1. Introduction
Assessment of adequate depth of anesthesia in equine patients during general anesthesia protocols, especially with the use of balanced anesthesia that combines inhalation and intravenous drugs, is still a challenge. The lack of neurologic-based research causes anesthetists to base their decisions regarding the quality of anesthesia upon hemodynamics, respiratory, and other parameters (e.g., muscle tone, eye reflexes).
Electroencephalography is known as a non-invasive technique of measuring the electrical potential and, therefore, the awareness of the Central Neurological System [
1,
2]. In horses, it has been used primarily in studies concerning sleep and neurological disorders [
3,
4,
5]. Recently, it has been presumed to be possibly the most objective method for anesthesia depth assessment in medicine [
6,
7] and in veterinary medicine [
8,
9,
10]. To date, there have been several studies performed to investigate this potential in horses [
11,
12,
13,
14,
15]. These, however, did not investigate intraoperative analysis of the raw EEG wave. The only algorithms (mathematical analysis of EEG that transformed the raw EEG into a unified number) available, which could work during anesthesia, were adapted from human medicine, and were subsequently confirmed in research as not applicable for horses [
16,
17,
18]. Possibly, the most interesting for the equine practitioner might be the recording of the spectral edge frequency of EEG waves for the left and right hemisphere. Quite promising are also the results of studies recording the DSA (density spectral area), which show the changes in the power of EEG waves in different frequency bands (delta (δ) 0.1-4 Hz, theta (θ) 4-8 Hz, alpha (α) 8-12 Hz, beta (β) 12–25 Hz, gamma (λ) >30 Hz) with colorful diagrams. The presence of waves of a certain frequency reflects the awareness; the main rule describes the lowest awareness with a presence of low-frequency waves [
13,
19,
20].
The aim of this pilot study was to investigate the method of EEG measurements in horses, starting from electrode mounting, checking device data collection in potential clinical conditions (including different positions of a horse during surgery, different anesthesia protocols, and different types of surgeries). Comparisons were also made by using various electrode placement locations and during subsequent anesthesia phases—sedation, maintenance, recovery. This would be used as the basics for further studies including methodical statistical analysis and comparison of these measurements to monitoring techniques commonly used in clinical practice.
The study was approved by the Local Ethical Committee.
2. Materials and Methods
Six horses were used for the study—an 11-month-old Arabian mare (A), a 9-year-old half-blood gelding (B), a 3-year-old half-blood stallion (C), a 3-year-old English blood stallion (D), a 7-year-old half-blood gelding (E), and a 12-year-old half-blood gelding (F). The types of procedures, positions of the horses, and types of anesthesia protocol, including drugs and their relevant doses, are shown in
Table 1.
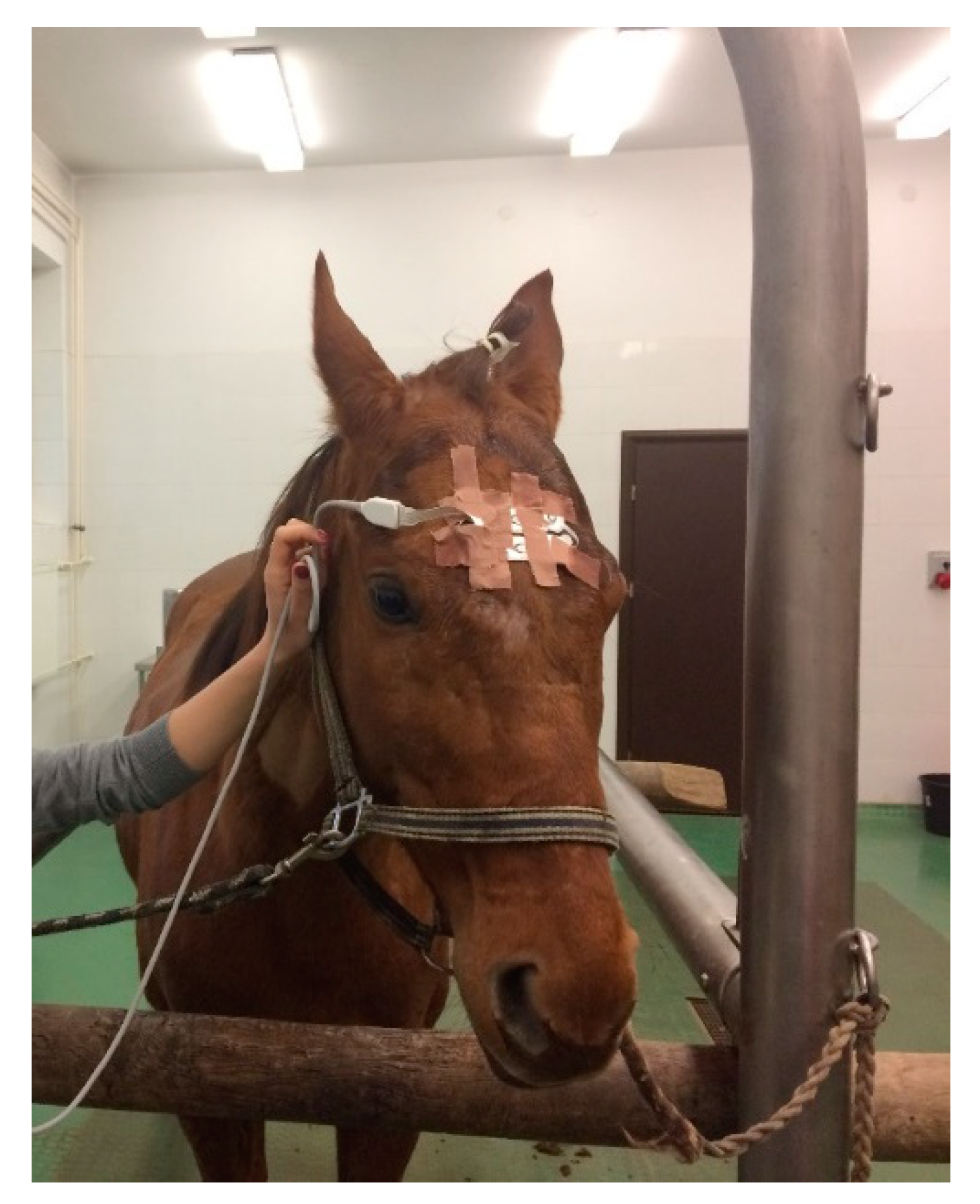
In each case, electrodes were placed on the frontal part of the horse skull (according to Giovagnoli 1996) with the use of gel and tape—two left (L1 and L2), two right (R1, R2), one ground electrode (GB) and one reference electrode (CT) in the center position. The placement area was not shaved. Once the electrodes were in place, the recording device (Root with Sedline
®, Masimo, Irvine CA, USA) was attached and the measurements commenced (
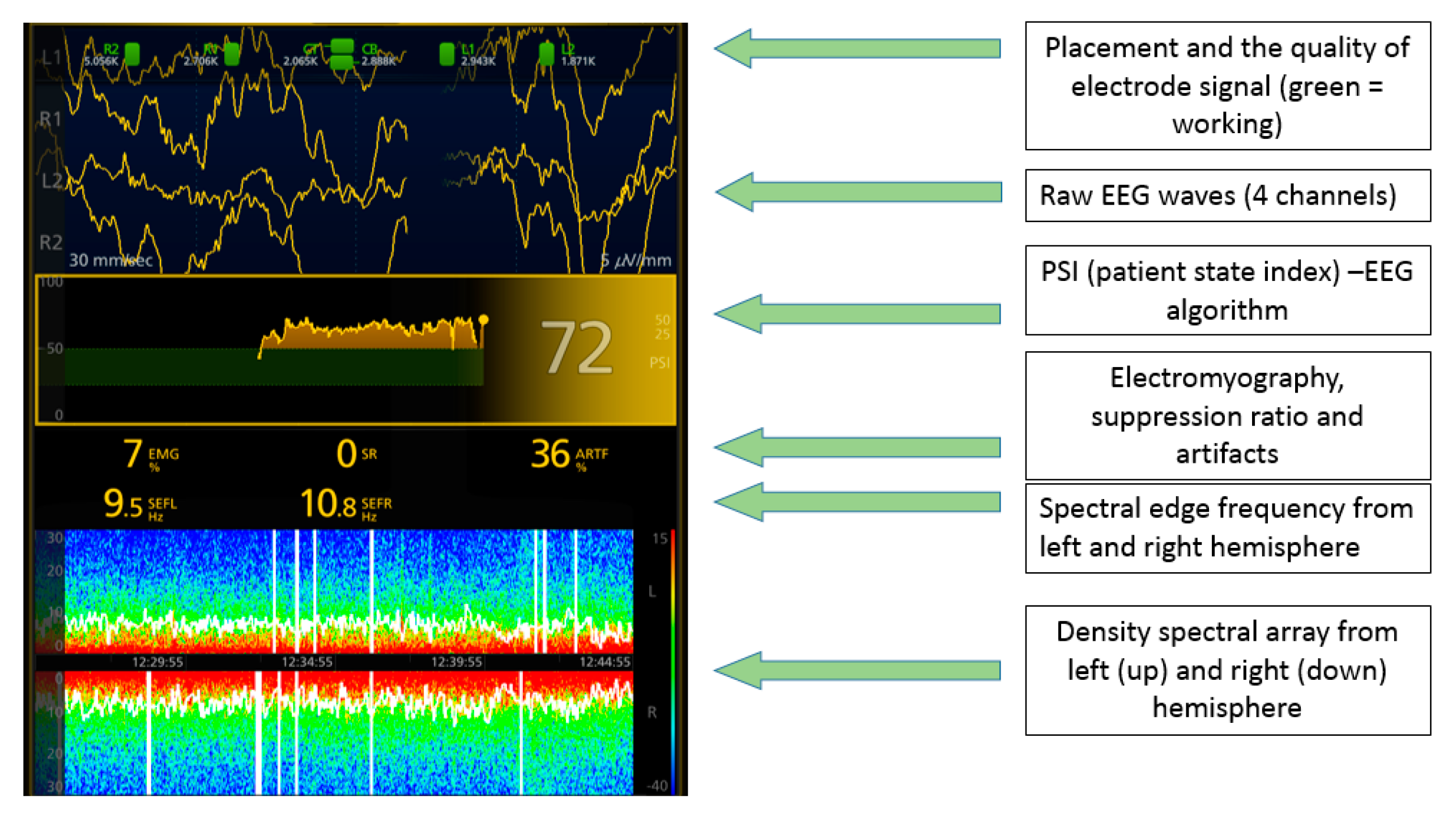
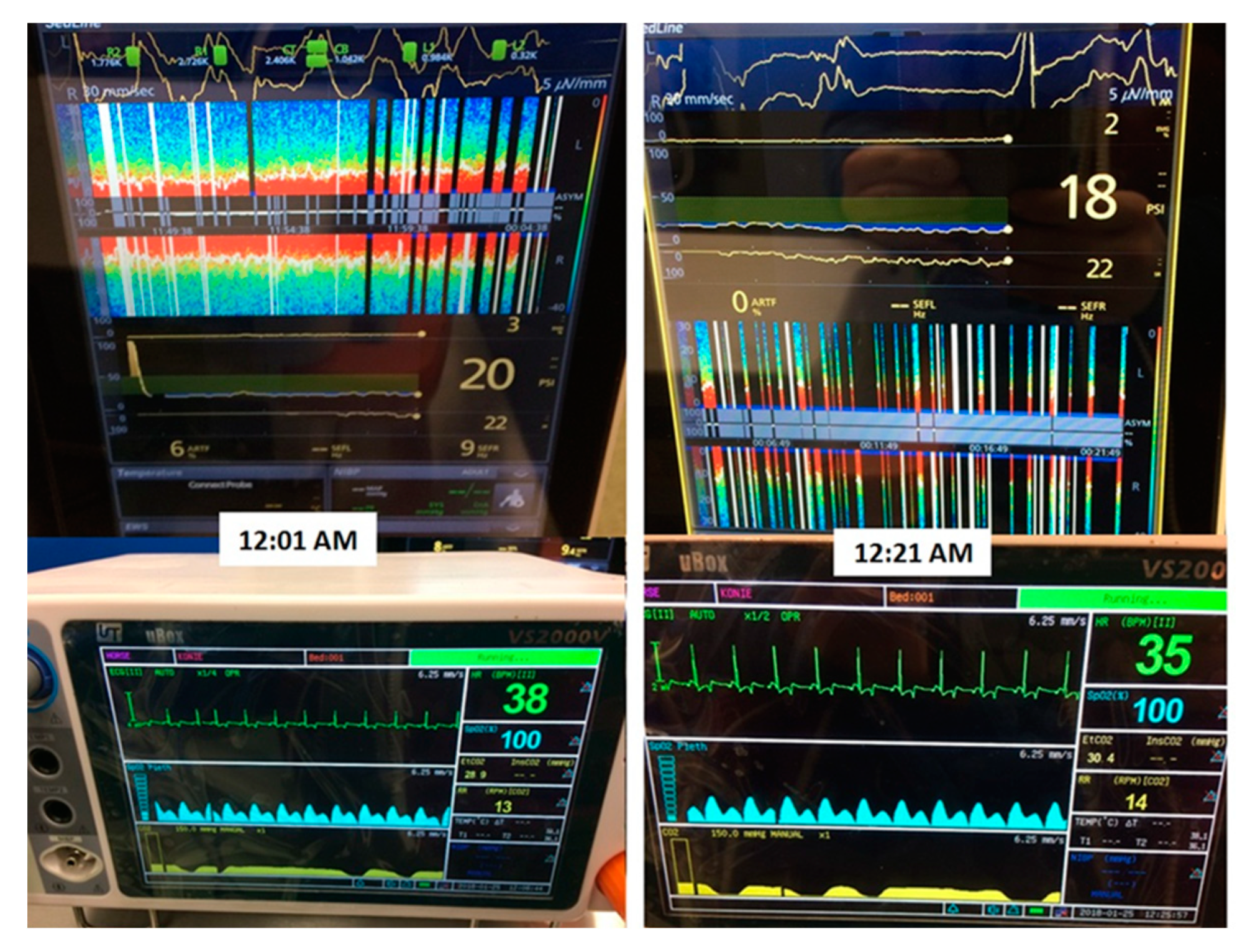
Figure 1). On the monitor, the raw EEG waves from 4 channels (L1, L2, R1, R2), spectral edge frequency (SEF) of the left and right hemisphere, EMG (electromyography) of the muscles that could influence the measurements, Patient State Index (mathematical algorithm counted from raw EEG), burst suppression ratio (SR), and density spectral area (showing the power of waves in certain frequency bands) could be seen. The impedance was also measured, and when it exceeded the marginal acceptable level and interfered with the EEG measurement (indicated by the electrode color on the screen changing from green to yellow), the electrodes were pressed slightly and additional gel was applied between the electrode and the skin surface. For each procedure, a life parameter monitor (Datex Ohmeda Cardiocap 5 or Veterinarian Patient Monitor Vs2000v) was attached for the measurement of pulse oximetry, ECG, capnography, breaths per minute count, and, in 3 cases, end tidal isoflurane and invasive blood pressure (
Figure 2). These measurements were conducted during sedation (Case D), maintenance of anesthesia, and recovery (Cases A, B, C, E, and F). The results were recorded as numbers for hemodynamic parameters on the anesthesia record chart and compared in the time of recording during anesthesia with changes visible on the DSA. This was to mimic the situation when the practitioner would monitor the patient during anesthesia and, at the same time, investigate the possibility of occurrence of any visible changes in response to inadequate anesthetic/analgesic administration (seen as changes in life parameters) during the surgical procedure.
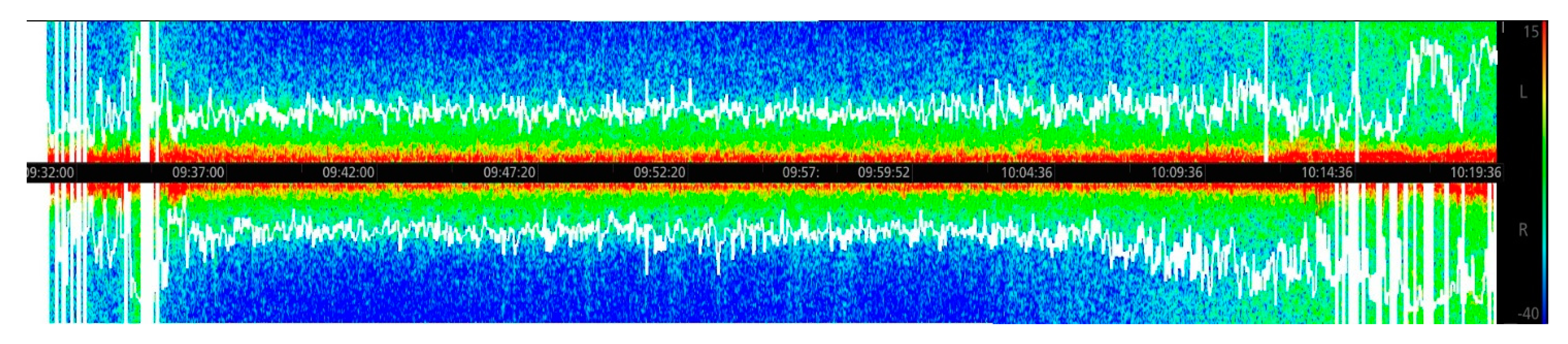
During all procedures, visual readings of the DSA were made, including the hue of the color that indicated the power of the wave (red color—high power, blue color—low power) and the role of the color on the scale—frequency. The scale was always visible on the monitoring screen. The DSA was shown as two horizontal stripes, representing the two hemispheres (
Figure 3).
3. Results
During all procedures it was clearly demonstrated that the levels of awareness, as illustrated by the high frequency and power of the waves shown by the EEG, were different according to the anesthetic protocol and the surgical procedure.
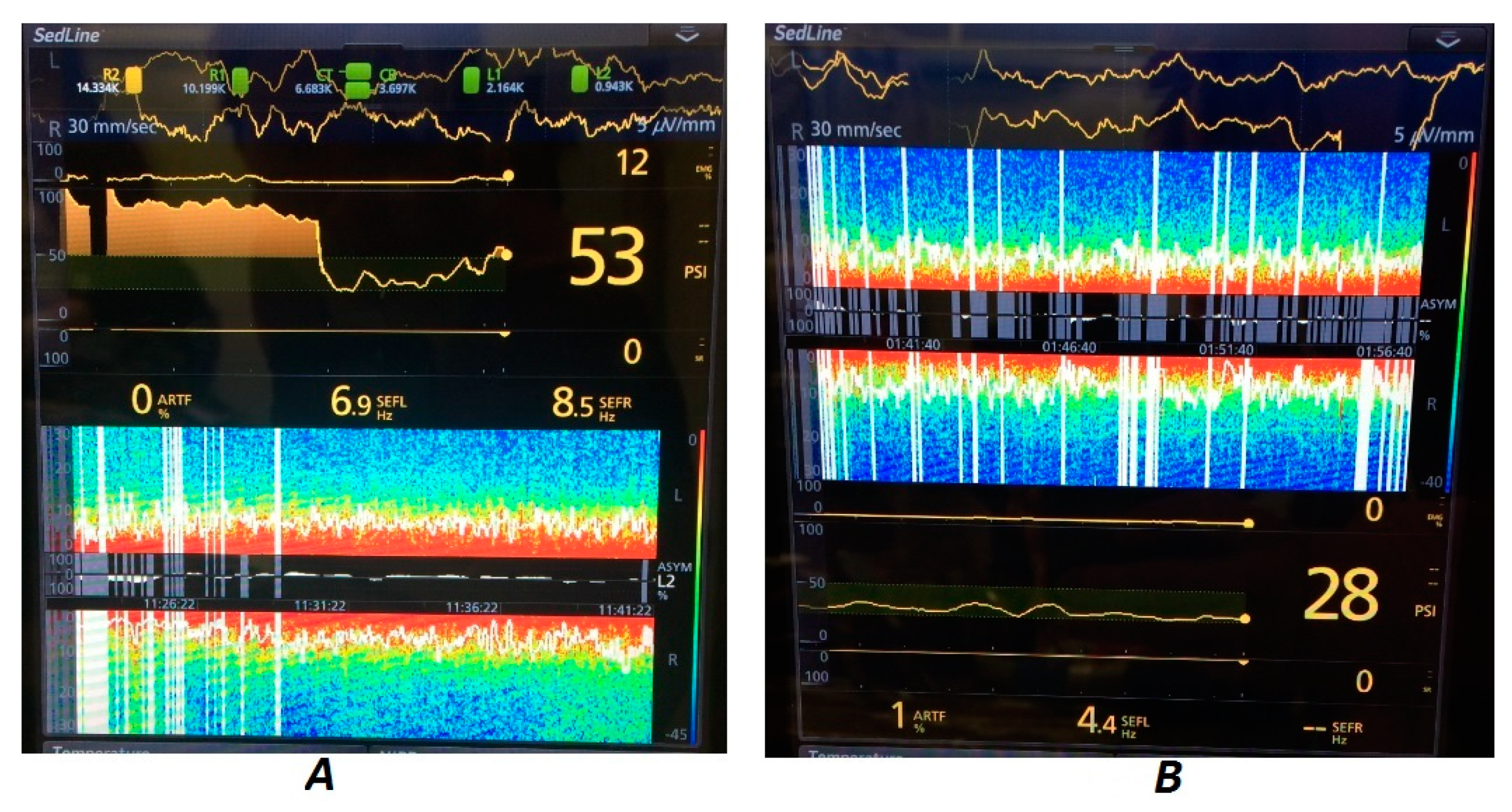
In the young patient brought to the CT (Horse A), the hemodynamic parameters were stable and within the normal range throughout the entire procedure. This underlined the changes visible on the DSA—while the SEF was quite stable, with time, there was a gradual rise in the power of the low-frequency (<3 Hz) waves. Apart from that, low power of higher-frequency waves (5–15 Hz) could be seen, with the power gradually dropping with elevating frequency. This was correlated with the high level of isoflurane set on the vaporizer (3%). Eventually, the strong and frequent burst suppression (flattening of the EEG wave) could be seen as black stripes within the DSA (and on the raw EEG) that correlated with maintaining a high isoflurane level. During this change, of all related parameters, only the heart rate dropped slightly (
Figure 4).
During colic surgery (Horse B), which lasted 3 h, there was extensive variability in the frequency and power of the EEG waves recorded. The horse was in a critical clinical condition before the surgery and its hemodynamic parameters changed significantly during the maintenance of anesthesia. The DSA was recorded without artifacts. In-tidal isoflurane concentrations and supplemental medications were adjusted as needed to maintain cardiopulmonary function and assure desirable pain management. Significant jumps in the SEF and power of higher-frequency waves could be seen during more painful moments—skin cut, enterotomy, and skin suturing at the end. Also, periods of high power of high-frequency waves could be seen (
Figure 5).
In the case of castration (Horse C), the SEF was also variable but the waves of significant (for overall arousal) power were present only within short frequencies. There were no periods of higher-frequency waves emerging during the whole procedure. The hemodynamic parameters were stable. The horse was kept in a light plane of anesthesia. There were sporadic artifacts visible. During the whole procedure, the appearing waves were stable in power within their frequency bands (
Figure 5).
In standing surgery (Horse D), the DSA was not demonstrated clearly enough to provide adequate interpretation as there was a large impedance between electrodes that caused the occurrence of the artifacts due to movement of the head, which seems to be a significant drawback in EEG measurements. The total power and amplitude of the wave was high, which reflected high awareness (
Figure 6).
During anesthesia for arthroscopy (Horse E), the DSA was visible without any artifacts. The SEF was stable and there were minor changes during maintenance. The power of the waves was greatest at low frequencies and was stable during the entire procedure. The hemodynamic parameters were also stable. The recovery showed a gradual return of increased brain activity by elevation of the power of the higher-frequency waves with spectral edge frequency growth in the same time period (
Figure 7).
In the case of total intravenous anesthesia (Horse F), the changes in the SEF were the most significant, particularly during the induction and recovery phases. The frequency of the waves was low, and the most commonly observed waves were very low frequency and in the delta frequency band. Of all procedures, in this horse the power in the frequency band was the most stable. There were no problems with recording the density spectral array, nor were there artifacts interfering with the readings. Anesthetic concentrations were adjusted as indicated (
Figure 8).
The most important findings of DSA changes during all procedures are shown in
Table 2.
4. Discussion
These preliminary studies, conducted on six different patients, showed that EEG monitoring with the use of surface electrodes in the Root with Sedline® system may be used in equine patients. The electrodes can be attached without shaving the skin and the presence of coating do not disturb the signal transmission. The glue on the electrodes might not be enough to maintain good contact, especially since the horse is able to sweat over the whole of the skin surface; thus, additional tape or bandaging is necessary for mounting. However, the measurements on DSA divided into two hemispheres do not provide as much information as desired, since it is difficult to attach the electrodes in perfect symmetrical placement.
DSA measurement might be difficult to obtain without general anesthesia in horses due to skin and muscle movement, which interferes with signal transmission and electrode mounting. The artifacts were disturbing especially during standing surgeries as the head was not still. However, during recoveries it was possible to observe the DSA up to the moment the horse started to stand, which might make this system useful for recovery predictions. This was also shown for TIVA anesthesia in Horse F—a situation where monitoring is normally limited and recovery prediction, especially in the field, is highly desirable for practitioners. The matter of successful recovery is one of the most difficult, particularly after long anesthesia during colics.
Observations of DSA revealed changes connected with procedures, possible pain, and the depth of anesthesia. In Horse A, the occurrence of burst suppression, independent of hemodynamic parameters, revealed too deep a level of anesthesia. It is worth mentioning that in the case of CT the horse did not receive any nociceptive stimuli. The variability of SEF in Horse B is an indicator of unstable anesthesia and possible difficulties that might occur during further anesthesia, which is understandable in a case of colic surgery in a horse in critical clinical condition. Also, the occurrence of higher power in higher-frequency bands can indicate periodical arousal due to surgical stimulation and/or inappropriate depth and analgesia level. There may be major changes in cardiopulmonary parameters occurring during various stages of the colic symptoms and adjustments during surgery. These anticipated levels of visceral pain create major challenges to maintaining the desired level of anesthesia and analgesia. Horses C and E are good examples of routine procedures in horses with good physical condition, and they showed a stable SEF matched with stable hemodynamic parameters. The level of anesthesia can be assessed in such cases by changes in the power of low- and medium-frequency waves. A procedure on a horse in a good clinical condition is described by the occurrence of stable frequency waves, which is the target of anesthesia depth assessment. Case D demonstrated the problem with standing surgery measurements due to head movement, but this problem might be better solved by head immobilization.
During field anesthesia, such as the case of Horse F, DSA might be especially helpful as in such cases there are limited possibilities of anesthesia monitoring compared to hospital conditions. Some modification of current monitors might be helpful. A comparison of Horse F and the other horses anesthetized with inhalant agent shows significant differences in the influence of the drugs on the Central Nervous System. Inhalants revealed the occurrence of various frequency waves, while total intravenous anesthesia seems to stabilize the waves on low and medium frequencies without significant changes. This also shows the complicated route of action of inhalant anesthetics, which has not been described until now.
The vital signs monitored and recorded were not as responsive as the EEG changes during the surgical procedures. Heart rate changes were more likely to respond sooner to potential surgical pain than blood pressure. Neither eye movement/position nor muscle relaxation were indicative of pain during the levels of surgical anesthesia maintained in these horses.
Comparing the changes between the phases of anesthesia can be a problem. The surface electrodes can be mounted before sedation in some horses, although during sedation there is not as much need for EEG monitoring as after induction of anesthesia. However, there is a need among the practitioners who perform standing surgery to be sure about the level of anesthesia in those procedures, so possibly the methodology of measurements in those cases should be improved. These kinds of surgeries are more and more widely used due to lower anesthetic risk.
The recording of EEG during induction is still a challenge as the movement of the horse from the standing to the recumbent position interferes with measurements, which likely results in artifacts. However, from the observations, during inductions, the frequency of the waves drops, which is understandable. The most important readings are recorded during anesthetic maintenance, which can show significant changes in the wave frequencies, thus indicating inappropriate levels of anesthetic during surgery.
There are some drawbacks to the method that was used in this research. There is still a need for better software for the EEG DSA machine, as the DSA can be saved only as a picture screenshot from the device during anesthesia but cannot be transferred to the computer and then mathematically analyzed. That is the reason that the study was based upon observations, rather than statistics. Moreover, making observations of the DSA and understanding the changes requires practice and the establishment of the relation to other parameters.
From a physiological point of view there are different expected responses to medications selected in balanced anesthesia and analgesia. This was demonstrated in this study.
5. Conclusions
EEG DSA measurements during general anesthesia have the potential to help clinicians provide better monitoring and anesthesia depth assessment in horses. Further investigation is needed to systematically record all the observations characteristic of specific events, protocols and surgeries. This preliminary study on different types of anesthesia protocols during multiple surgeries revealed its usefulness in monitoring changes, possibly faster than those shown by hemodynamic parameters. EEG monitoring is not a new concept, but as new monitors are available their recordings should be analyzed together with other parameters due to the complex nervous system responses to anesthetic agents and species differences. The Root with Sedline EEG monitor shows promising results; however, more investigation into the unique issues of equine anesthesia and surgery is necessary.
Animal Consent
The Local Ethical Committee was asked to verify the research and claimed that the research does not need the Official Ethical Committee Consent and application for it.