Photoacoustic Imaging for Management of Breast Cancer: A Literature Review and Future Perspectives
Abstract
1. Introduction
2. Methods
3. Results
3.1. Description of Studies
3.1.1. Phantom Studies
3.1.2. Animal Studies
Endogenous Contrast Agents
Exogenous Contrast Agents
3.1.3. Ex Vivo Studies
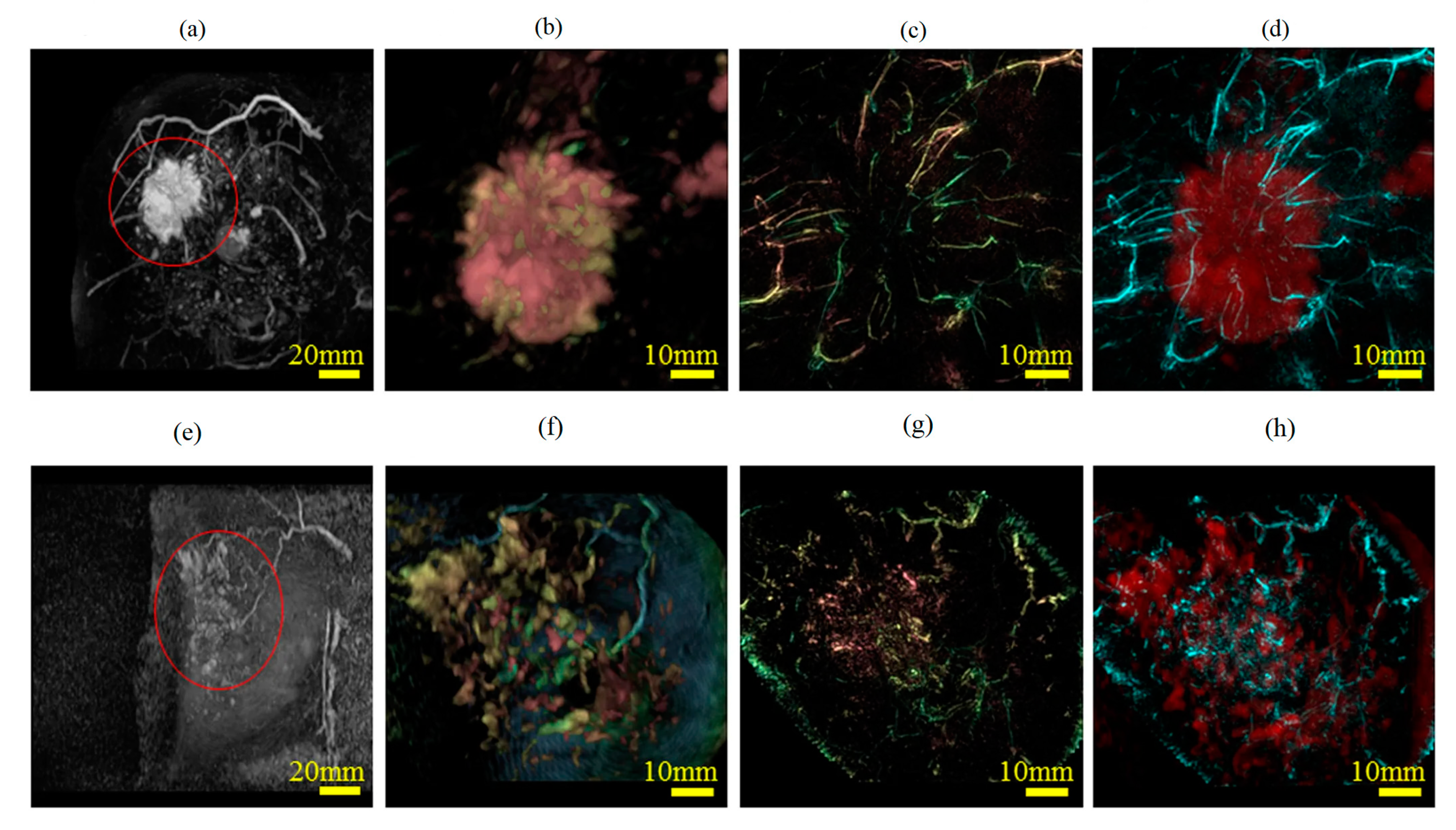
3.1.4. In Vivo Studies
4. Discussion
4.1. Comparison of PA Imaging with Other Imaging Modalities
4.1.1. PA Imaging Versus X-ray Mammography and US Imaging
4.1.2. PA Imaging Versus MRI
4.1.3. PA Imaging Versus Histology
4.2. Patient Population in Clinical Studies
4.3. Comparison of PA Imaging System Configurations
4.4. Spectroscopic Imaging
4.5. Breast Cancer Treatment Monitoring
4.6. Questions Raised by Manohar’s Research Group and Answers
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- American Cancer Society. Types of Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/types-of-breast-cancer.html (accessed on 17 February 2017).
- American Cancer Society, Inc. Surveillance Research. Estimated Number* of New Cancer Cases and Deaths by Sex, US, 2017. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/estimated-number-of-new-cancer-cases-and-deaths-by-sex-us-2017.pdf (accessed on 17 February 2017).
- American Cancer Society. Cancer Facts & Figures 2018. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf (accessed on 26 September 2018).
- American Cancer Society. Cancer Facts & Figures 2019. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf (accessed on 15 January 2019).
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, E.; Pisano, E.; Schnall, M.; Sener, S. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 2007, 57, 75–89. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Breast Cancer Early Detection and Diagnosis. Available online: https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html#written_by (accessed on 1 December 2017).
- World Health Organization. Guide to Cancer Early Diagnosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Newton, E.; Lee, M. Breast Cancer Screening. Available online: https://emedicine.medscape.com/article/1945498-overview (accessed on 7 March 2019).
- Xia, W.; Steenbergen, W.; Manohar, S. Photoacoustic mammography: Prospects and promises. Breast Cancer 2014, 3, 387–390. [Google Scholar] [CrossRef]
- Gøtzsche, P.C.; Nielsen, M. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 2009, 4, 1–73. [Google Scholar]
- Köşüş, N.; Köşüş, A.; Duran, M.; Simavlı, S.; Turhan, N. Comparison of standard mammography with digital mammography and digital infrared thermal imaging for breast cancer screening. J. Turk. Ger. Gynecol. Assoc. 2010, 11, 152. [Google Scholar] [CrossRef]
- Buist, D.S.; Porter, P.L.; Lehman, C.; Taplin, S.H.; White, E. Factors contributing to mammography failure in women aged 40–49 years. J. Natl. Cancer Inst. 2004, 96, 1432–1440. [Google Scholar] [CrossRef]
- Berg, W.A.; Gutierrez, L.; NessAiver, M.S.; Carter, W.B.; Bhargavan, M.; Lewis, R.S.; Ioffe, O.B. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer 1. Radiology 2004, 233, 830–849. [Google Scholar] [CrossRef]
- Mukherjee, S.D.; Hodgson, N.; Lovrics, P.J.; Dhamanaskar, K.; Minuk, T.; Chambers, S.; Sussman, J. A Retrospective Study Evaluating the Impact of Preoperative Breast MRI on Surgical Decision-Making in Young Patients (≤50 Years) with Invasive Breast Cancer. Breast Cancer Basic Clin. Res. 2016, 10, 53. [Google Scholar] [CrossRef]
- Johns Hopkins HealthCare. Medical Policy: Magnetic Resonance Imaging (MRI) of the Breast. Available online: https://www.hopkinsmedicine.org/johns_hopkins_healthcare/downloads/Policies/cms_13_04_magnetic_resonance_imaging_2017.pdf (accessed on 14 July 2019).
- Taif, S.A. Breast magnetic resonance imaging indications in current practice. Asian Pac. J. Cancer Prev. 2014, 15, 569–575. [Google Scholar] [CrossRef][Green Version]
- Gunawardena, D. Current Status of Breast MRI–Clinical applications. Sri Lanka J. Radiol. 2015, 1, 1–5. [Google Scholar] [CrossRef]
- Menezes, G.L.; Knuttel, F.M.; Stehouwer, B.L.; Pijnappel, R.M.; van den Bosch, M.A. Magnetic resonance imaging in breast cancer: A literature review and future perspectives. World J. Clin. Oncol. 2014, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C. The current status of breast MR imaging part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 2007, 244, 356–378. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, P.A.; Dietzel, M.; Vag, T.; Burmeister, H.; Gajda, M.; Camara, O.; Pfleiderer, S.O.; Kaiser, W.A. Clinical MR Mammography: Impact of Hormonal Status on Background Enhancement and Diagnostic Accuracy. Röntgenstr 2011, 183, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, C.; Campassi, C.; Goloubeva, O.; Wooten, K.; Kesmodel, S.; Bellevance, E.; Feigenberg, S.; Ioffe, O.; Tkaczuk, K.H. Breast magnetic resonance imaging (MRI) surveillance in breast cancer survivors. SpringerPlus 2015, 4, 459. [Google Scholar] [CrossRef] [PubMed]
- American Society of Clinical Oncology. Breast MRI. Available online: http://www.cancer.net/navigating-cancer-care/diagnosing-cancer/tests-and-procedures/breast-mri (accessed on 18 February 2017).
- Breast Cancer.org. Understanding Breast Calcifications. Available online: https://www.breastcancer.org/symptoms/testing/types/mammograms/mamm_show/calcifications (accessed on 14 July 2019).
- Heijblom, M.; Piras, D.; Brinkhuis, M.; Van Hespen, J.; Van den Engh, F.; Van der Schaaf, M.; Klaase, J.; van Leeuwen, T.; Steenbergen, W.; Manohar, S. Photoacoustic image patterns of breast carcinoma and comparisons with Magnetic Resonance Imaging and vascular stained histopathology. Sci. Rep. 2015, 5, 11778. [Google Scholar] [CrossRef]
- Song, N. Quantitative Photoacoustic Tomography for Breast Cancer Screening; Ecole Centrale Marseille: Marseille, France, 2014. [Google Scholar]
- Gupta, M.K.; Qin, R.-Y. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. WJG 2003, 9, 1144. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [PubMed]
- Wang, L.V. Photoacoustic imaging and spectroscopy; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Tam, A.C. Applications of photoacoustic sensing techniques. Rev. Mod. Phys. 1986, 58, 381. [Google Scholar] [CrossRef]
- Rosencwaig, A. Photoacoustics and photoacoustic spectroscopy; Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Cox, B.; Laufer, J.G.; Arridge, S.R.; Beard, P.C. Quantitative spectroscopic photoacoustic imaging: A review. J. Biomed. Opt. 2012, 17, 0612021. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic Clinical Imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Heijblom, M.; Piras, D.; Engh, F.M.; Schaaf, M.; Klaase, J.M.; Steenbergen, W.; Manohar, S. The state of the art in breast imaging using the Twente Photoacoustic Mammoscope: Results from 31 measurements on malignancies. Eur. Radiol. 2016, 1–14. [Google Scholar] [CrossRef]
- Heijblom, M.; Piras, D.; Maartens, E.; Huisman, E.J.; van den Engh, F.M.; Klaase, J.M.; Steenbergen, W.; Manohar, S. Appearance of breast cysts in planar geometry photoacoustic mammography using 1064-nm excitation. J. Biomed. Opt. 2013, 18, 126009. [Google Scholar] [CrossRef] [PubMed]
- Neuschler, E.I.; Butler, R.; Young, C.A.; Barke, L.D.; Bertrand, M.L.; Böhm-Vélez, M.; Destounis, S.; Donlan, P.; Grobmyer, S.R.; Katzen, J. A Pivotal Study of Optoacoustic Imaging to Diagnose Benign and Malignant Breast Masses: A New Evaluation Tool for Radiologists. Radiology 2017, 287, 398–412. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. The PIONEER-0 Study of the Imagio Breast Imaging System. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01943916 (accessed on 6 March 2019).
- Toi, M.; Asao, Y.; Matsumoto, Y.; Sekiguchi, H.; Yoshikawa, A.; Takada, M.; Kataoka, M.; Endo, T.; Kawaguchi-Sakita, N.; Kawashima, M. Visualization of tumor-related blood vessels in human breast by photoacoustic imaging system with a hemispherical detector array. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Kruger, R.A.; Lam, R.B.; Reinecke, D.R.; Del Rio, S.P.; Doyle, R.P. Photoacoustic angiography of the breast. Med. Phys. 2010, 37, 6096–6100. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Jain, R.K.; Safabakhsh, N.; Sckell, A.; Chen, Y.; Jiang, P.; Benjamin, L.; Yuan, F.; Keshet, E. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: Role of vascular endothelial growth factor. Proc. Natl. Acad. Sci. USA 1998, 95, 10820–10825. [Google Scholar] [CrossRef]
- Yuan, F.; Salehi, H.A.; Boucher, Y.; Vasthare, U.S.; Tuma, R.F.; Jain, R.K. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994, 54, 4564–4568. [Google Scholar]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T. Characterisation of Contrast Agents for Photoacoustic Imaging; UCL (University College London): London, UK, 2017. [Google Scholar]
- Asao, Y.; Hashizume, Y.; Suita, T.; Nagae, K.-i.; Fukutani, K.; Sudo, Y.; Matsushita, T.; Kobayashi, S.; Tokiwa, M.; Yamaga, I. Photoacoustic mammography capable of simultaneously acquiring photoacoustic and ultrasound images. J. Biomed. Opt. 2016, 21, 116009. [Google Scholar] [CrossRef] [PubMed]
- Menke, J. Photoacoustic breast tomography prototypes with reported human applications. Eur. Radiol. 2015, 25, 2205–2213. [Google Scholar] [CrossRef]
- Zackrisson, S.; van de Ven, S.; Gambhir, S. Light in and sound out: Emerging translational strategies for photoacoustic imaging. Cancer Res. 2014, 74, 979–1004. [Google Scholar] [CrossRef]
- Valluru, K.S.; Willmann, J.K. Clinical photoacoustic imaging of cancer. Ultrasonography 2016, 35, 267. [Google Scholar] [CrossRef]
- Gargiulo, S.; Albanese, S.; Mancini, M. State-of-the-Art Preclinical Photoacoustic Imaging in Oncology: Recent Advances in Cancer Theranostics. Contrast Media Mol. Imaging 2019, 2019, 5080267. [Google Scholar] [CrossRef]
- Manohar, S.; Dantuma, M. Current and future trends in photoacoustic breast imaging. Photoacoustics 2019, 16, 100134. [Google Scholar] [CrossRef]
- Nyayapathi, N.; Xia, J. Photoacoustic imaging of breast cancer: A mini review of system design and image features. J. Biomed. Opt. 2019, 24, 121911. [Google Scholar] [CrossRef]
- Esenaliev, R.O.; Karabutov, A.A.; Tittel, F.K.; Fornage, B.D.; Thomsen, S.L.; Stelling, C.; Oraevsky, A.A. Laser optoacoustic imaging for breast cancer diagnostics: Limit of detection and comparison with x-ray and ultrasound imaging. In Proceedings of the BiOS’97, Part of Photonics West, San Jose, CA, USA, 18 August 1997; pp. 71–82. [Google Scholar]
- Web of Science. Available online: http://apps.webofknowledge.com/UA_GeneralSearch_input.do?product=UA&SID=C3Dt5TrJjHRCHy7DaYF&search_mode=GeneralSearch (accessed on 27 September 2018).
- PubMed. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 28 September 2018).
- IEEE. IEEE Xplore Digital Library. Available online: https://ieeexplore.ieee.org/search/searchresult.jsp (accessed on 28 September 2018).
- Nature. Available online: https://www.nature.com/search/advanced? (accessed on 28 September 2018).
- Manohar, S.; Kharine, A.; van Hespen, J.C.; Steenbergen, W.; van Leeuwen, T.G. The Twente Photoacoustic Mammoscope: System overview and performance. Phys. Med. Biol. 2005, 50, 2543. [Google Scholar] [CrossRef]
- Ku, G.; Fornage, B.D.; Jin, X.; Xu, M.; Hunt, K.K.; Wang, L.V. Thermoacoustic and photoacoustic tomography of thick biological tissues toward breast imaging. Technol. Cancer Res. Treat. 2005, 4, 559–565. [Google Scholar] [CrossRef]
- Oraevsky, A.A.; Andreev, V.A.; Karabutov, A.A.; Fleming, R.D.; Gatalica, Z.; Singh, H.; Esenaliev, R.O. Laser optoacoustic imaging of the breast: Detection of cancer angiogenesis. In Proceedings of the BiOS’99 International Biomedical Optics Symposium, San Jose, CA, USA, 15 July 1999; pp. 352–363. [Google Scholar]
- Andreev, V.G.; Karabutov, A.A.; Solomatin, S.V.; Savateeva, E.V.; Aleinikov, V.; Zhulina, Y.V.; Fleming, R.D.; Oraevsky, A.A. Optoacoustic tomography of breast cancer with arc-array transducer. In Proceedings of the BiOS 2000 The International Symposium on Biomedical Optics, San Jose, CA, USA, 19 May 2000; pp. 36–47. [Google Scholar]
- Oraevsky, A.A.; Karabutov, A.A.; Solomatin, S.V.; Savateeva, E.V.; Andreev, V.A.; Gatalica, Z.; Singh, H.; Fleming, R.D. Laser optoacoustic imaging of breast cancer in vivo. In Proceedings of the BiOS 2001 The International Symposium on Biomedical Optics, San Jose, CA, USA, 15 June 2001; pp. 6–15. [Google Scholar]
- Oraevsky, A.A.; Savateeva, E.V.; Solomatin, S.V.; Karabutov, A.A.; Andreev, V.G.; Gatalica, Z.; Khamapirad, T.; Henrichs, P.M. Optoacoustic imaging of blood for visualization and diagnostics of breast cancer. In Proceedings of the International Symposium on Biomedical Optics, San Jose, CA, USA, 10 June 2002; pp. 81–94. [Google Scholar]
- Ermilov, S.A.; Conjusteau, A.; Mehta, K.; Lacewell, R.; Henrichs, P.M.; Oraevsky, A.A. 128-channel laser optoacoustic imaging system (LOIS-128) for breast cancer diagnostics. In Proceedings of the Biomedical Optics, San Jose, CA, USA, 6 March 2006; pp. 608609–608612. [Google Scholar]
- Ermilov, S.A.; Khamapirad, T.; Conjusteau, A.; Leonard, M.H.; Lacewell, R.; Mehta, K.; Miller, T.; Oraevsky, A.A. Laser optoacoustic imaging system for detection of breast cancer. J. Biomed. Opt. 2009, 14, 024007. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Manohar, S.; Kolkman, R.G.; Steenbergen, W.; van Leeuwen, T.G. Imaging of tumor vasculature using Twente photoacoustic systems. J. Biophotonics 2009, 2, 701–717. [Google Scholar] [CrossRef]
- Xia, W.; Piras, D.; Singh, M.K.; van Hespen, J.C.; van Leeuwen, T.G.; Steenbergen, W.; Manohar, S. Design and evaluation of a laboratory prototype system for 3D photoacoustic full breast tomography. Biomed. Opt. Express 2013, 4, 2555–2569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pramanik, M.; Ku, G.; Li, C.; Wang, L.V. Design and evaluation of a novel breast cancer detection system combining both thermoacoustic (TA) and photoacoustic (PA) tomography. Med. Phys. 2008, 35, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Erpelding, T.N.; Jankovic, L.; Liu, C.; Wang, L.V. Performance characterization of an integrated ultrasound, photoacoustic, and thermoacoustic imaging system. J. Biomed. Opt. 2012, 17, 0560101–0560106. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Yang, S.; Xing, D. Three-dimensional photoacoustic imaging system in line confocal mode for breast cancer detection. Appl. Phys. Lett. 2010, 97, 213702. [Google Scholar] [CrossRef]
- Xi, L.; Li, X.; Yao, L.; Grobmyer, S.; Jiang, H. Design and evaluation of a hybrid photoacoustic tomography and diffuse optical tomography system for breast cancer detection. Med. Phys. 2012, 39, 2584–2594. [Google Scholar] [CrossRef]
- Kruger, R.A.; Kuzmiak, C.M.; Lam, R.B.; Reinecke, D.R.; Del Rio, S.P.; Steed, D. Dedicated 3D photoacoustic breast imaging. Med. Phys. 2013, 40, 113301. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Manivasagan, P.; Quang Bui, N.; Oh, Y.-O.; Lim, I.G.; Park, S.; Oh, J. Cytotoxic Induction and Photoacoustic Imaging of Breast Cancer Cells Using Astaxanthin-Reduced Gold Nanoparticles. Nanomaterials 2016, 6, 78. [Google Scholar] [CrossRef]
- Wilson, K.E.; Bachawal, S.V.; Tian, L.; Willmann, J.K. Multiparametric spectroscopic photoacoustic imaging of breast cancer development in a transgenic mouse model. Theranostics 2014, 4, 1062–1071. [Google Scholar] [CrossRef]
- Zhang, T.; Cui, H.; Fang, C.-Y.; Jo, J.; Yang, X.; Chang, H.-C.; Forrest, M.L. In vivo photoacoustic imaging of breast cancer tumor with HER2-targeted nanodiamonds. In Proceedings of the SPIE NanoScience+ Engineering, San Diego, CA, USA, 20 September 2013; p. 881504. [Google Scholar]
- Balasundaram, G.; Ho, C.J.H.; Li, K.; Driessen, W.; Dinish, U.; Wong, C.L.; Ntziachristos, V.; Liu, B.; Olivo, M. Molecular photoacoustic imaging of breast cancer using an actively targeted conjugated polymer. Int. J. Nanomed. 2015, 10, 387. [Google Scholar] [CrossRef]
- Xiao, W.; Li, Y.; Hu, C.; Huang, Y.; He, Q.; Gao, H. Melanin-originated carbonaceous dots for triple negative breast cancer diagnosis by fluorescence and photoacoustic dual-mode imaging. J. Colloid Interface Sci. 2017, 497, 226–232. [Google Scholar] [CrossRef]
- Xia, J.; Feng, G.; Xia, X.; Hao, L.; Wang, Z. NH4HCO3 gas-generating liposomal nanoparticle for photoacoustic imaging in breast cancer. Int. J. Nanomed. 2017, 12, 1803. [Google Scholar] [CrossRef]
- Wilson, K.E.; Bachawal, S.V.; Abou-Elkacem, L.; Jensen, K.; Machtaler, S.; Tian, L.; Willmann, J.K. Spectroscopic Photoacoustic Molecular Imaging of Breast Cancer using a B7-H3-targeted ICG Contrast Agent. Theranostics 2017, 7, 1463. [Google Scholar] [CrossRef]
- Song, K.H.; Stein, E.W.; Margenthaler, J.A.; Wang, L.V. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. J. Biomed. Opt. 2008, 13, 054033. [Google Scholar] [CrossRef]
- Song, K.H.; Kim, C.; Cobley, C.M.; Xia, Y.; Wang, L.V. Near-infrared gold nanocages as a new class of tracers for photoacoustic sentinel lymph node mapping on a rat model. Nano Lett. 2008, 9, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Kim, C.; Maslov, K.; Wang, L.V. Noninvasive in vivo spectroscopic nanorod-contrast photoacoustic mapping of sentinel lymph nodes. Eur. J. Radiol. 2009, 70, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Kim, C.; Maslov, K.; Shung, K.K.; Wang, L.V. High-speed dynamic 3D photoacoustic imaging of sentinel lymph node in a murine model using an ultrasound array. Med. Phys. 2009, 36, 3724–3729. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, M.; Song, K.H.; Swierczewska, M.; Green, D.; Sitharaman, B.; Wang, L.V. In vivo carbon nanotube-enhanced non-invasive photoacoustic mapping of the sentinel lymph node. Phys. Med. Biol. 2009, 54, 3291. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Pramanik, M.; Senpan, A.; Ghosh, S.; Wickline, S.A.; Wang, L.V.; Lanza, G.M. Near infrared photoacoustic detection of sentinel lymph nodes with gold nanobeacons. Biomaterials 2010, 31, 4088–4093. [Google Scholar] [CrossRef]
- Akers, W.J.; Kim, C.; Berezin, M.; Guo, K.; Fuhrhop, R.; Lanza, G.M.; Fischer, G.M.; Daltrozzo, E.; Zumbusch, A.; Cai, X. Noninvasive photoacoustic and fluorescence sentinel lymph node identification using dye-loaded perfluorocarbon nanoparticles. ACS Nano 2010, 5, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Erpelding, T.N.; Jankovic, L.; Pashley, M.D.; Wang, L.V. Deeply penetrating in vivo photoacoustic imaging using a clinical ultrasound array system. Biomed. Opt. Express 2010, 1, 278–284. [Google Scholar] [CrossRef]
- Kim, C.; Song, K.H.; Gao, F.; Wang, L.V. Sentinel lymph nodes and lymphatic vessels: Noninvasive dual-modality in vivo mapping by using indocyanine green in rats—volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging 1. Radiology 2010, 255, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Erpelding, T.N.; Maslov, K.; Jankovic, L.; Akers, W.J.; Song, L.; Achilefu, S.; Margenthaler, J.A.; Pashley, M.D.; Wang, L.V. Handheld array-based photoacoustic probe for guiding needle biopsy of sentinel lymph nodes. J. Biomed. Opt. 2010, 15, 046010. [Google Scholar] [CrossRef] [PubMed]
- Erpelding, T.N.; Kim, C.; Pramanik, M.; Jankovic, L.; Maslov, K.; Guo, Z.; Margenthaler, J.A.; Pashley, M.D.; Wang, L.V. Sentinel lymph nodes in the rat: Noninvasive photoacoustic and US imaging with a clinical US system. Radiology 2010, 256, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Cai, X.; Yalaz, C.; Senpan, A.; Omanakuttan, K.; Wickline, S.A.; Wang, L.V.; Lanza, G.M. Photoacoustic sentinel lymph node imaging with self-assembled copper neodecanoate nanoparticles. ACS Nano 2012, 6, 1260–1267. [Google Scholar] [CrossRef]
- Luke, G.P.; Bashyam, A.; Homan, K.A.; Makhija, S.; Chen, Y.-S.; Emelianov, S.Y. Silica-coated gold nanoplates as stable photoacoustic contrast agents for sentinel lymph node imaging. Nanotechnology 2013, 24, 455101. [Google Scholar] [CrossRef]
- Grootendorst, D.J.; Jose, J.; Fratila, R.M.; Visscher, M.; Velders, A.H.; Ten Haken, B.; Van Leeuwen, T.G.; Steenbergen, W.; Manohar, S.; Ruers, T.J. Evaluation of superparamagnetic iron oxide nanoparticles (Endorem®) as a photoacoustic contrast agent for intra-operative nodal staging. Contrast Media Mol. Imaging 2013, 8, 83–91. [Google Scholar] [CrossRef]
- Liu, X.; Law, W.C.; Jeon, M.; Wang, X.; Liu, M.; Kim, C.; Prasad, P.N.; Swihart, M.T. Cu2–xSe nanocrystals with localized surface plasmon resonance as sensitive contrast agents for in vivo photoacoustic imaging: Demonstration of sentinel lymph node mapping. Adv. Healthc. Mater. 2013, 2, 952–957. [Google Scholar]
- Koo, J.; Jeon, M.; Oh, Y.; Kang, H.W.; Kim, J.; Kim, C.; Oh, J. In vivo non-ionizing photoacoustic mapping of sentinel lymph nodes and bladders with ICG-enhanced carbon nanotubes. Phys. Med. Biol. 2012, 57, 7853. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Zhang, Y.; Jeon, M.; Liu, C.; Song, L.; Lovell, J.F.; Kim, C. Dual-color photoacoustic lymph node imaging using nanoformulated naphthalocyanines. Biomaterials 2015, 73, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cheng, J.; Chen, Y.; Yu, S.; Liu, F.; Sun, Y.; Chen, Y.; Ran, H. Phase-transition nanodroplets for real-time photoacoustic/ultrasound dual-modality imaging and photothermal therapy of sentinel lymph node in breast cancer. Sci. Rep. 2017, 7, 45213. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, K.; Periyasamy, V.; Pramanik, M. Hand-held Clinical Photoacoustic Imaging System for Real-time Non-invasive Small Animal Imaging. 2017. Available online: https://www.jove.com/video/56649/hand-held-clinical-photoacoustic-imaging-system-for-real-time-non (accessed on 21 January 2020).
- Cha, M.G.; Lee, S.; Park, S.; Kang, H.; Lee, S.G.; Jeong, C.; Lee, Y.-S.; Kim, C.; Jeong, D.H. A dual modal silver bumpy nanoprobe for photoacoustic imaging and SERS multiplexed identification of in vivo lymph nodes. Nanoscale 2017, 9, 12556–12564. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.R.; Kang, J.; Kwak, J.Y.; Chang, J.H.; Kim, S.I.; Youk, J.H.; Moon, H.J.; Kim, M.J.; Kim, E.-K. Photoacoustic imaging of breast microcalcifications: A preliminary study with 8-gauge core-biopsied breast specimens. PLoS ONE 2014, 9, e105878. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.T.; Zhang, R.; Hai, P.; Zhang, C.; Pleitez, M.A.; Aft, R.L.; Novack, D.V.; Wang, L.V. Fast label-free multilayered histology-like imaging of human breast cancer by photoacoustic microscopy. Sci. Adv. 2017, 3, e1602168. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Vaartjes, S.E.; van Hespen, J.C.; Klaase, J.M.; van den Engh, F.M.; Steenbergen, W.; Van Leeuwen, T.G. Initial results of in vivo non-invasive cancer imaging in the human breast using near-infrared photoacoustics. Opt. Express 2007, 15, 12277–12285. [Google Scholar] [CrossRef]
- Menezes, G.L.; Pijnappel, R.M.; Meeuwis, C.; Bisschops, R.; Veltman, J.; Lavin, P.T.; van de Vijver, M.J.; Mann, R.M. Downgrading of Breast Masses Suspicious for Cancer by Using Optoacoustic Breast Imaging. Radiology 2018, 288, 355–365. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Imaging with Opto-acoustics to downgrade BI-RADS classification relative to other Diagnostic Methodologies (MAESTRO). Available online: https://clinicaltrials.gov/ct2/show/record/NCT02364388 (accessed on 6 March 2019).
- Lin, L.; Hu, P.; Shi, J.; Appleton, C.M.; Maslov, K.; Li, L.; Zhang, R.; Wang, L.V. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 2018, 9, 2352. [Google Scholar] [CrossRef]
- Garcia-Uribe, A.; Erpelding, T.N.; Krumholz, A.; Ke, H.; Maslov, K.; Appleton, C.; Margenthaler, J.A.; Wang, L.V. Dual-modality photoacoustic and ultrasound imaging system for noninvasive sentinel lymph node detection in patients with breast cancer. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Kitai, T.; Torii, M.; Sugie, T.; Kanao, S.; Mikami, Y.; Shiina, T.; Toi, M. Photoacoustic mammography: Initial clinical results. Breast Cancer 2014, 21, 146–153. [Google Scholar] [CrossRef]
- Fakhrejahani, E.; Torii, M.; Kitai, T.; Kanao, S.; Asao, Y.; Hashizume, Y.; Mikami, Y.; Yamaga, I.; Kataoka, M.; Sugie, T. Clinical report on the first prototype of a photoacoustic tomography system with dual illumination for breast cancer imaging. PLoS ONE 2015, 10, e0139113. [Google Scholar] [CrossRef]
- Li, X.; Heldermon, C.D.; Yao, L.; Xi, L.; Jiang, H. High resolution functional photoacoustic tomography of breast cancer. Med. Phys. 2015, 42, 5321–5328. [Google Scholar] [CrossRef]
- Zalev, J.; Clingman, B.; Herzog, D.; Miller, T.; Ulissey, M.; Stavros, A.T.; Oraevsky, A.; Lavin, P.; Kist, K.; Dornbluth, N.C. Opto-acoustic image fusion technology for diagnostic breast imaging in a feasibility study. In Proceedings of the SPIE Medical Imaging, Orlando, FL, USA, 17 March 2015; p. 941909. [Google Scholar]
- Neuschler, E.I.; Lavin, P.T.; Tucker, F.L.; Barke, L.D.; Bertrand, M.L.; Böhm-Vélez, M.; Destounis, S.; Dogan, B.E.; Grobmyer, S.R.; Katzen, J. Downgrading and upgrading gray-scale ultrasound BI-RADS categories of benign and malignant masses with optoacoustics: A pilot study. Am. J. Roentgenol. 2018, 211, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Menezes, G.L.; Mann, R.M.; Meeuwis, C.; Bisschops, B.; Veltman, J.; Lavin, P.T.; van de Vijver, M.J.; Pijnappel, R.M. Optoacoustic imaging of the breast: Correlation with histopathology and histopathologic biomarkers. Eur. Radiol. 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dogan, B.E.; Menezes, G.L.; Butler, R.S.; Neuschler, E.I.; Aitchison, R.; Lavin, P.T.; Tucker, F.L.; Grobmyer, S.R.; Otto, P.M.; Stavros, A.T. Optoacoustic imaging and gray-scale US features of breast cancers: Correlation with molecular subtypes. Radiology 2019, 292, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Piras, D.; Xia, W.; Steenbergen, W.; van Leeuwen, T.G.; Manohar, S. Photoacoustic imaging of the breast using the twente photoacoustic mammoscope: Present status and future perspectives. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 730–739. [Google Scholar] [CrossRef]
- Heijblom, M.; Piras, D.; Ten Tije, E.; Xia, W.; Van Hespen, J.; Klaase, J.; Van den Engh, F.; Van Leeuwen, T.; Steenbergen, W.; Manohar, S. Breast imaging using the Twente Photoacoustic Mammoscope (PAM): New clinical measurements. In Proceedings of the European Conference on Biomedical Optics, Munich Germany, 22–26 May 2011; p. 80870N. [Google Scholar]
- Heijblom, M.; Piras, D.; Xia, W.; Van Hespen, J.; Klaase, J.; Van den Engh, F.; Van Leeuwen, T.; Steenbergen, W.; Manohar, S. Visualizing breast cancer using the Twente photoacoustic mammoscope: What do we learn from twelve new patient measurements? Opt. Express 2012, 20, 11582–11597. [Google Scholar] [CrossRef]
- Erguvan-Dogan, B.; Whitman, G.J.; Kushwaha, A.C.; Phelps, M.J.; Dempsey, P.J. Bi-RADS-MRI: A primer. Am. J. Roentgenol. 2006, 187, W152–W160. [Google Scholar] [CrossRef]
- Oraevsky, A. 3D optoacoustic tomography: From molecular targets in mouse models to functional imaging of breast cancer. In Proceedings of the conference on Lasers and Electro-Optics (CLEO), San Jose, CA, USA, 8–13 June 2014; pp. 1–2. [Google Scholar]
- Oraevsky, A.; Su, R.; Nguyen, H.; Moore, J.; Lou, Y.; Bhadra, S.; Forte, L.; Anastasio, M.; Yang, W. Full-View 3d Imaging System for Functional and Anatomical Screening of the Breast; SPIE: San Francisco, CA, USA, 11 April 2018; Volume 10494. [Google Scholar]
- Raikhlin, A.; Curpen, B.; Warner, E.; Betel, C.; Wright, B.; Jong, R. Breast MRI as an adjunct to mammography for breast cancer screening in high-risk patients: Retrospective review. Am. J. Roentgenol. 2015, 204, 889–897. [Google Scholar] [CrossRef]
- Becker, A.; Masthoff, M.; Claussen, J.; Ford, S.J.; Roll, W.; Burg, M.; Barth, P.J.; Heindel, W.; Schäfers, M.; Eisenblätter, M. Multispectral optoacoustic tomography of the human breast: Characterisation of healthy tissue and malignant lesions using a hybrid ultrasound-optoacoustic approach. Eur. Radiol. 2017, 28, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Diot, G.; Metz, S.; Noske, A.; Liapis, E.; Schroeder, B.; Ovsepian, S.V.; Meier, R.; Rummeny, E.; Ntziachristos, V. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer Res. 2017, 23, 6912–6922. [Google Scholar] [CrossRef] [PubMed]
- Abeyakoon, O.; Morscher, S.; Dalhaus, N.; Ford, S.J.; Mendichovszky, I.A.; Manavaki, R.; Wallis, M.; Moyle, P.; Woitek, R.; Patterson, A. Optoacoustic Imaging Detects Hormone-Related Physiological Changes of Breast Parenchyma. Ultraschall Med.-Eur. J. Ultrasound 2019, 40, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Heldermon, C.; Jiang, H. Monitoring neoadjuvant chemotherapy in breast cancer using quantitative photoacoustic tomography. In Proceedings of the Biomedical Optics, Miami, FL, USA, 26–30 April 2014; p. BS3A. 66. [Google Scholar]
- Hysi, E.; Wirtzfeld, L.A.; May, J.P.; Undzys, E.; Li, S.-D.; Kolios, M.C. Photoacoustic signal characterization of cancer treatment response: Correlation with changes in tumor oxygenation. Photoacoustics 2017, 5, 25–35. [Google Scholar] [CrossRef]
- Heijblom, M.; Steenbergen, W.; Manohar, S. Clinical photoacoustic breast imaging: The Twente experience. IEEE Pulse 2015, 6, 42–46. [Google Scholar] [CrossRef]
- Yamaga, I.; Kawaguchi-Sakita, N.; Asao, Y.; Matsumoto, Y.; Yoshikawa, A.; Fukui, T.; Takada, M.; Kataoka, M.; Kawashima, M.; Fakhrejahani, E. Vascular branching point counts using photoacoustic imaging in the superficial layer of the breast: A potential biomarker for breast cancer. Photoacoustics 2018, 11, 6–13. [Google Scholar] [CrossRef]
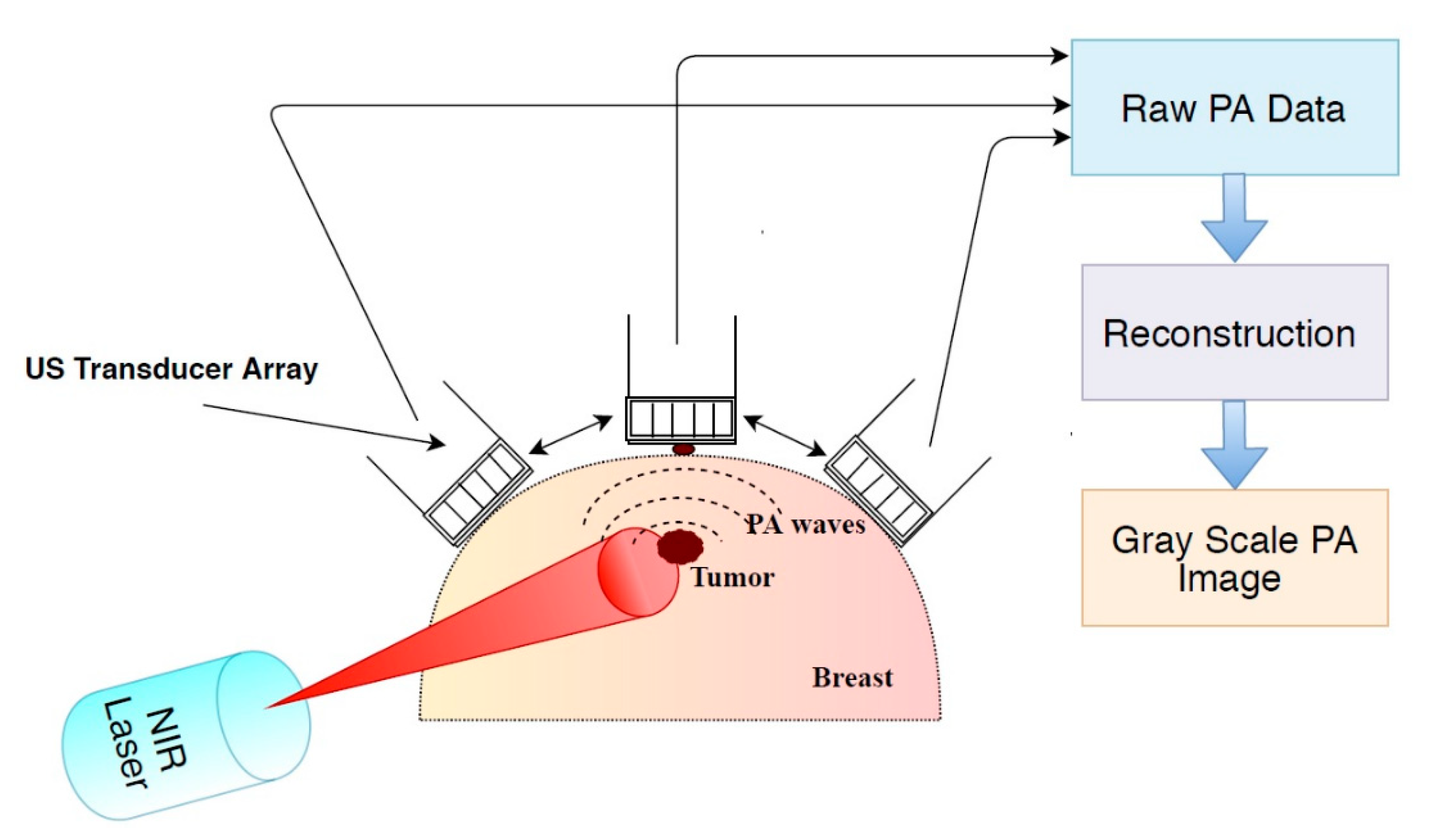
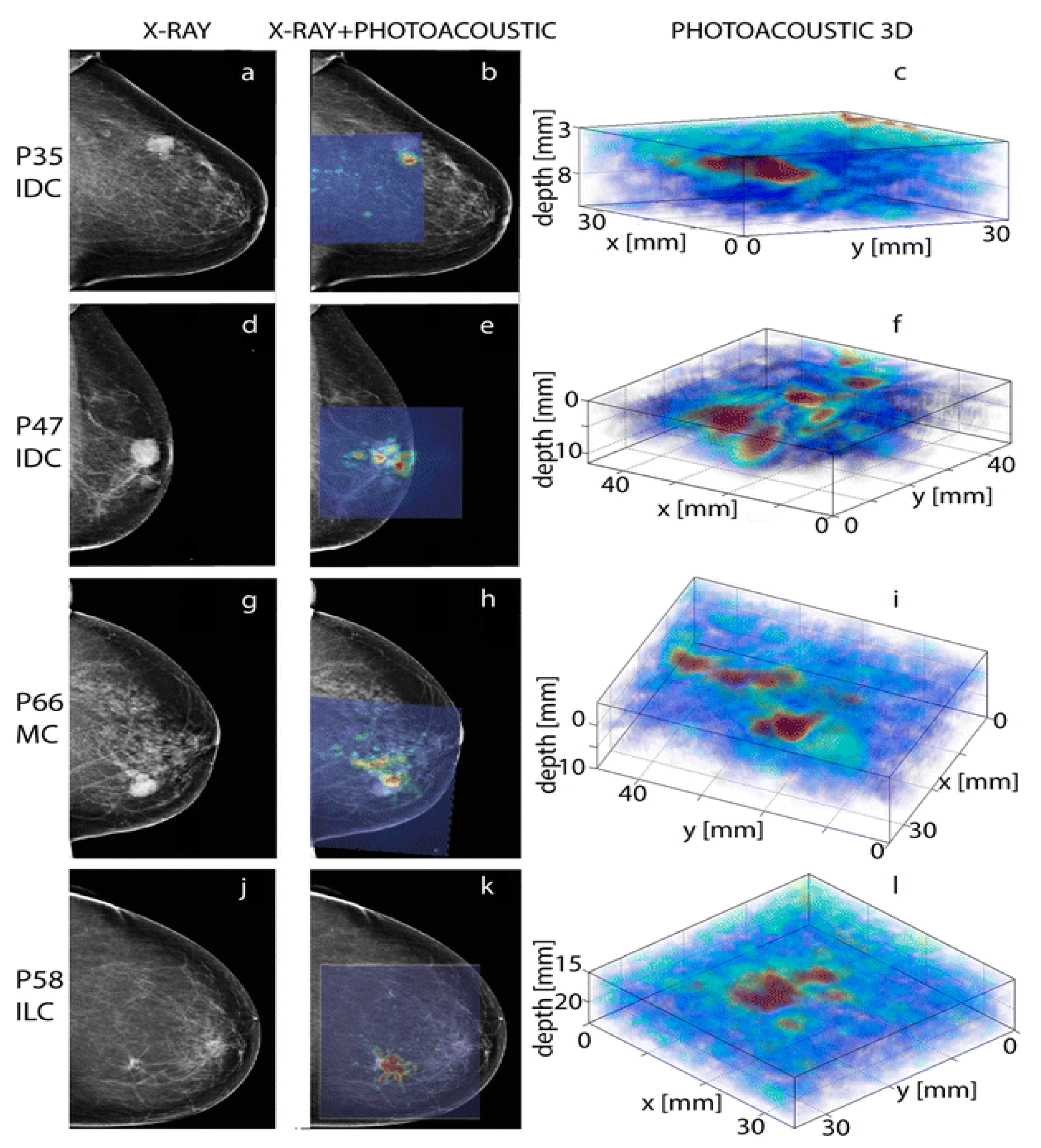
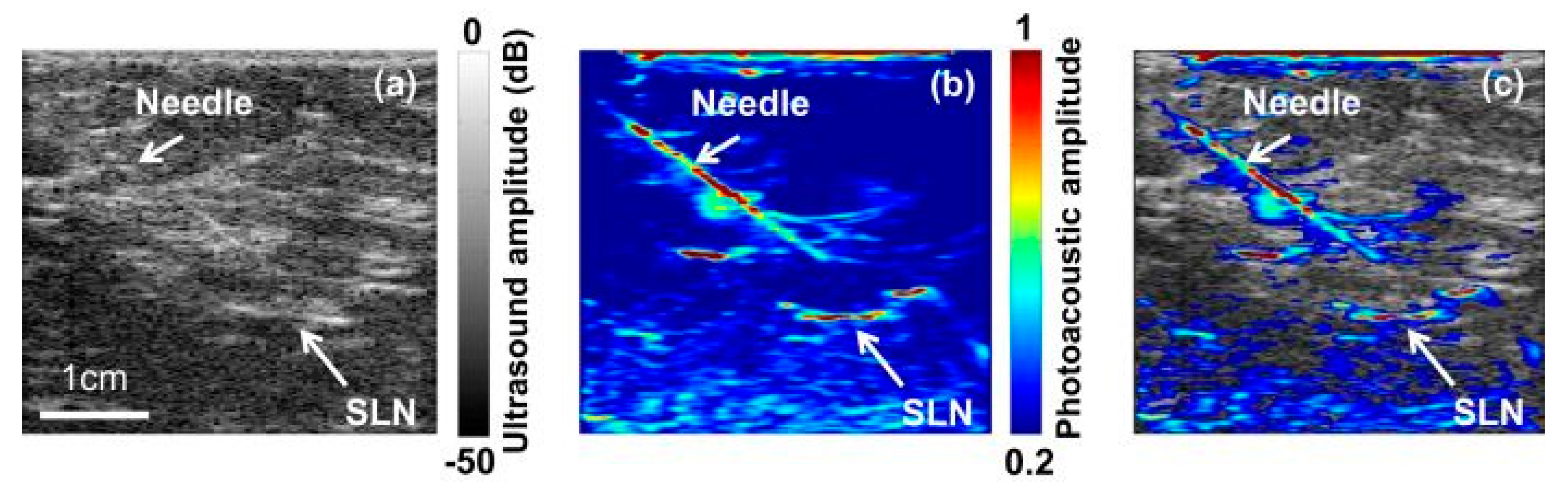
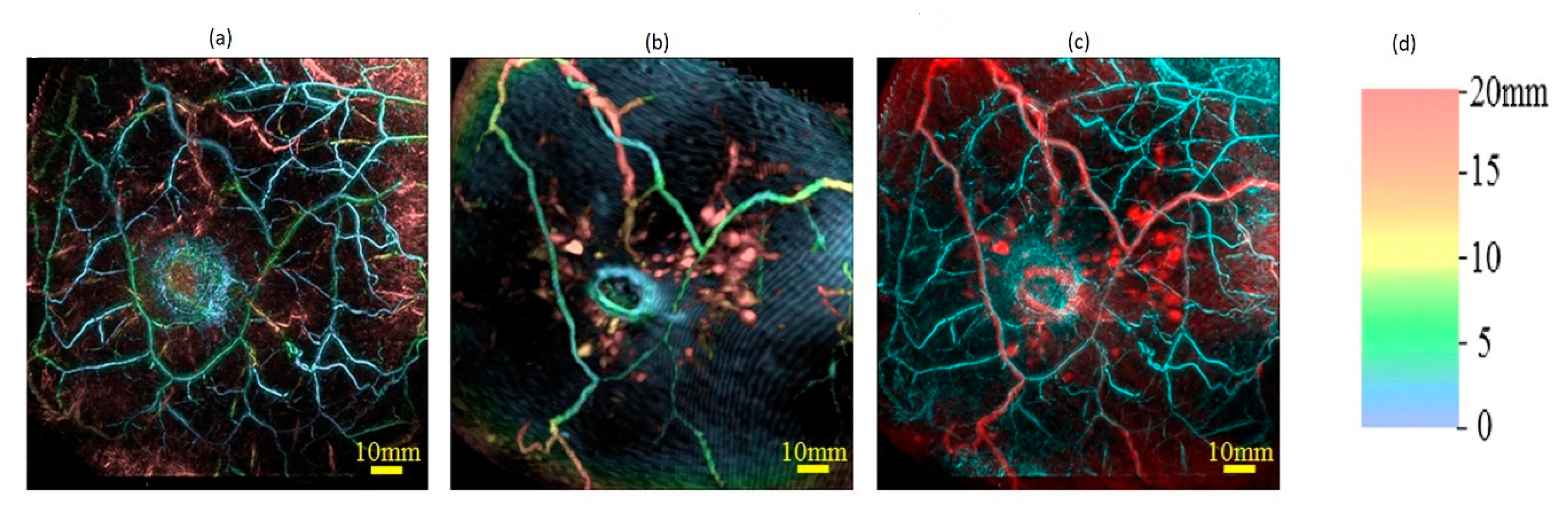
Based on the study results reported by various research groups beginning from 1997 to 2019, this review provides:
|
| Electronic Database | Web of Science [55] | PubMed [56] | IEEE [57] | Nature [58] | Total | Remarks | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phrase1 | Phrase2 | Phrase1 | Phrase2 | Phrase1 | Phrase2 | Phrase1 | Phrase2 | |||
| Number of Articles | 424 | 128 | 190 | 32 | 38 | 9 | 103 | 30 | 924 | Initial Search |
| PA/OA for other cancers | 79 | 30 | 23 | 1 | 7 | 2 | 20 | 5 | 167 | 431 Articles Excluded |
| PA/OA imaging systems | 62 | 38 | 10 | 2 | 4 | 2 | 16 | 8 | 142 | |
| BC with other imaging system | 24 | 2 | 5 | 0 | 2 | 0 | 5 | 0 | 38 | |
| Others | 19 | 7 | 1 | 0 | 2 | 0 | 45 | 10 | 84 | |
| Remaining Articles | Articles from initial search (924)—Articles excluded (431) | 493 | ||||||||
| Articles considered for this review | Remaining articles (493)—(Duplicates)—(Articles involving studies not having ethics committee/institutional board approval) | 66 | ||||||||
| Breast Imaging | 47,514 | 42,036 | 16,907 | 18,740 | 125,197 | |||||
| Phantom Study | Materials Mimicking Breast Tissue | Materials Mimicking Tumor/Blood Vessel/Target |
|---|---|---|
| Esenaliev et al. [54] | 10% gelatin (bulk collagen gels) 1064 nm (µa = 0.11 cm−1, µs′ = 2.92 cm−1) | Gel spheres colored with the bovine hemoglobin (µa = 0.75 cm−1) |
| Oraevsky et al. [61] | 10% gelatin (µa = 0.13 cm−1) | Gel spheres colored with the bovine hemoglobin |
| Andreev et al. [62] | Gelatin | Gel spheres colored with the bovine hemoglobin (µa = 1.0 cm−1) |
| Oraevsky et al. [63] | Gelatin | Blood vessels filled with rat blood (µa varies from 0.8 to 4.0 cm−1) |
| Oraevsky et al. [64] | 10% gelatin (µa = 0.13 cm−1) | Polyethelene tubes filled with the blood of sheep (µa in the range of 0.45 to 1.0 cm−1). |
| Ermilov et al. [65] | Polyvinyl chloride-plastisol (PVCP) | Pencil leads |
| Ermilov et al. [66] | India ink dissolved in aqueous milk solution | A thin-wall rubber shell filled with India ink dissolved in aqueous milk solution |
| Manohar et al. [59] | Poly vinyl alcohol (PVA) gel | Dyed, rigid PVA gel spheres |
| Jose et al. [67] | Intralipid, PVA | A silicon rubber tube filled with flowing human blood and dyed, rigid PVA gel spheres |
| Xia et al. [68] | Agar/intralipid gel | Plastic tube filled with agar gel and spherical objects made of agar gel |
| Ku et al. [60] | Chicken breast muscle | Raw blood in 1.5 mm diameter tube |
| Pramanik et al. [69] | Porcine fat | water-based agar gel 2% agar, 2% salt and 96% water): clear gel/gel mixed with black India ink |
| Ke et al. [70] | Chicken breast | Low-density polyethylene (LDPE) tubes filled with either oxygenated bovine blood or methylene blue dye |
| Ye et al. [71] | 13% gelatin, 12.5% milk, and 74.5% water | Carbon points |
| Xi et al. [72] | Intralipid + India ink | Thin metal wires and ex-vivo tumor tissue |
| Kruger et al. [73] | Carbon/graphite fiber embedded in agar | Point absorber (a small spot of ink placed on the tip of a clear, thin polyethylene thread) |
| Bharathiraja et al. [74] | Gelatin | Astaxanthin-mediated gold nanoparticles (Atx-AuNPs)-treated breast cancer cells |
| Study | Contrast Agent | Animals | Tumor/Target |
|---|---|---|---|
| Zhang et al. [76] | Radiation damaged nanodiamond | Female Balb/c mice | 1. HER2 positive tumor model (4T1.2 neu) treated with PEG-NDs 2. HER2 negative tumor model (4T1.2) treated with HER2-PEG-DNDs |
| Balasundaram et al. [77] | Folate-CP dots | Mice xenograft models | MCF-7 breast cancer cells |
| Xiao et al. [78] | Melanin carbonaceous dots (MCDs) | Female Balb/c mice | Triple negative breast cancer cell line 4T1 |
| Xia et al. [79] | Liposome-encapsulating ammonium bicarbonate (NH4HCO3) | Female nude mice | MDA-MB-231 tumor cells |
| Wilson et al. [80] | B7-H3-ICG | Transgenic mouse model | FVB/N-Tg(MMTVPyMT)634Mul that develops invasive breast cancers |
| Song et al. [81] | Methylene Blue | Adult male Sprague Dawley rats | Sentinel lymph node (SLN) detection |
| Song et al. [82] | Au nanocages | Sprague-Dawley rats | |
| Song et al. [83] | Au nanorods | Adult male Sprague Dawley rats | |
| Song et al. [84] | Evans blue | Murine model | |
| Pramanik et al. [85] | Single-walled carbon nanotubes (SWNT) | Rat model | |
| Pan et al. [86] | Au nanobeacons | Rodent model | |
| Akers et al. [87] | Perfluorocarbon (PFC) nanoparticles loaded with NIR dye PPCy-C8 | Rats | |
| Kim et al. [88] | Methylene blue | Sprague Dawley rats | |
| Kim et al. [89,90] | ICG | Rats | |
| Erpelding et al. [91] | Methylene blue | Rats | |
| Pan et al. [92] | Copper neodecanoate nanoparticles | Rodent model | |
| Luke et al. [93] | Silica-coated gold nanoparticles (Si-AuNPs). | Mouse model | |
| Grootendorst et al. [94] | Super-paramagnetic iron oxide nanoparticles (Endorem) | Rats | |
| Liu et al. [95] | Copper Selenide nanocrystals (Cu2-xSe NCs) | Rat model | |
| Koo et al. [96] | SWNTs conjugated with ICG | Rat | |
| Lee et al. [97] | Nano-formulated napthalocyanines (nanonaps) | Rats | |
| Yang et al. [98] | Carbon nanoparticles added with liquid-gas phase-transition nanodroplets (CNPs) | New Zealand white rabbits | |
| Sivasubramanian et al. [99] | Methylene blue | Rat | |
| Cha et al. [100] | Silica-coated silver bumpy nanoshell probes | Rat |
| Research Group | Optical Wavelength(s) (nm) | Detector | Reconstruction | Resolution | Penetration Depth | Field-of-View | N | Study Type | Clinical Study |
|---|---|---|---|---|---|---|---|---|---|
| S. Manohar Biomedical Photonic Imaging Group Twente PAM [35,36] | 1064 | Planar, 590 elements, 1 MHz, 130% bandwidth | Acoustic back-projection algorithm | 3 mm (axial and lateral) | 25–30 mm | 90 × 80 mm2 | 31 | In vivo | Yes |
| Kruger OptoSonics, Inc., 3D PAT [40] | 800 | Hemispherical, 128 elements, 5 MHz | Filtered back-projection algorithm | ~250 µm (spatial) | 40 mm | 64 × 64 × 50 mm3 | 01 | In vivo | No |
| Kruger OptoSonics, Inc., 3D PAM [73] | 756 | Spherical aperture, 512 elements, 2 MHz, 70% bandwidth | Filtered back-projection algorithm | 0.42 mm (spatial) | 53 mm | 1335 mL (breast size) | 04 | In vivo | No |
| Oraevsky Tomowave Laboratories, Inc. LOUIS-3D [119,120] # | 1064 | Arc, 96 elements, bandwidth 50 KHz to 6 MHz | ~0.28 mm | 100 | In vivo | Pilot clinical study | |||
| L.V. Wang Optical Imaging Laboratory Tri-modality TAT/PAT/US [70] | 650 | Ultrasound phased array, 80 elements, bandwidth 1 MHz to 5 MHz | Delay and Sum/Fourier beam- forming algorithm | 640 µm (axial), 720 µm (lateral), and 3.5 mm (elevational) | 66 mm | NA | Animal (Chicken Breast tissue) | No | |
| L.V. Wang Caltech Optical Imaging Laboratory [106] | 1064 | Full-ring, 512 elements, 2.25 MHz, 95% bandwidth | 3D back-projection algorithm | 255 µm spatial | 32 mm | Accommodate breast sizes ranging from B cup to DD cup | 08 | In vivo | Pilot study |
| Toi Kyoto University, Japan PAM-03 [39] | 755, 795 | Hemispherical, 512 elements, 2 MHz, 70% bandwidth | Universal back-projection algorithm | 27 mm | 30 | In vivo | Yes | ||
| IMAGIOTM Seno Medical Instruments Inc. IMAGIOTM Seno Medical Instruments Inc. [37,38] # | 1064 (Nd:YAG laser), 757 (Alexandrite laser) | Linear, 128 elements, bandwidth 4 MHz to 16 MHz | Back-projection of filtered and weighted optoacoustic signals | Nd: YAG laser: 0.42 mm (axial) 0.73 (lateral) Alexandrite laser: 0.47 mm (Axial) 0.81 mm (lateral) | 2105 | In vivo | Yes | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, A.P.; Bokde, N.; Sinha, S. Photoacoustic Imaging for Management of Breast Cancer: A Literature Review and Future Perspectives. Appl. Sci. 2020, 10, 767. https://doi.org/10.3390/app10030767
Rao AP, Bokde N, Sinha S. Photoacoustic Imaging for Management of Breast Cancer: A Literature Review and Future Perspectives. Applied Sciences. 2020; 10(3):767. https://doi.org/10.3390/app10030767
Chicago/Turabian StyleRao, A. Prabhakara, Neeraj Bokde, and Saugata Sinha. 2020. "Photoacoustic Imaging for Management of Breast Cancer: A Literature Review and Future Perspectives" Applied Sciences 10, no. 3: 767. https://doi.org/10.3390/app10030767
APA StyleRao, A. P., Bokde, N., & Sinha, S. (2020). Photoacoustic Imaging for Management of Breast Cancer: A Literature Review and Future Perspectives. Applied Sciences, 10(3), 767. https://doi.org/10.3390/app10030767






