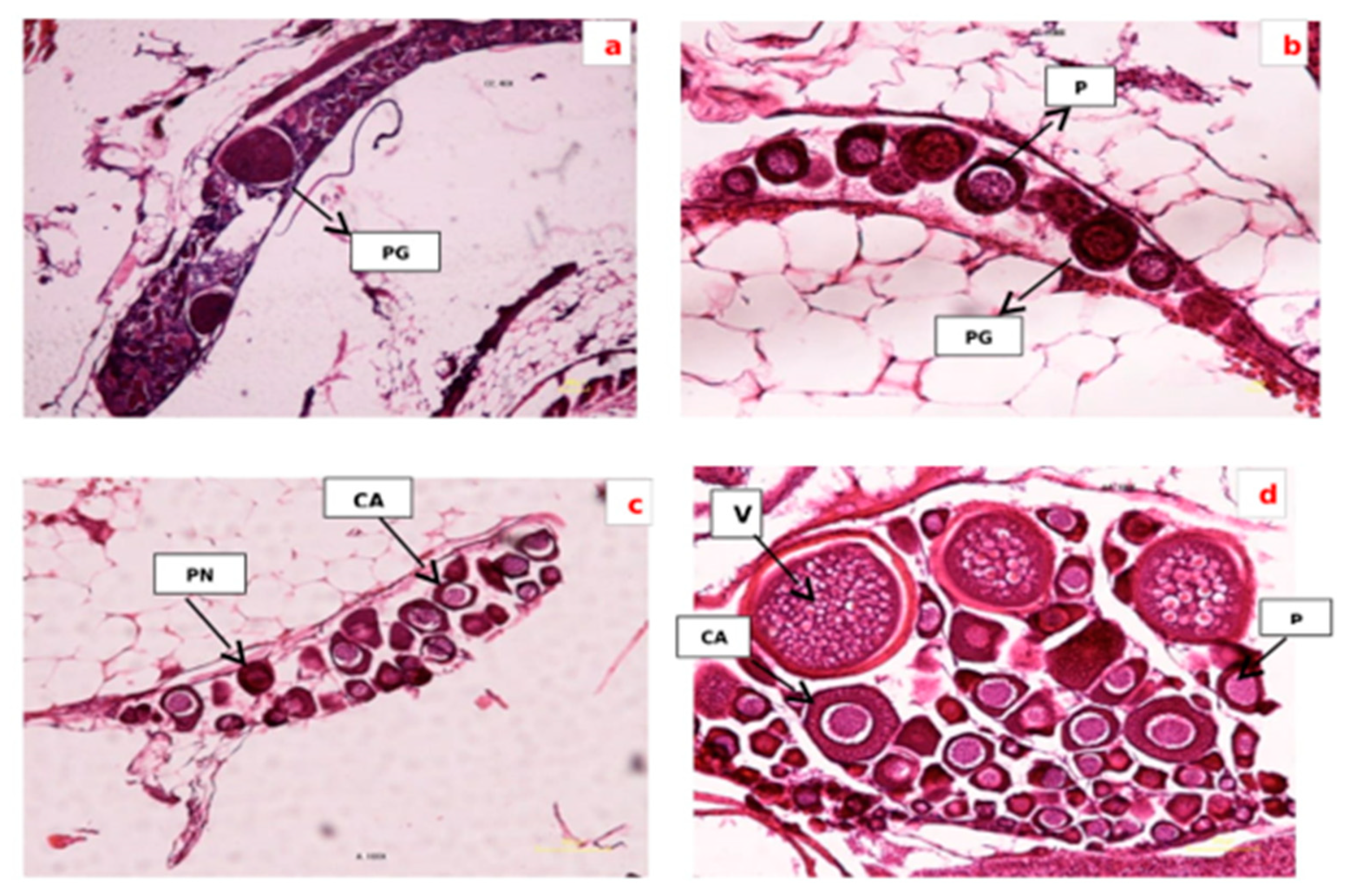
The control treatment was often immature oocytes and in primary growth (Figure 1a). Oocytes developed from the primary growth stage to the cortical alveolus stage with increasing doses from 5 to 10 g−1 kg (Figure 1b,c). Oocytes exhibited maximum growth, vitellogenic and mature oocyte in the group receiving 15 g−1 kg of plant extract (Figure 1d). The oocyte’s growth was similar in the teleosts. In most of the teleosts, the growth of oogenesis might be in four, five, six and eight phases. The oocyte’s development in the zebrafish was manifested in a series of variations, and their division was during four stages. Throughout the oocyte growth, the oocyte was enlarged due to proteolysis and hydration of the yolk protein. The vitelline envelope started to form in the cortical alveolus stages and develop in the vitellogenic stages, and was obviously detected in the mature oocytes.
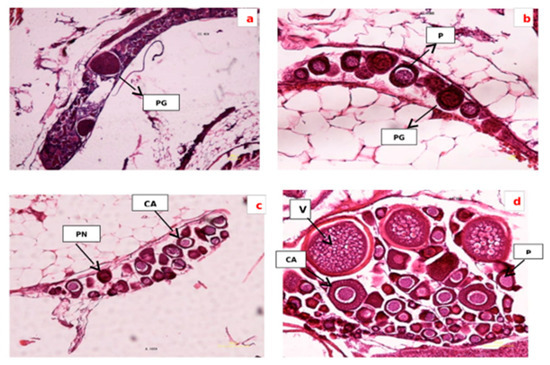
Figure 1.
Section of ovarian tissue in different groups: (a) control group. Egg is in primary growth stage (PG); (b) treated by Vitex agnus-castus at dose 5 g/1 kg, the oocytes are in primary growth stage (PG) and perinucleoar stage (PN); (c) treated by Vitex agnus-castus at dose 10 g/1 kg, dominant phase oocytes contain cortical alveolar stage (CA); (d) treated by Vitex agnus-castus at dose 15 g/1 kg, the vitellogenin stage is apparent (GV), (100 µm, HandE).
4. Discussion
Indirect processes, which include feminizing by synthetic sex steroids have generally been used to produce all-female stocks, but they have some problems, such as high costs and toxicity. Hence, the main purpose of this study was the advance of indirect techniques for the production of all-female populations of Danio rerio through the use of natural steroids. Therefore, Vitex agnus-castus hydroalcoholic extract was used as a natural steroid. The present research showed that Vitex agnus-castus extract was effective in successful growth performance in Zebrafish. Therefore, it appears that Vitex agnus-castus could proficiently be used as a natural alternative to synthetic hormones for sex reversal in aquaculture industry.
The results achieved confirmed that the effect of Vitex agnus-castus was not significant on the survival rate (p > 0.05), but growth indices were affected meaningfully with the increasing of Vitex agnus-castus doses (p < 0.05). The findings in this research were in agreement with the results of Turan and Akyurt, (2005) [36] on African catfish, Ahilan and Nithiyapriyatharshini (2015) [37] on goldfish and Mooraki et al. (2014) [38] on Cyprinus carpio. They reported that growth performance was positively affected by herbal extract in the food. According to the significant differences among the treatments of the control group and experimental groups in terms of growth performance, it can be concluded that the Vitex agnus-castus has beneficial and nutrient materials that can positively affect growth indices.
Phytoestrogens play a serious role in developing adult reproductive cycles and sex differentiation in the vertebrates. Analysis of the results obtained from a comparison of different treatments in terms of the histological changes of the ovaries, mean GSI, mean oocyte diameter and absolute fecundity displayed that the extract of Vitex agnus-castus enhanced the final maturity of the Zebrafish, and this effect was more obvious at a higher dose. Comparison of the treatments and control group, in terms of the histological changes of the ovaries, mean GSI, mean oocyte diameter and absolute fecundity a indicated significant difference (p < 0.05), whereby T3 was better than other treatments. Also, the weight and length of larvae increased at T3 (p < 0.05).
Constructed on the present study, Vitex agnus-castus extract (15 g kg−1 food) was most effective infertility and weight gain (Table 3). With esteem to determining the optimum concentration for growing the growth performance and feminization of Zebrafish life stage in this revision, it was found that the level of 15 g−1 kg food is suggested as the best concentration. It has been stated that gonad development in mice was significantly affected by injection or oral additive Vitex agnus-castus extract among herbal extract effects [39,40], but insufficient studies have been carried out to estimate the herbal extract on fish gonad development.
Microscopic studies showed that Vitex agnus-castus extract influenced the maturity in a shorter time. Therefore, the treatment groups (especially in group T3) were incomparable with the control group, where the maturation of fish was earlier and with more quantity of egg (Figure 1). Subsequently, the treatment group T3 was matured 15 days earlier than the control group. The increase in fertility rate in the test groups may be due to the increase in the level of the estrogenic compound, which could be caused by the action of the extract in the nourishment. Thus, it is essential to use the phytoestrogens such as the Vitex agnus-castus extract before the beginning of the reproduction season. Findings in the present study are consistent with the findings of Nazari and Roozbehani (2015) [41]. Their research on Poecilia reticulata indicates that Fennel Foeniculum vulgar extract influenced by the maturity of the ovum in a shorter time. Thus, the level of the estrogenic hormone was increased in treatment groups during the experiment by feeding. An increase in Fennel extract in the diet enhanced reproductive activity and the offspring was born in a shorter time.
Oocyte diameters were observed between 0.54–0.75 mm, that highest was in T3. According to the results, treatment T3 for precocious puberty in zebrafish is suggested as model species in aquaculture. Also, reproductive performance was significantly better in T3 than the other treatments (p < 0.05).
Nazari and Roozbehani (2015) [41] described that Guppy larvae (Poecilia reticulata) were positively affected by phytoestrogens. The larvae attained the best enactment and displayed the fastest development of gonad using the herbal extract. Abbasi et al. (2006) [42] reached maximum growth and oocyte maturation of Epinephelus Coiodes larvae fed with phytoestrogen.
Considering not sufficient and certain data for the effect of Vitex agnus-castus extract on fishes, there is low contrasting recommendations. In the present study, the mean egg diameter, absolute fecundity, GSI, survival, length, and weight of larvae were increased with an increased dose of Vitex agnus-castus extract in the diet (p < 0.05). The highest measured egg diameter was 0.03±0.75 mm that it was in agreement with Aytekin and Yüce (2008) [43]. As confirmed by other research, herbal extract gets improved the growth and reproductive indices. Naji et al. (2014) [44] compared the reproductive effect of extract Vitex agnus-castus and 17β estradiol (E2) on ovarian tissue of immature female three spot gourami (Trichogaster trichopterus). Their results showed that oocytes diameter, fecundity, and fertility enhancement were the most identical for a treated group with extract of Vitex agnus-castus. Their results are in agreement with our results.
In this experiment the ratio of female to male improved (p < 0.05). Treatment T3 was leaded to 87.23 percent of female production with increasing concentration of extract in the diet. Naji et al. (2014) [44] investigated the Effects of Origanum vulgare extract on ovary morphology and histology in immature Trichogaster trichopterus. They found that Origanum vulgare can hasten oocyte maturation. Also, histological studies indicated that Origanum vulgare increased the maturation and growth of oocytes.
The former studies on the phytoestrogens compound indicated that phytoestrogens have diverse effects on the different fish species especially on ornamental species. Brown et al. (2014) [45] indicated that Genistein as a phytoestrogen has a limited effect on reproductive endpoints in a female fighting fish Betta splenden, while other researches showed contrast results. Phytoestrogens increased vitellogenin synthesis in Tilapia’s primary hepatocytes [46].
Dada and Ajilore (2009) [47] used the ethanol extract of Garcinia kola seeds to enhance fertility in C. gariepinus. Fish were fed with complemented diets in different concentrations (0.25, 0.5, 1.0 and 2.0 g−1 kg diet) of ethanol extract of G. kola seeds for 56 days and a significant difference (p < 0.05) was observed in fecundity. The egg’s diameter was improved with the dose of ethanol extract in the fish diet, which is in agreement with our results. The increased fecundity of fed fish with herbal supplemented diet was due to the presence of bioflavonoids and xanthone in this plant [48]. The combinations are powerful antioxidants and can increase estrogen production, thereby leading to egg production and maturation. Yılmaz et al. (2009) [11] assessed that Genesis (G, a commercial mixture of phytoestrogens) including phytostrogens, which increased growth rate and sex separation in the Sharptooth catfish Clarias gariepinus. They concluded that the usage of higher doses and treatment durations of Genesis could be more effective for all-female production of the Sharptooth catfish. Moreover, phytoestrogens were able to motivate vitellogenin synthesis in Siberian sturgeon Acipenser baeri [49] and common carp, Cyprinus carpio [50]. Thus, it seems that phytoestrogens could effectively be used as natural alternatives to synthetic hormones for sex reversal in fish culture.
5. Conclusions
Vitex agnus-castus contains vital fatty acids, flavonoids diterpenes, iridoid glycosides, and essential oils. Its contraceptive effects are recommended in relation to crucial fatty acids and flavonoids. The results showed that the oocyte diameter regularly increased and the final development of the Zebrafish hastened with increasing Vitex agnus-castus.. Also, this phytoestrogen enhanced the growth parameters in Zebrafish as a model in aquaculture. The observations showed that Vitex agnus-castus as a phytoestrogen can be considered as potential as a natural additive to fish feed in commercial aquaculture to increase the fertility rate.
Author Contributions
T.E.G., R.F.R., M.P. (Mojtaba Pouladi) M.L., performed the experiments, prepared the pitures and wrote the manuscript; M.P. (Maria Pagano) reviewed and edited the manuscript; C.F. supervised and validated the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thanks the staff at the aquaculture lab of Gorgan University of Agricultural Science and Natural Resources for their kind help during the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bartoskova, M.; Dobsikova, R.; Stancova, V.; Zivna, D.; Blahova, J.; Marsalek, P.; Zelnickova, L.; Bartos, M.; Di Tocco, F.C.; Faggio, C. Evaluation of ibuprofen toxicity for zebrafish (Danio rerio) targeting on selected biomarkers of oxidative stress. Neuroendocrinol. Lett. 2013, 34, 102–108. [Google Scholar] [PubMed]
- Fiorino, E.; Sehonova, P.; Plhalova, L.; Blahova, J.; Svobodova, Z.; Faggio, C. Effects of glyphosate on early life stages: Comparison between Cyprinus carpio and Danio rerio. Environ. Sci. Pollut. Res. 2018, 25, 8542–8549. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Yousefi, S.; Capillo, G.; Paknejad, H.; Khalili, M.; Tabarraei, A.; Van Doan, H.; Spanò, N.; Faggio, C. Mucosal immune parameters, immune and antioxidant defence related genes expression and growth performance of zebrafish (Danio rerio) fed on Gracilaria gracilis powder. Fish Shellfish Immunol. 2018, 83, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Plhalova, L.; Blahova, J.; Divisova, L.; Enevova, V.; Casuscelli di Tocco, F.; Faggio, C.; Tichy, F.; Vecerek, V.; Svobodova, Z. The effects of subchronic exposure to NeemAzal T/S on zebrafish (Danio rerio). Chem. Ecol. 2018, 34, 199–210. [Google Scholar] [CrossRef]
- Sehonova, P.; Tokanova, N.; Hodkovicova, N.; Kroupova, H.K.; Tumova, J.; Blahova, J.; Marsalek, P.; Plhalova, L.; Doubkova, V.; Dobsikova, R. Oxidative stress induced by fluoroquinolone enrofloxacin in zebrafish (Danio rerio) can be ameliorated after a prolonged exposure. Environ. Toxicol. Pharmacol. 2019, 67, 87–93. [Google Scholar] [CrossRef]
- Fenner-Crisp, P.A.; Maciorowski, A.F.; Timm, G.E. The endocrine disruptor screening program developed by the US Environmental Protection Agency. Ecotoxicology 2000, 9, 85–91. [Google Scholar] [CrossRef]
- Huet, M.-C. OECD activity on endocrine disrupters test guidelines development. Ecotoxicology 2000, 9, 77–84. [Google Scholar] [CrossRef]
- Beck, V.; Rohr, U.; Jungbauer, A. Phytoestrogens derived from red clover: An alternative to estrogen replacement therapy? J. Steroid Biochem. Mol. Biol. 2005, 94, 499–518. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999, 129, 758S–767S. [Google Scholar] [CrossRef]
- Clotfelter, E.D.; Rodriguez, A.C. Behavioral changes in fish exposed to phytoestrogens. Environ. Pollut. 2006, 144, 833–839. [Google Scholar] [CrossRef]
- Yılmaz, E.; Çek, Ş.; Mazlum, Y. The effects of combined phytoestrogen administration on growth performance, sex differentiation and body composition of sharptooth catfish Clarias gariepinus (Burchell, 1822). Turk. J. Fish. Aquat. Sci. 2009, 9, 33–37. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Sheikhzadeh, N.; Roshanaei, K.; Dargahi, N.; Faggio, C. Can dietary ginger (Zingiber officinale) alter biochemical and immunological parameters and gene expression related to growth, immunity and antioxidant system in zebrafish (Danio rerio)? Aquaculture 2019, 507, 341–348. [Google Scholar] [CrossRef]
- Rashidian, G.; Bahrami Gorji, S.; Farsani, M.N.; Prokić, M.D.; Faggio, C. The oak (Quercus brantii) acorn as a growth promotor for rainbow trout (Oncorhynchus mykiss): Growth performance, body composition, liver enzymes activity and blood biochemical parameters. Nat. Prod. Res. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, H.; Hoseinifar, S.H.; Faggio, C.; Chitmanat, C.; Mai, N.T.; Jaturasitha, S.; Ringø, E. Effects of corncob derived xylooligosaccharide on innate immune response, disease resistance, and growth performance in Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 2018, 495, 786–793. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Sringarm, K.; Jaturasitha, S.; Yuangsoi, B.; Dawood, M.A.O.; Esteban, M.Á.; Ringø, E.; Faggio, C. Effects of Assam tea extract on growth, skin mucus, serum immunity and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae. Fish Shellfish Immunol. 2019, 93, 428–435. [Google Scholar] [CrossRef]
- Knight, D.C.; Eden, J.A. Phytoestrogens—A short review. Maturitas 1995, 22, 167–175. [Google Scholar] [CrossRef]
- Varner, J.; Bonner, J. Plant Biochemistry; Academic Press: Cambridge, MA, USA, 1966. [Google Scholar]
- Hobs, C. The Women’s Herb; Healthy Living Publications: Summertowns, UK, 2003; pp. 203–207. [Google Scholar]
- Nasri, S.; Ebrahimi, S. Medical effect of Vitex agnus-castus. J. Babol Univ. Med. Sci. 2006, 7, 49–53. [Google Scholar]
- Nyiligira, E.; Viljoen, A.M.; Van Heerden, F.R.; Van Zyl, R.L.; Van Vuuren, S.F.; Steenkamp, P.A. Phytochemistry and in vitro pharmacological activities of South African Vitex (Verbenaceae) species. J. Ethnopharmacol. 2008, 119, 680–685. [Google Scholar] [CrossRef]
- Cossuta, D.; Simándi, B.; Vági, E.; Hohmann, J.; Prechl, A.; Lemberkovics, É.; Kéry, Á.; Keve, T. Supercritical fluid extraction of Vitex agnus-castus fruit. J. Supercrit. Fluids 2008, 47, 188–194. [Google Scholar] [CrossRef]
- Roemheld-Hamm, B. Chasteberry. Am. Fam. Phys. 2005, 72, 821–824. [Google Scholar]
- Usui, T. Pharmaceutical prospects of phytoestrogens. Endocr. J. 2006, 53, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.L.; Kerr, J.; O’DONNELL, L.; Jones, M.E.E.; Drummond, A.E.; Davis, S.R.; Simpson, E.R.; Findlay, J.K. Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. FASEB J. 2002, 16, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Brion, F.; Tyler, C.R.; Palazzi, X.; Laillet, B.; Porcher, J.-M.; Garric, J.; Flammarion, P. Impacts of 17β-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile-and adult-life stages in zebrafish (Danio rerio). Aquat. Toxicol. 2004, 68, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Fenske, M.; Segner, H. Aromatase modulation alters gonadal differentiation in developing zebrafish (Danio rerio). Aquat. Toxicol. 2004, 67, 105–126. [Google Scholar] [CrossRef] [PubMed]
- Örn, S.; Holbech, H.; Madsen, T.H.; Norrgren, L.; Petersen, G.I. Gonad development and vitellogenin production in zebrafish (Danio rerio) exposed to ethinylestradiol and methyltestosterone. Aquat. Toxicol. 2003, 65, 397–411. [Google Scholar] [CrossRef]
- Cerezuela, R.; Cuesta, A.; Meseguer, J.; Esteban, M.Á. Effects of inulin on gilthead seabream (Sparus aurata L.) innate immune parameters. Fish Shellfish Immunol. 2008, 24, 663–668. [Google Scholar] [CrossRef]
- Luz, R.K.; Martínez-Álvarez, R.M.; De Pedro, N.; Delgado, M.J. Growth, food intake regulation and metabolic adaptations in goldfish (Carassius auratus) exposed to different salinities. Aquaculture 2008, 276, 171–178. [Google Scholar] [CrossRef]
- Mozaffarian, V. Encyclopedia of Iranian Plants; Farhang Moaser Publ.: Tehran, Iran, 1996; 671P. [Google Scholar]
- Ramezanloo, F.; Najafizadeh, P.; Naji, T.; Amin, G.H.; Mousavi, Z.; Vahabzadeh, G. The effect of hydroalcoholic extract of leaves of vitex on the gestation indices of male rats. J. Pharm. Health Care Sci. 2017, 5, 61–69. [Google Scholar]
- Cheraghi Niroumand, M.; Heydarpour, F.; Farzaei, M.H. Pharmacological and therapeutic effects of Vitex agnus-castus L.: A Review. Pharmacogn. Rev. 2018, 12, 103–114. [Google Scholar]
- Fawcette, J.K.; Scott, J.E. Practical Clinical Biochemistry; Academic Publishers: Kolkata, India, 1960. [Google Scholar]
- Fereidouni, M.S.; Akbary, P.; Soltanian, S. Survival rate and biochemical parameters in Mugil cephalus (Linnaeus, 1758) larvae fed garlic (Allium sativum L.) extract. Am. J. Mol. Biol. 2015, 5, 7. [Google Scholar] [CrossRef]
- Worthington, C.C. Worthington Enzyme Manual Related Biochemical; American Chemical Society: Freehold, NJ, USA, 1991. [Google Scholar]
- Turan, F.; Akyurt, I. Effects of red clover extract on growth performance and body composition of African catfish Clarias gariepinus. Fish. Sci. 2005, 71, 618–620. [Google Scholar] [CrossRef]
- Ahilan, B.; Nithiyapriyatharshini, A. Influence of herbal additives on the growth and disease resistance of goldfish, carassius auratus (linnaeus). J. Aquac. Trop. 2015, 30, 23. [Google Scholar]
- Mooraki, N.; Dadgar, S.H.; Sadegh, N.M. The effect of using parsley (petroselinum sativum) on growth performance of koi fish (Cyprinus carpio). J. Aquac. Develop. 2014, 8(2), 63–72. [Google Scholar]
- Daniele, C.; Coon, J.T.; Pittler, M.H.; Ernst, E. Vitex agnus-castus. Drug Saf. 2005, 28, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, W.; Jarry, H.; Christoffel, V.; Spengler, B.; Seidlova-Wuttke, D. Chaste tree (Vitex agnus-castus)–pharmacology and clinical indications. Phytomedicine 2003, 10, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Roozbehani, S. Influence of fennel Foeniculum Vulgar extract on fertility, growth rate and histology of Gonads on Guppy Poecilia reticulata. Turk. J. Fish. Aquat. Sci. 2015, 15, 457–463. [Google Scholar] [CrossRef]
- Abbasi, F.; Oryan, S.H.; Matinfar, A.B. Histology and morphology of Ovary of Epinephelus Coiodes in Persian Gulf. J. Pajouhesh Sazand 2006, 66, 84–94. [Google Scholar]
- Aytekin, Y.; Yüce, R. Ovary maturatıon stages and histological investigation of ovary of the Zebrafish (Danio rerio). Braz. Arch. Biol. Technol. 2008, 51, 513–522. [Google Scholar]
- Naji, T.; Ghafouri, S.; Sahafi, H.H. The Histological Effects of Cucurbita pepo, Silybum marianum, Linum usitatissmum, Vitex agnus-castus 17β estradiol on ovarian tissue in three Spot Gorami (Trichogaster trichopterus). Bull. Env. Pharmacol. Life Sci. 2014, 3, 120–127. [Google Scholar]
- Brown, A.C.; Stevenson, L.M.; Leonard, H.M.; Nieves-Puigdoller, K.; Clotfelter, E.D. Phytoestrogens β-sitosterol and genistein have limited effects on reproductive endpoints in a female fish, Betta splendens. Biomed Res. Int. 2014, 2014, 681396. [Google Scholar] [CrossRef]
- Turker, H.; Takemura, A. Effects of environmental contaminants and natural substances on vitellogenesis in tilapia primary hepatocytes. Turk. J. Fish. Aquat. Sci. 2011, 11, 539–545. [Google Scholar] [CrossRef]
- Dada, A.A.; Ajilore, V.O. Use of ethanol extracts of Garcinia kola as fertility enhancer in female catfish Clarias gariepinus broodstock. Int. J. Fish. Aquac. 2009, 1, 1–5. [Google Scholar]
- Zamani, M.; Neghab, N.; Torabian, S. Therapeutic effect of Vitex agnus-castus in patients with premenstrual syndrome. Acta Med. Iran. 2012, 101–106. [Google Scholar]
- Pelissero, C.; Bennetau, B.; Babin, P.; Le Menn, F.; Dunogues, J. The estrogenic activity of certain phytoestrogens in the Siberian sturgeon Acipenser baeri. J. Steroid Biochem. Mol. Biol. 1991, 38, 293–299. [Google Scholar] [CrossRef]
- Turker, H.; Bozcaarmutlu, A. Effect of total isoflavones found in soybean on vitellogenin production in common carp. Kafkas Univ. Vet. Fak. Derg. 2009, 15, 561–568. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).