4.1. Depth and Temperature Calibrations of Samples
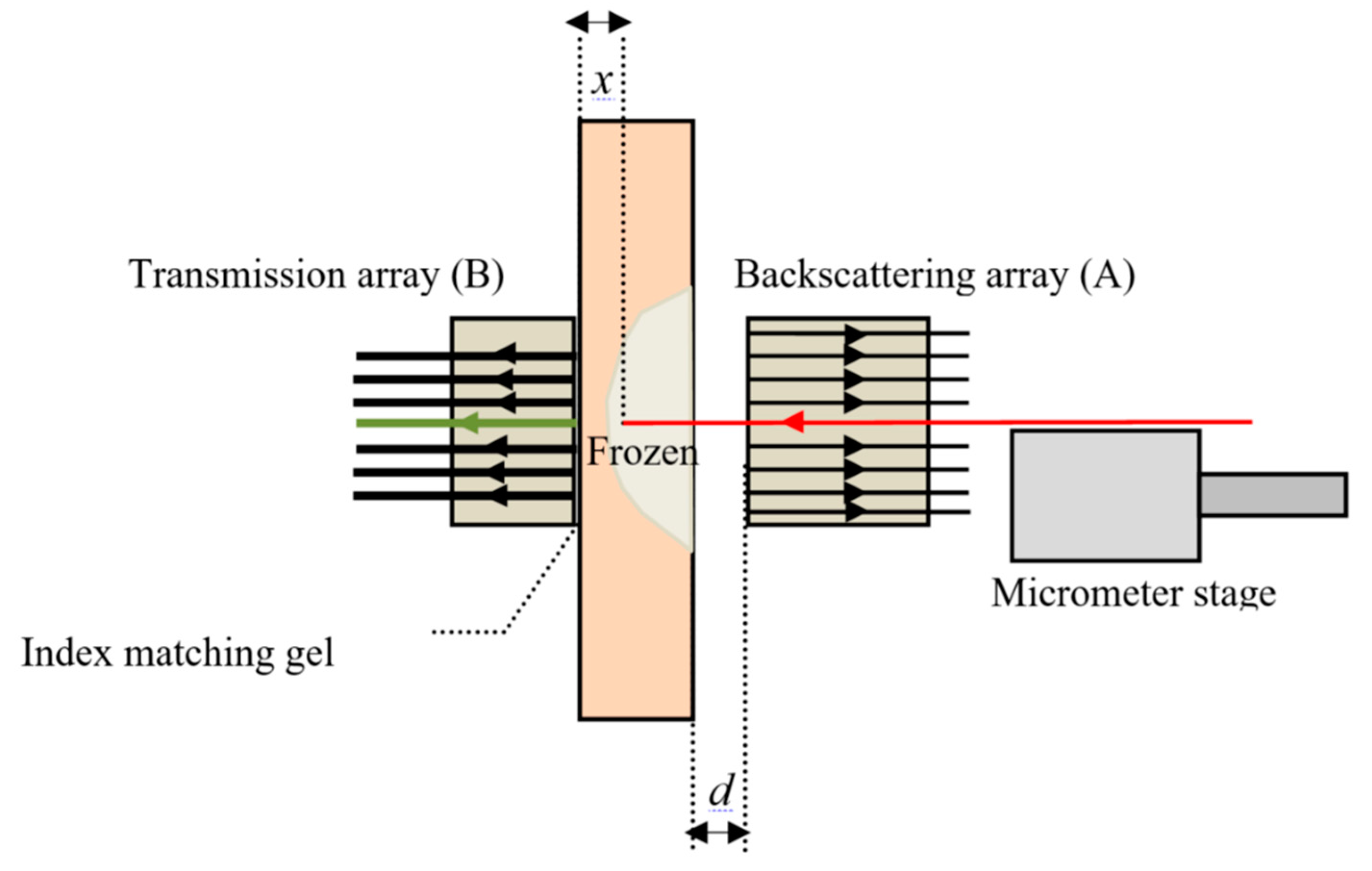
The calibration procedure, described previously, correlates backscattered and transmitted light detected by fiber arrays A and B for different skin depths of the illumination fiber. Initially the illumination fiber was in contact with detection fiber (T4 in array-B), i.e., to a distance of
x = 0. The tissue was frozen measuring backscattered and transmitted intensities from arrays-A and -B. When the tissue thawed, the illumination fiber was retracted in steps by 0.1 to 0.2 mm, and the process was repeated until the fiber reached the surface of the tissue. Typical results for successive freeze/thaw cycles of a 3.0 mm thick tissue sample are shown in
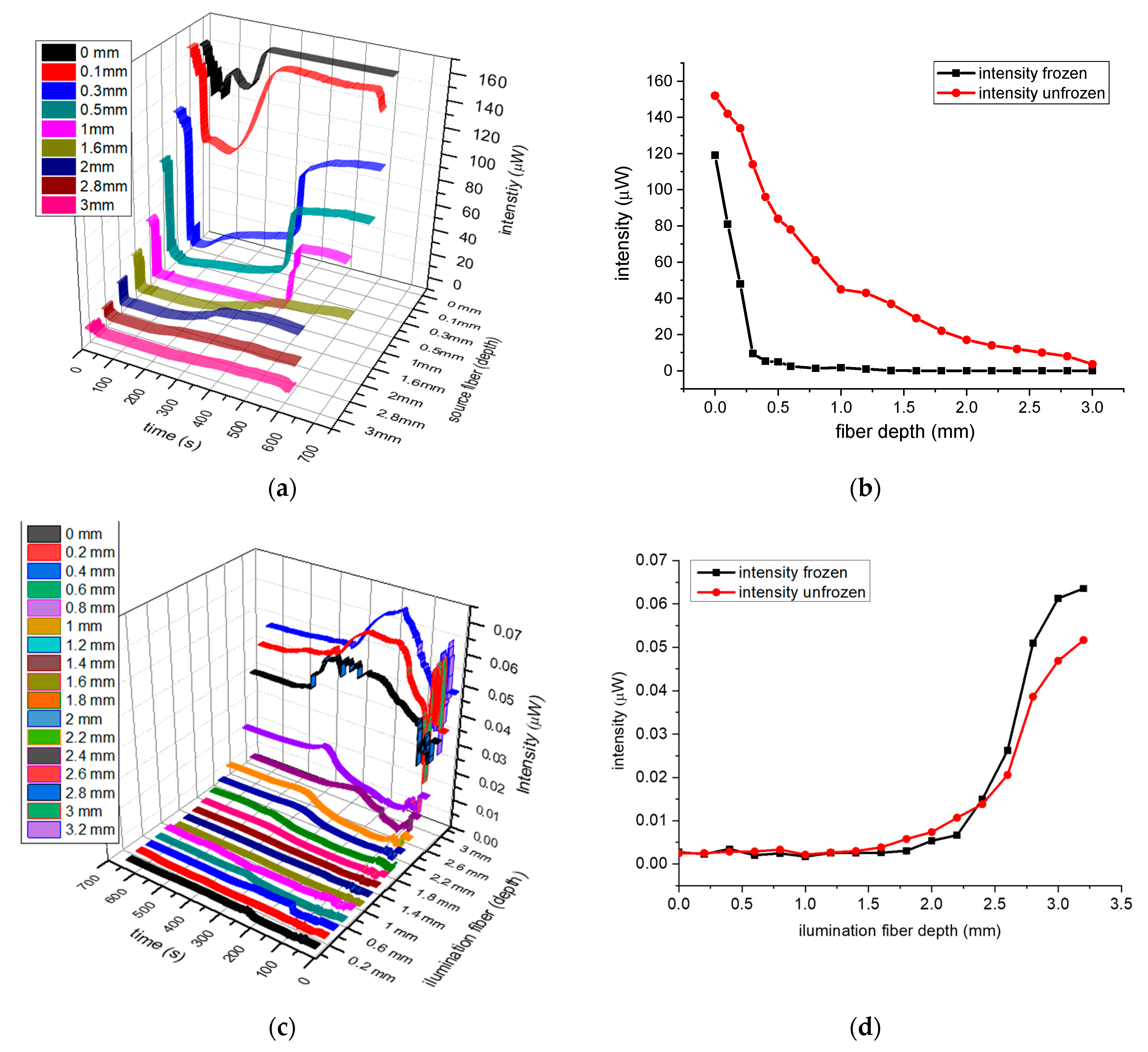
Figure 3a,c, with the transmitted signals from fiber-T4 in array-B and BS4 in array-A, are plotted as a function of time (timeline) for different tissue depths.
Generally, the traces in
Figure 3a exhibiting an initial rapid reduction in intensity, due to the ice formation in the tissue which reduce the transmitted light, followed by a near constant low transmission, which is dependent on the skin separation
x, between the two fibers. Thawing takes several minutes, with tissue gradually returning from pale white to its original color, and the transmitted intensity gradually returns to its initial value. The transmitted intensities as a function of skin thicknesses
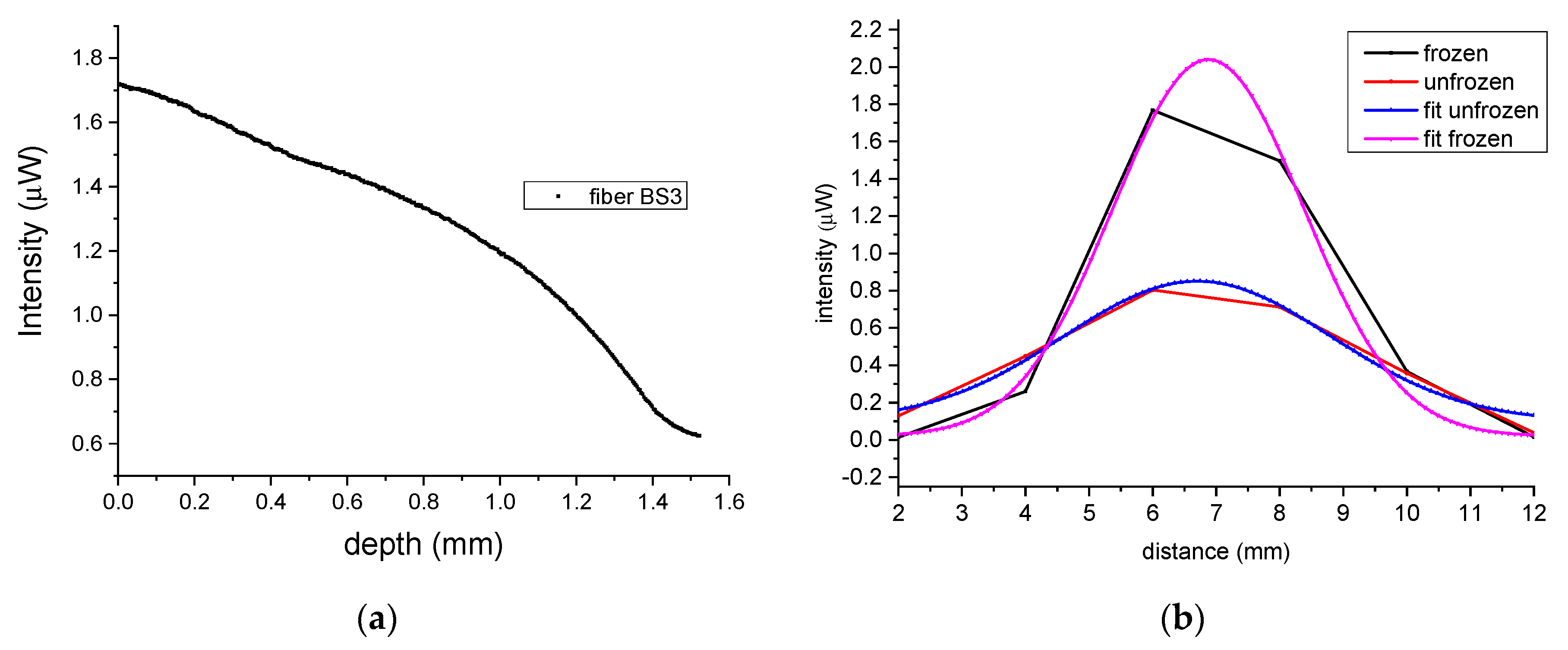
x for frozen and unfrozen tissue are plotted in
Figure 3b, with the red trace being that of the unfrozen skin, while the black trace is for the frozen one. Based on the results in
Figure 3b, it can be seen that light diffused much further in unfrozen tissue (red trace) in contrast to the optical diffusion in frozen tissue, which was greatly reduced reaching near zero when the depth was about 1.4 mm to 1.5 mm (black trace). Similarly, the graphs in
Figure 3c show the backscattering timelines for the different depths
x.
Figure 3d shows the backscattered intensities for frozen (black) and unfrozen tissue (red) as a function of distances
x. Here, the variation in intensities was reciprocal to those of transmitted signals, which is to be expected as light cannot be transmitted through frozen tissue, and therefore is backscattered.
When the illumination fiber is fully retracted,
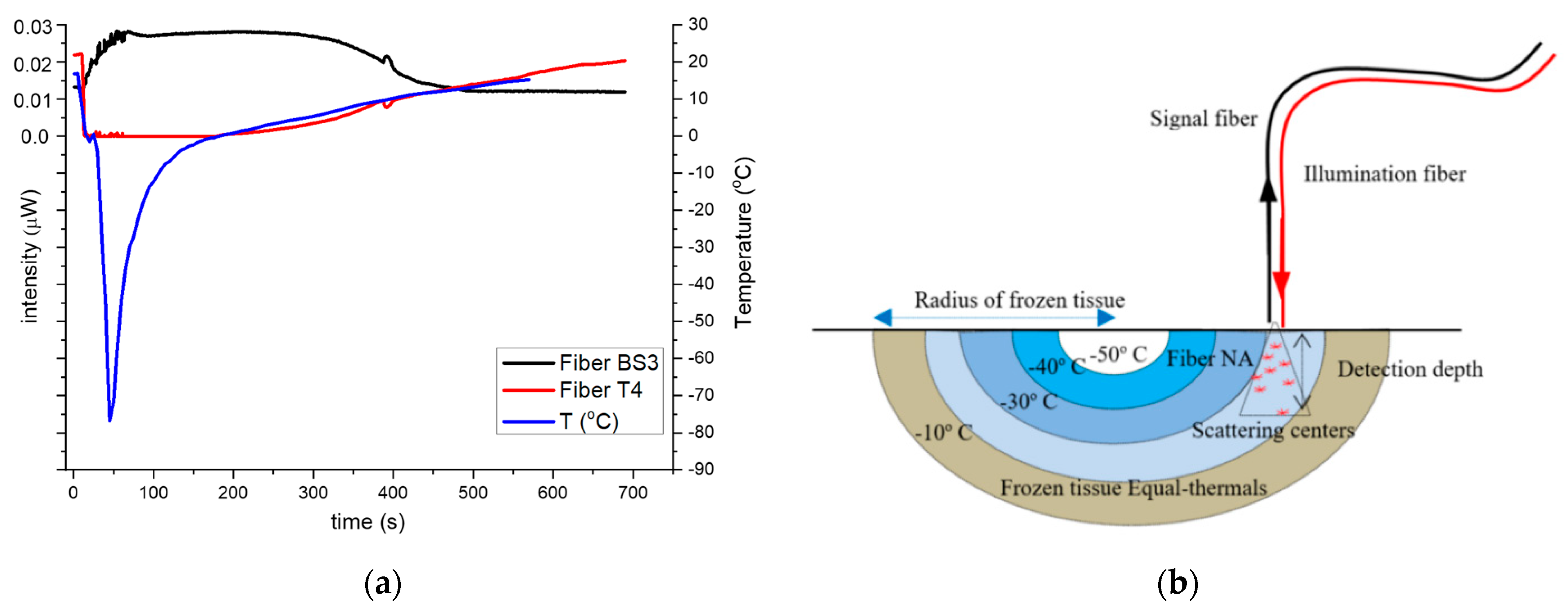
x = 3.0 mm and in contact with the skin surface, the timeline for transmitted, backscattered intensities and temperature variation for a freeze/thaw cycle is shown in
Figure 4a. When cryogen is applied, the temperature drops rapidly to about −80 °C followed by a rapid increase up to 0 °C, increasing slowly (around 0.05 deg/s) thereafter which attributed to the latent heat of freezing of the frozen tissue (blue trace
Figure 4a). The backscattered intensity variations, (in black), can be roughly divided in three regions related to the temperatures of the tissue, ignoring the first 100 s where temperature equalization happens. It is high and nearly constant when the tissue is frozen in the temperature range of −70 °C to −3 °C (100–300 s), lower and constant for the unfrozen in the 5 °C to 20 °C temperature range (450–700 s) and finally, in the region of −2 °C to 3 °C, the optical signals exhibit a progressive drop (300–450 s).
Conversely, the transmitted timeline signal (red trace) is, as expected, the reciprocal of the backscattered signal. This is attributed to the shrinking ice-hemisphere “ice ball” under the fiber sensor, as shown schematically in
Figure 4b, which leads to a corresponding gradual reduction in scattering. In other words, the reducing frozen volume under the detection cone (NA) of the fiber array due to the shrinking ice-hemisphere emulates a progressive reduction in the frozen tissue thickness. During thawing, both signals remain constant, as the maximum detectible optical transmission in frozen tissue is about 1.5 mm (based on the transmission calibration procedure,
Section 4.1), as light emanating from lower depths cannot be detected. Therefore, irrespective of the frozen skin depth, the backscattered signal that can be detected by array-A, comes from a maximum depth of 1.5 mm or less, while for the same reason the transmitted signal remains low until the frozen tissue also retreats to 1.5 mm or less.
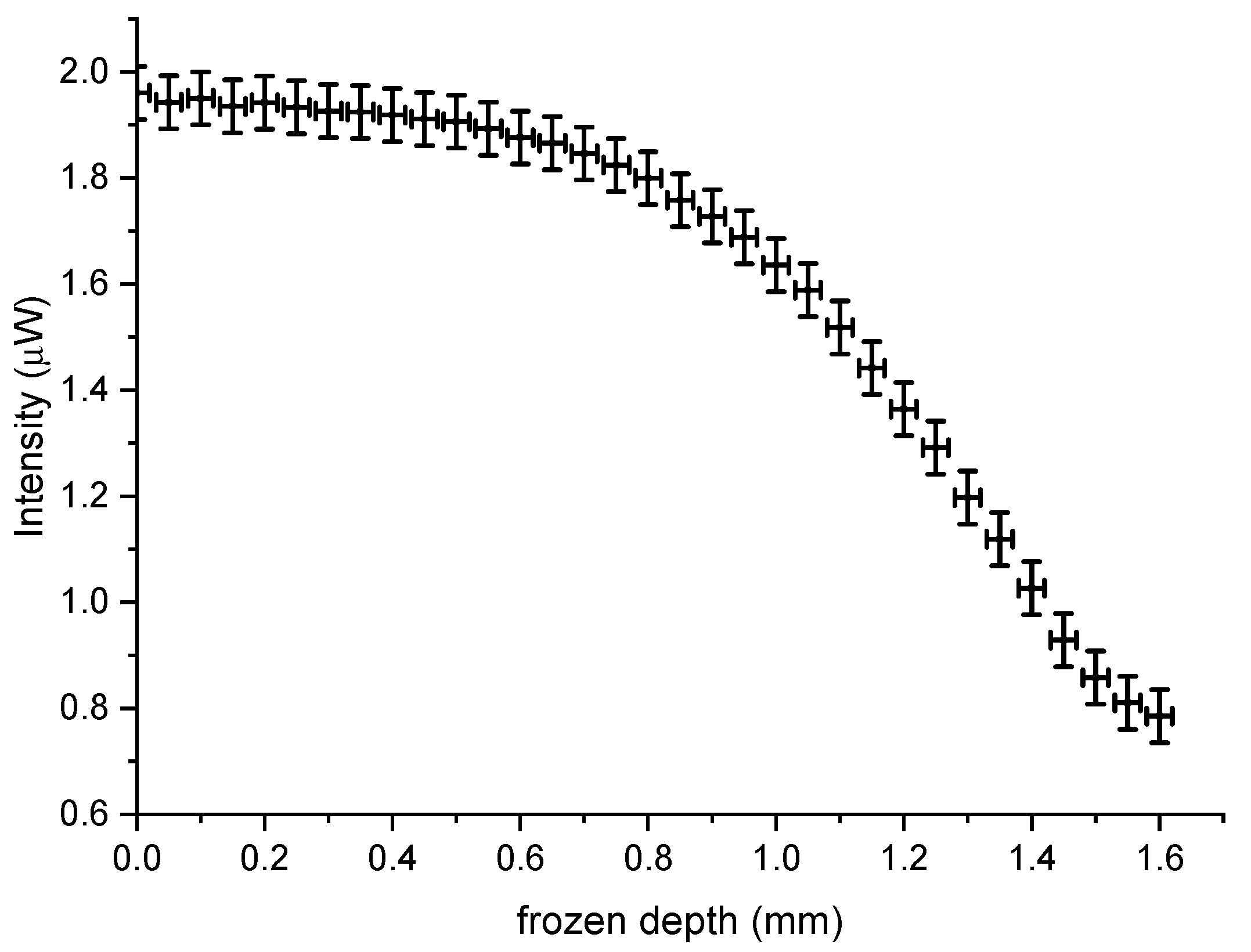
Hence, the freezing depth can be determined as a function of backscattering intensity and used to measure primarily the thawing process for a maximum 1.5 mm to 0 mm (tissue surface) with an accuracy of about ±0.1 mm, which was based on the transmission calibration (
Section 4.1) and are shown in
Figure 5. The detectable depth of freezing is thus dependent on the optical diffusion in frozen and unfrozen tissue and the sensitivity of the data acquisition system.
4.2. Measurement of the Frozen Skin Thickness Based on Backscattering Intensity Variations
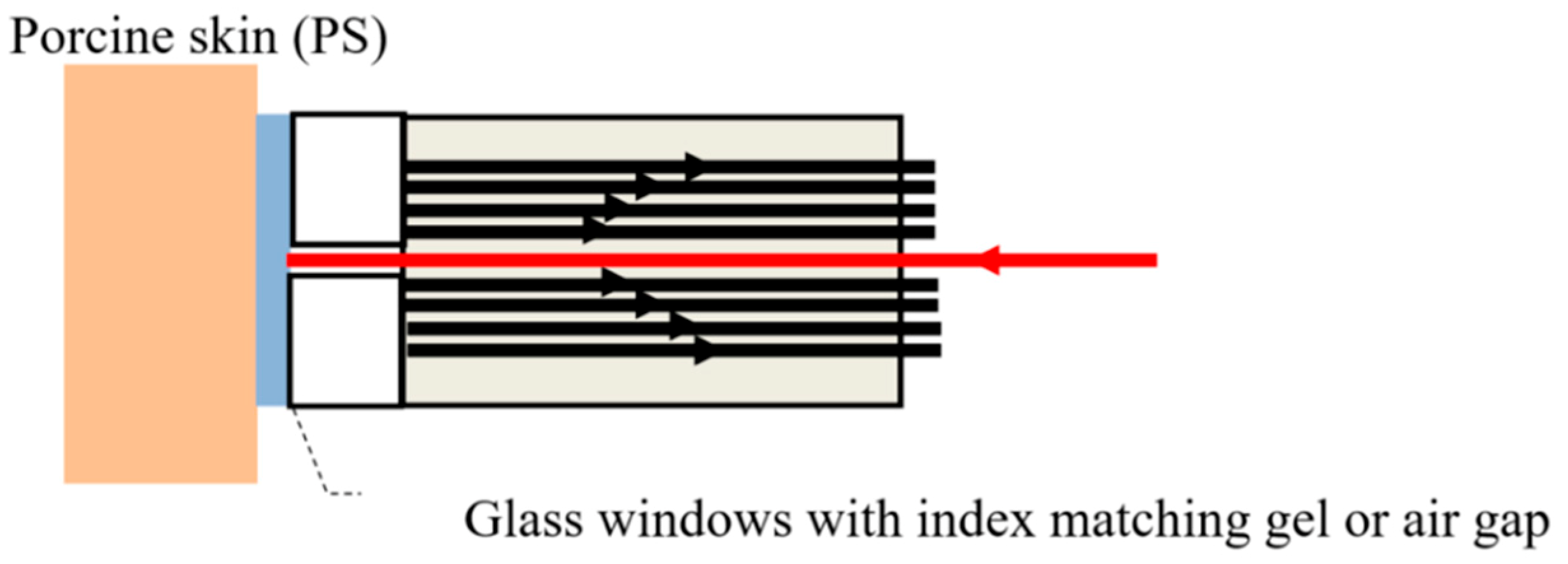
In living tissue there is only access to the skin’s surface; therefore, only backscattered signals can be used for determining the frozen depth and the sensor architecture includes fiber array-A only. For these experiments, the sensor head, shown in
Figure 2, was positioned in contact with the skin’s surface. The signal fibers were placed behind a 2 mm window, while the illumination fiber was level with the front facet. To eliminate frozen dew, the fiber array was placed in contact with the tissue, and IPA was applied prior to data acquisition to minimize surface backscattering. Based on these results and the calibration procedure described previously (
Figure 4b), the depth of thawing could be measured from the backscattering signal, from a maximum depth of 1.5 mm up to the surface of the skin.
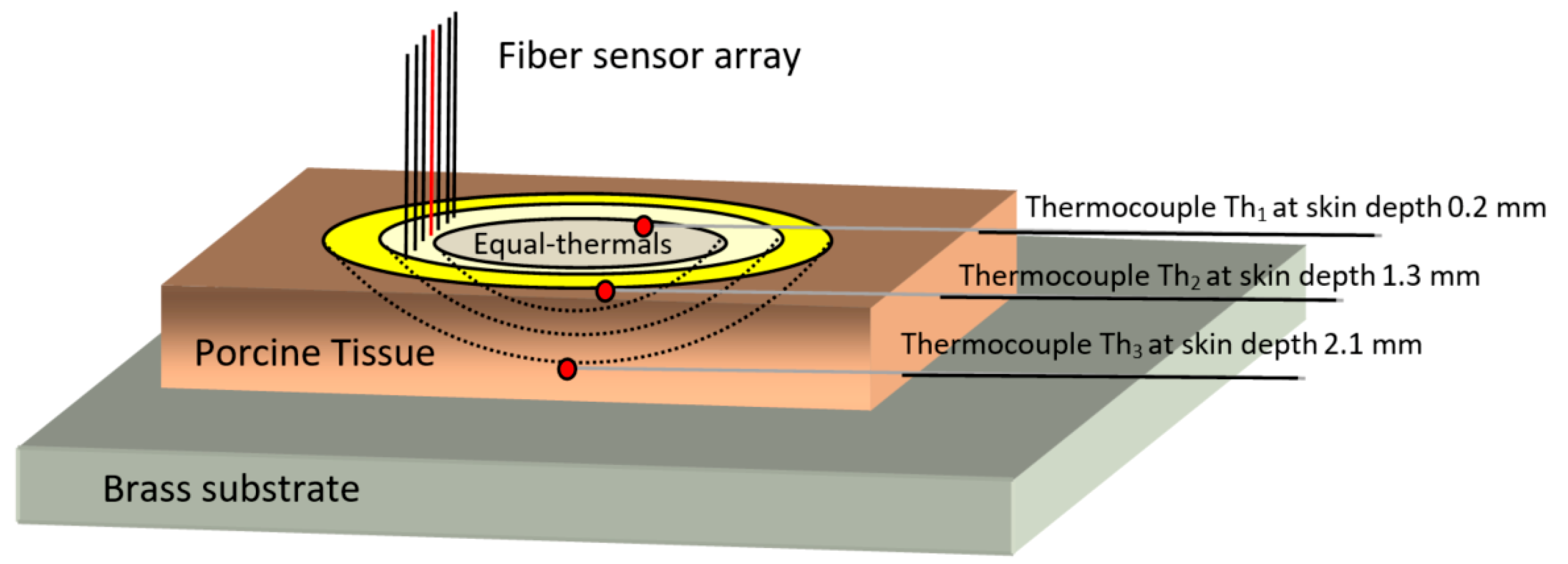
To further study the thawing process, the tissue was mounted in contact with a brass substrate, maintained at a constant 34 °C as shown schematically in
Figure 6. Porcine ex vivo skin tissue samples, consisting of the epidermis/dermis and a thin subcutaneous fatty layer, approximately 3 mm thick, were cut in sections of 5 × 4 cm. Three modified thermocouples (0.3 mm heads) were embedded at different vertical depths from the skin surface, as measured from the surface by a bespoke needle gauge micrometer, with Th
1 at a depth of 0.2 mm, Th
2 at 1.3 mm, and Th
3 at 2.1 mm being deepest and in close proximity to the brass plate. The lateral separation was 3 mm as shown in
Figure 6. The fiber-array axis was vertical and positioned laterally about 2 mm away from the thermocouples, to avoid any optical interference or reflections from the embedded metallic wires.
The experimental procedure involved retracting the fiber-array, to allow access of cryogen, and freezing an area of about 2.5 to 3 cm
2 of tissue, to about −80 °C. After applying IPA, the fiber array-sensor was re-positioned on the skin surface, and data from the signal-fibers and the embedded thermocouples were obtained. Thawing lasted about 85 to 100 s, with the skin’s surface temperature, as measured by Th
1, changing from −80 °C to +10 °C. The process was repeated over ten times in different positions of the skin and different skin sections yielding near identical results. As in the previous experiments, the signals from the inner fibers gave higher intensities, dropping off with time as the sample thaws [
10,
11]. However, the intensity timelines of the outer signal-fibers displayed decreasing signals as the tissue freezes and increasing as the tissue thaws. This is attributed to increased backscattering, which reduces optical diffusion in frozen tissue with less light reaching the outer fibers.
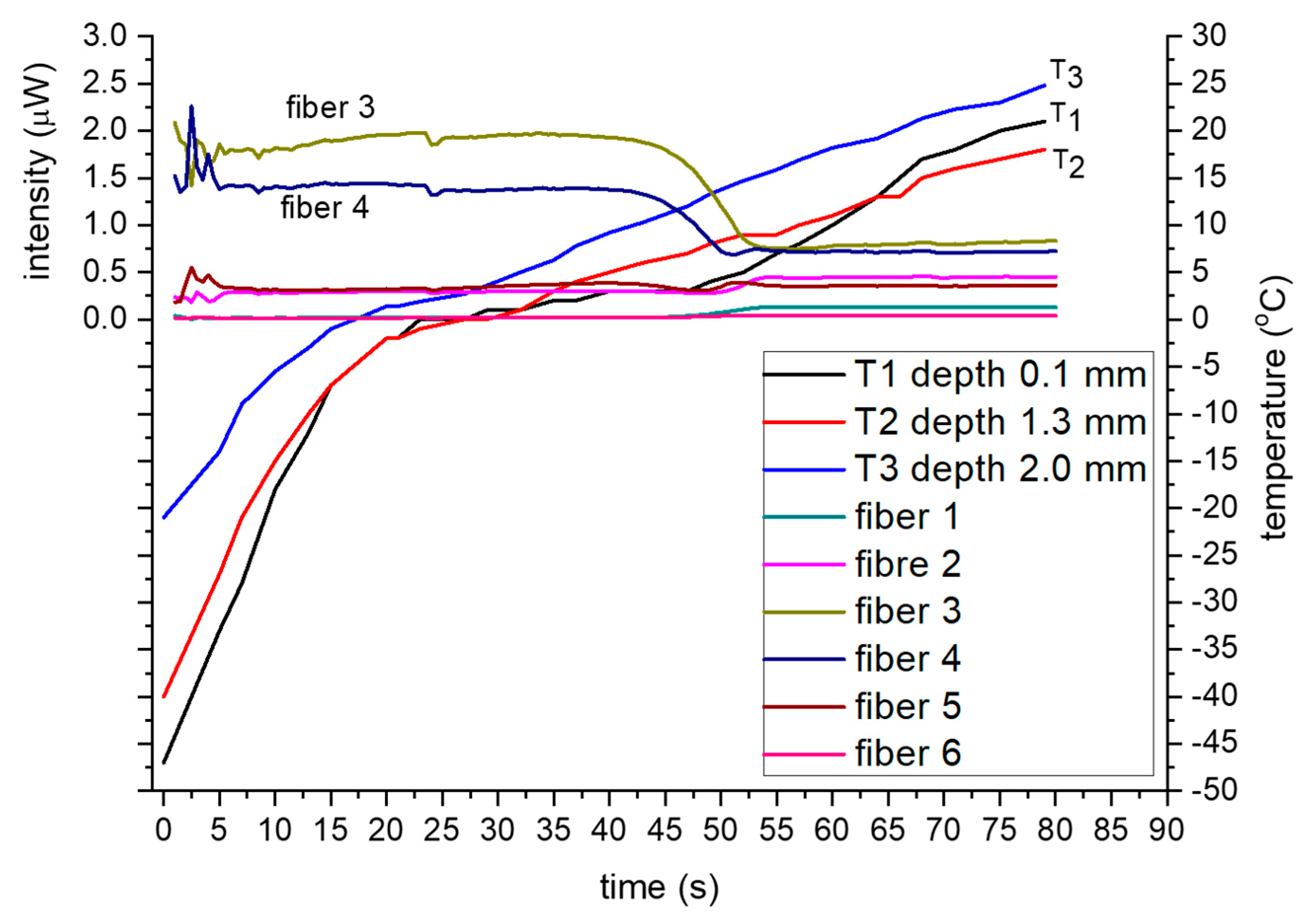
Figure 7 shows the timelines of the backscattering intensity together with the temperature variations ranging from about −50 °C to +10 °C.
In all experiments, Th
1 reached the lowest temperature, which was expected, as it is virtually on the skin surface hence closer to the application point of the cryogen. Similarly, the temperature gradient around 0 °C was low and nearly constant, for all the thermocouples, which is attributed to the latent heat of ice melting, i.e., remained constant until the ice was melted. According to
Figure 7, the intensity timelines for the two inner fibers (3,4) are higher and nearly constant when the tissue is frozen, temperature range −50 °C to 0 °C as measured at near surface by T
1 (0–25 s), and lower and constant for the unfrozen (5 °C to 20 °C, 55–80 s) temperature range. However, for the near 0 °C region (0 °C to 3 °C, 40–55 s), the optical signals exhibit a distinct and progressive reduction with time, i.e., from the high intensity, associated with frozen tissue, to a low intensity associated with unfrozen.
Based on the above results and the calibration of transmission (
Figure 3b) and backscattered timelines (
Figure 3d), the depth of freezing for the fiber array sensor was determined to be within 1.5 ± 0.2 mm as shown in
Figure 8a. This result can be deduced by the correlation of the calibration curve (
Figure 5) which defined the maximum depth as 1.5 mm and the equivalent region of 40–45 s in
Figure 7 and is also verified in an independent experiment outlined below (
Section 4.3). Given that human skin is about 1.5 mm thick, the method can therefore be used to determining the frozen depth during thawing using a similar configuration and methodology as described above.
In addition, the optical diffusion in tissue can also be determined by analyzing the geometry and the intensities timelines of the outer signal fibers during the freeze/thaw cycle. As light backscatters more in frozen tissue, it diffuses less so the outer fibers of the array have lower intensities when the tissue is frozen. In
Figure 8b, as the inter-fiber separation is known, the relative intensities are directly related to the optical diffusion via the FWHM measured by the fiber array, which for frozen tissue is 3.5 mm while in unfrozen tissue this extends to nearly 5 mm.
4.3. Verification of Optical Method Using Ultra-Sound Measurements
To verify the measurements obtained with the optical backscattering technique an independent measurement was implemented, using a dermatological ultrasound unit in conjunction with the fiber optical sensor. In a similar experimental arrangement, as the one distributed is
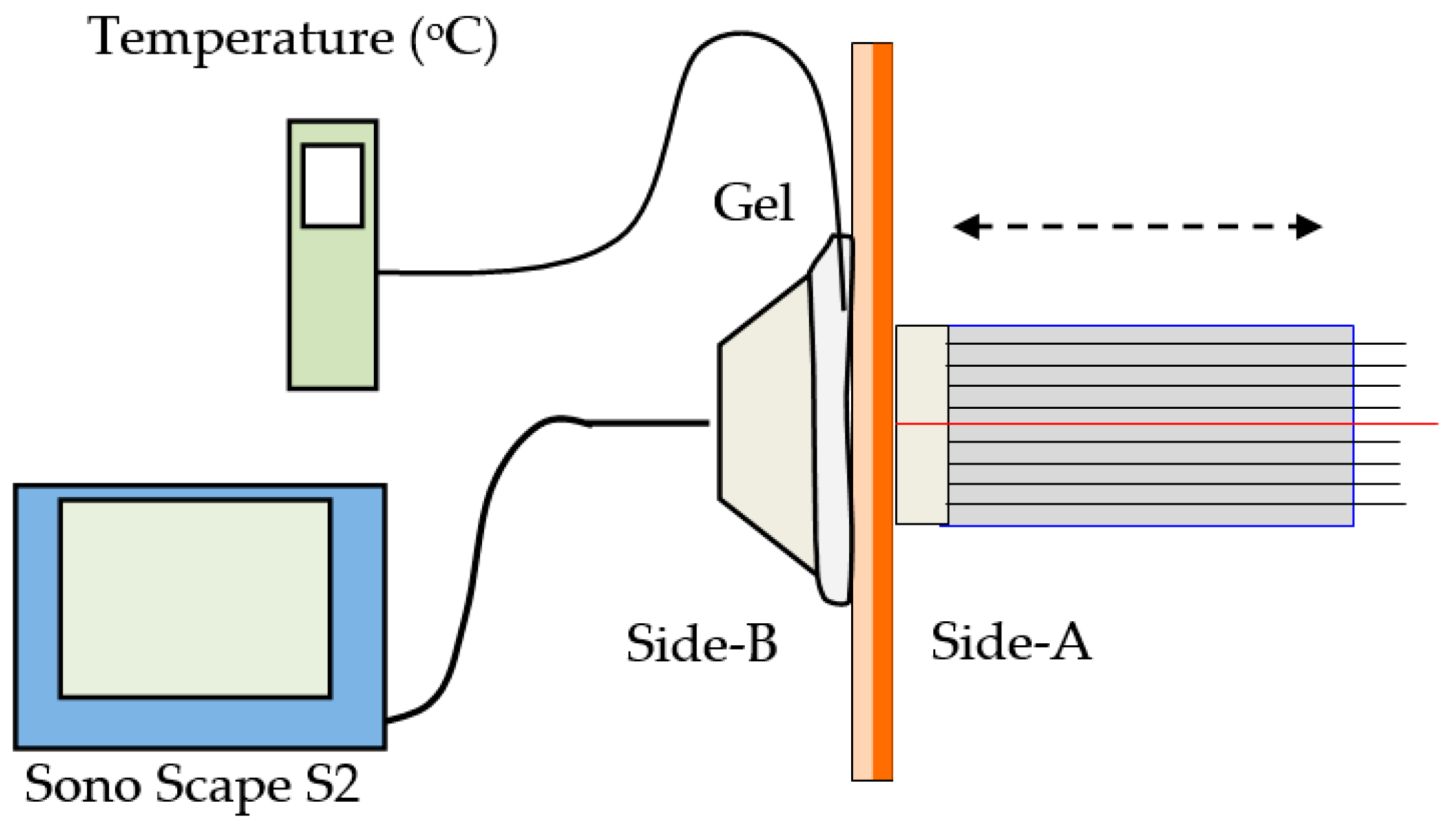
Section 3, a tissue consisting of the epidermis and a fatty layer with a combined thickness of about 3.1 mm, and held tight on a frame, allowing access from both sides. The fiber array sensor was positioned in contact with the tissue, on side-A, while an ultrasonic sensor (model SonoScape S2, ShenZhen Sonoscape Co. Ltd., Shenzhen, China), was positioned directly opposite on side-B of the tissue, as shown schematically in
Figure 9. Coupling gel on side-B, bridged the small gap of the acoustic sensor, transmitting the ultrasound in the tissue. As in the previous experiments, the tissue was frozen by cryogen spray, applied on side-A. During freezing, the fiber sensor was retracted and repositioned on the frozen tissue after the application of IPA which eliminated frozen dew on the surface. The temperature was monitored by a thermocouple, mounted on side-B, in contact with the skin and inside the gel between the tissue and the ultrasound head as shown in
Figure 9.
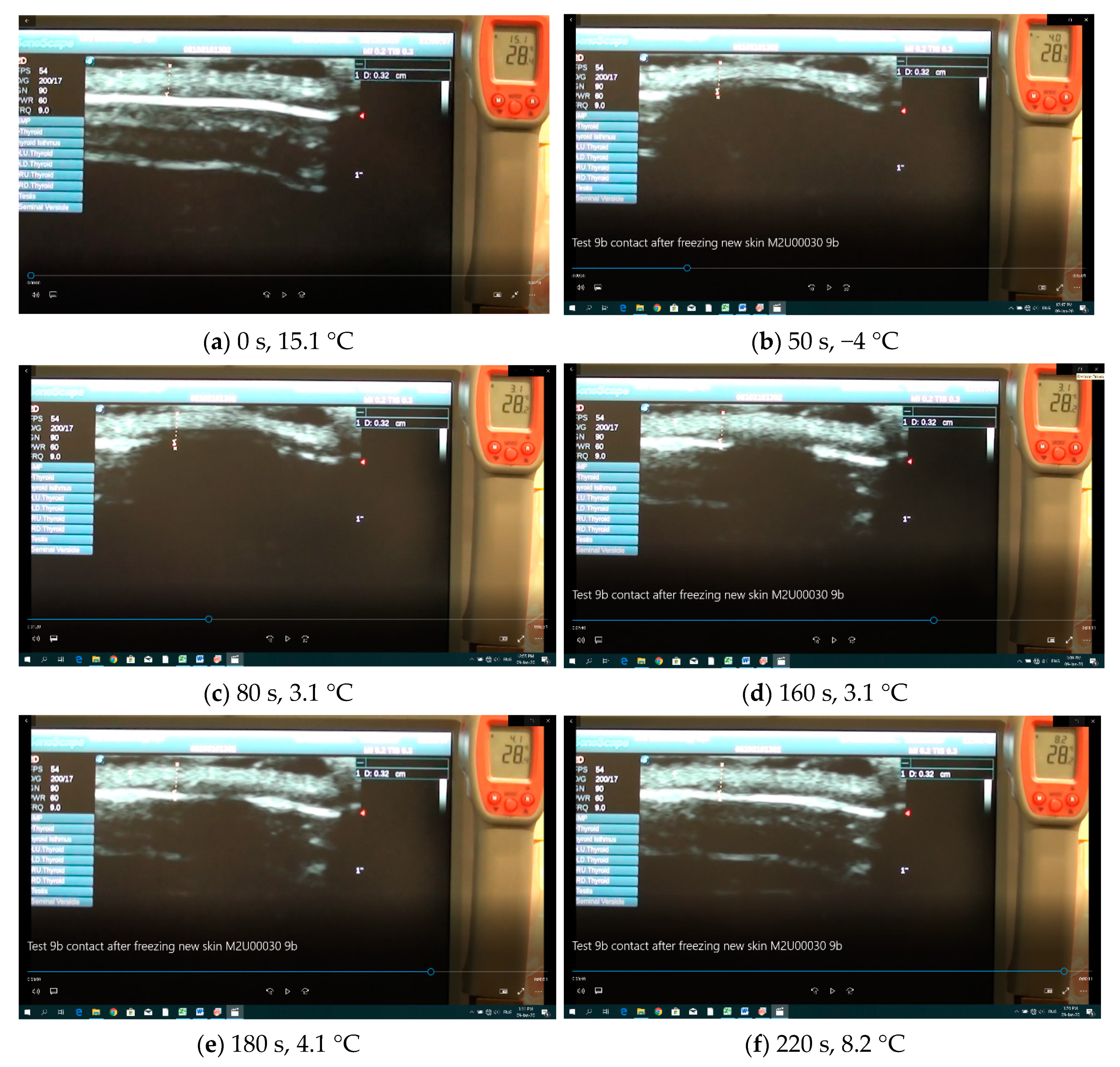
As before, the optical data from the fiber array sensor were detected by the data acquisition system stored in a computer. However, due to the limited memory of the ultrasound unit, the screen of the unit was continuously recorded by a digital video camera which also recorded the temperature of the thermometer. By synchronizing the optical signals with the video from the ultrasound unit, it was possible to correlate the results from the two systems and measure the depth of freezing. Specifically, by isolating sequential screen shots, taken every 10 s, we measured the frozen structures of the tissue with ImageJ (
https://imagej.nih.gov/ij/, v.1.52, U.S. National Institutes of Health, Bethesda, Maryland, USA). The resolution of this ultrasound system was about 0.1 mm to 0.2 mm with typical results shown in
Figure 10a–f.
Specifically,
Figure 10a–f shows distinct frames of the video taken before freezing (
Figure 10a) and during thawing (
Figure 10b–f). Furthermore, in
Figure 10a, the two red markers designate the unfrozen tissue which was about 3.2 mm thick (also indicated by the vertical trace). The white dense layer is the epidermis while the less dense region above is the fatty layer of the porcine specimen. The faint white structures below the epidermis are artifacts related to ultrasound reflections. The temperature behind the specimen was measured by a radio sparer (RS) digital dual thermometer measuring both Infrared (IR) radiation, (large digits) and temperature from the K-type thermocouple (small digits), used in these experiments. The ultrasound detector was placed on the upper side of the tissue and the fiber optic sensor on the lower side.
Figure 10b shows the same specimen after it was frozen and the fiber optic sensor was repositioned in contact with the tissue having reached front (side-A) and back (side-B) temperatures of about −7 °C and a −110 °C, respectively. There are several observations to be noted in these frames: (a) as the tissue freezes, ultrasound does not reflect back to the sensor and the frozen tissue appears dark thus designating the depth of freezing; (b) to avoid freezing the gel, which is water based, cooling was terminated when the temperature on the back facet reached −5 °C to −7 °C; (c) as the tissue thawed it reappeared on the ultrasound screen (
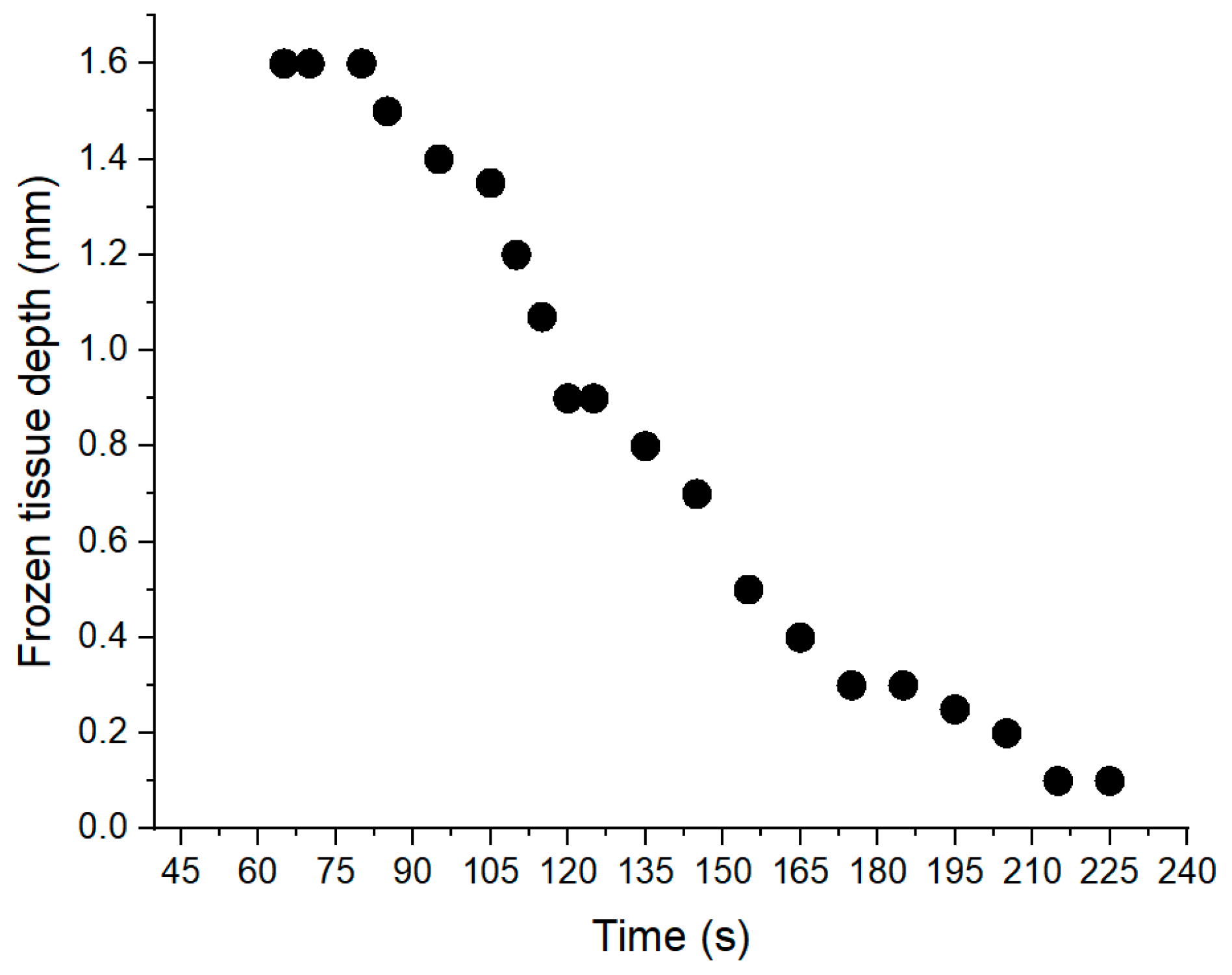
Figure 10c–f). Using ImageJ, the frozen tissue boundaries were measured, and the results are shown in
Figure 11, where the depth of frozen tissue is shown as a function of time. It is important to note, that as can be seen from this graph the maximum depth measured by ultrasound was also about 1.6 mm.
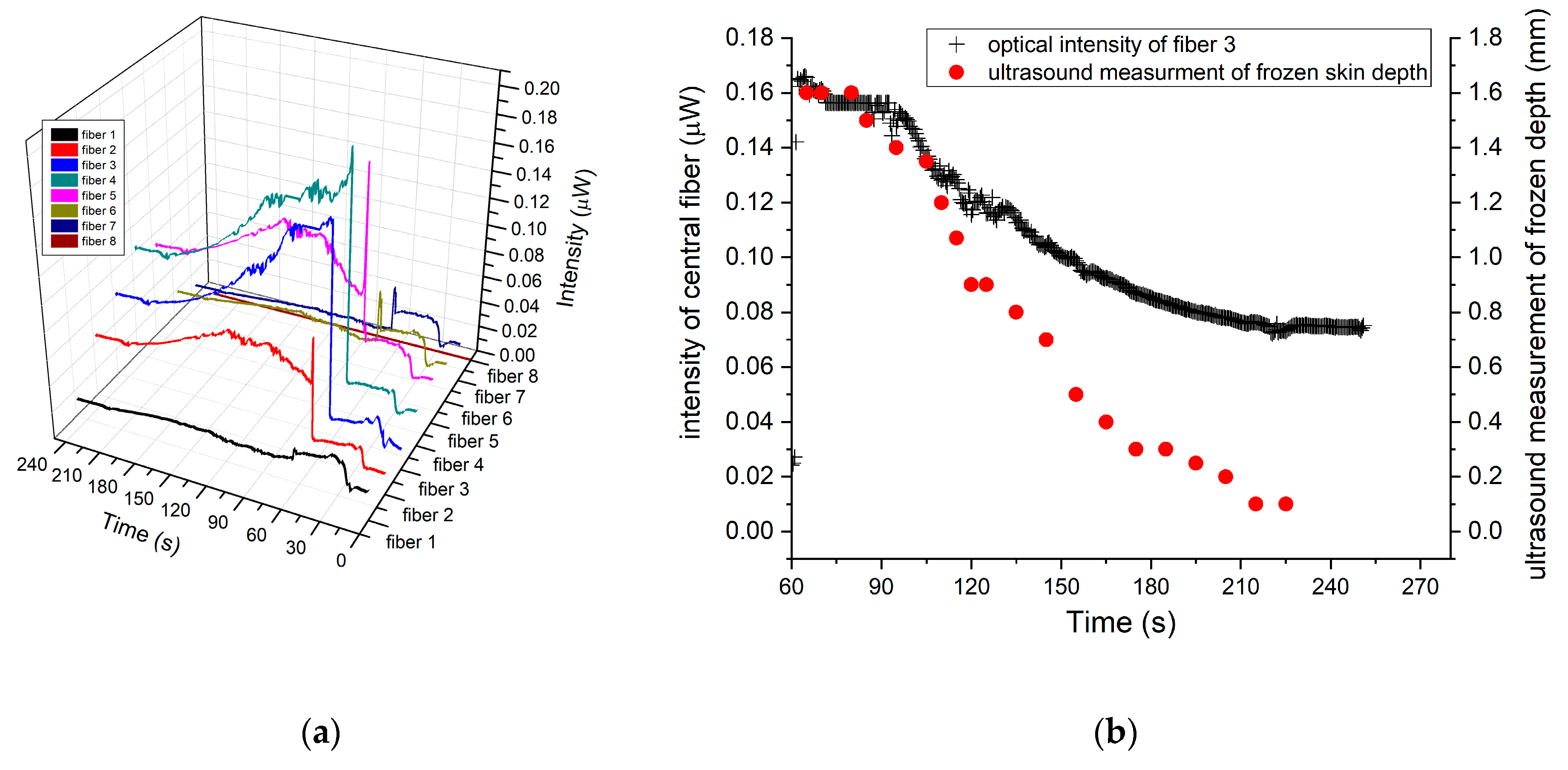
To correlate the ultrasound measurements with the backscattering optical intensity, the fiber optic sensor on side-A was placed in contact with the frozen tissue, and the synchronized intensity timeline for both measurements were recorded. In
Figure 12a, the backscattering optical intensity timeline for the fibers array sensor is shown, while
Figure 12b shows the synchronized timelines for both the backscattering intensity and the depth of frozen tissue measured by ultrasound.
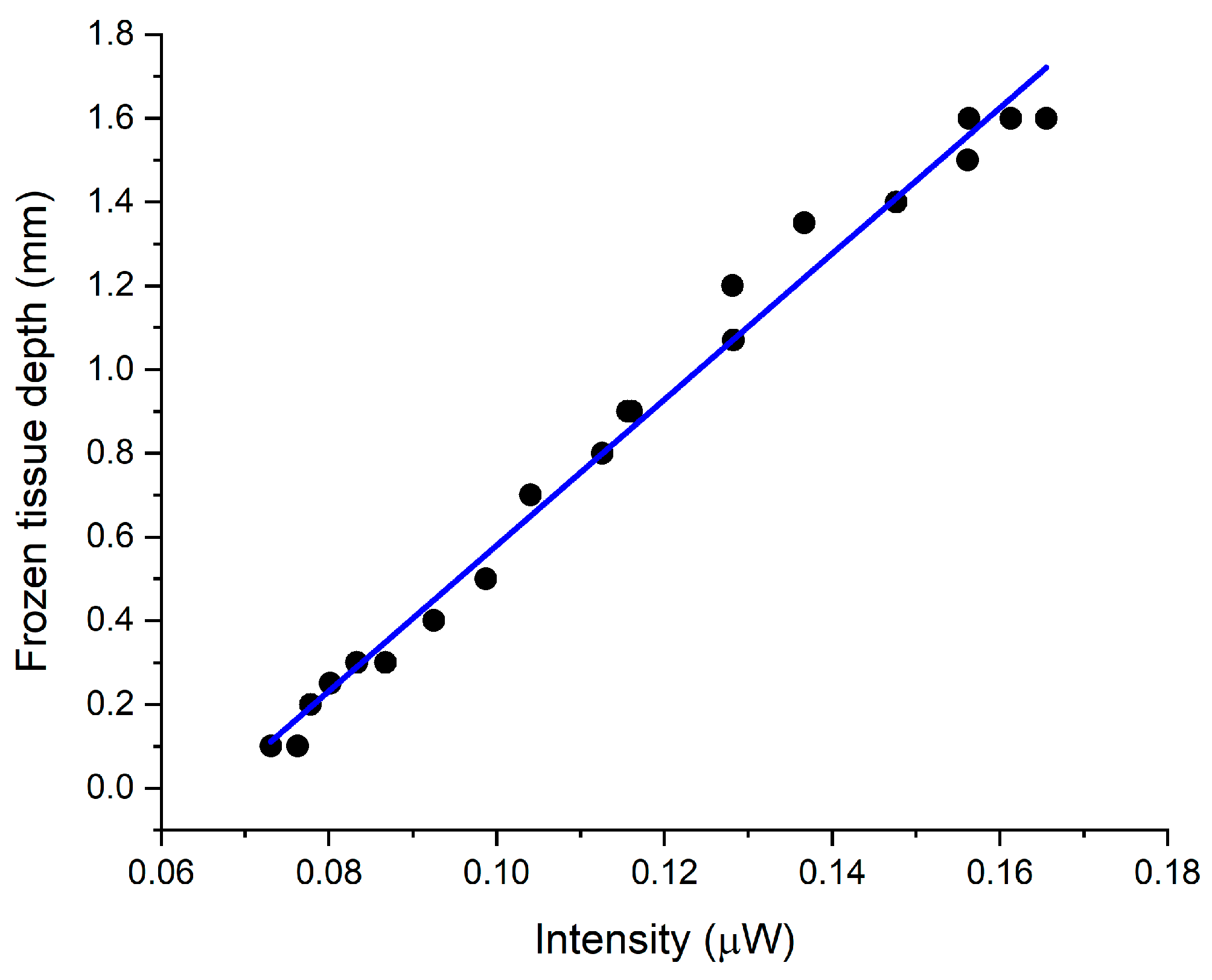
In
Figure 12b, the synchronized timelines begin at 60 s which was the time the fiber array sensor was placed in contact with the frozen skin. Furthermore, no changes in intensity were recorded in the central fibers (3 and 4), until 90 s, corresponding to the time required for the frozen depth, under the fiber array sensor, to be reduced to about 1.6 mm which is the maximum detectible frozen depth. Moreover, the ultrasound frozen skin depth measurements exhibited a similar timeline which was linearly correlated (
R2 = 0.98) to the optical intensity measured by fiber sensor (
Figure 13).
The results shown in
Figure 13 are in agreement with the previous calibrations experiments in
Figure 8a, and effectively verify, by an independent method, that the backscattered intensity from the frozen tissue can be used to accurately measure the depth of freezing.