The Potential of a Tailored Biomimetic Hydrogel for In Vitro Cell Culture Applications: Characterization and Biocompatibility
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the 3BE Hydrogel
2.2. Spectroscopic Characterization Analysis
2.3. Isolation of HFMSCs
2.4. Multilineage Differentiation of HFMSCs
2.5. Cell Seeding in the 3BE Hydrogel
2.6. Cell Live/Dead Observation
2.7. Cell Live/Dead Assay
2.8. Statistical Analysis
3. Results
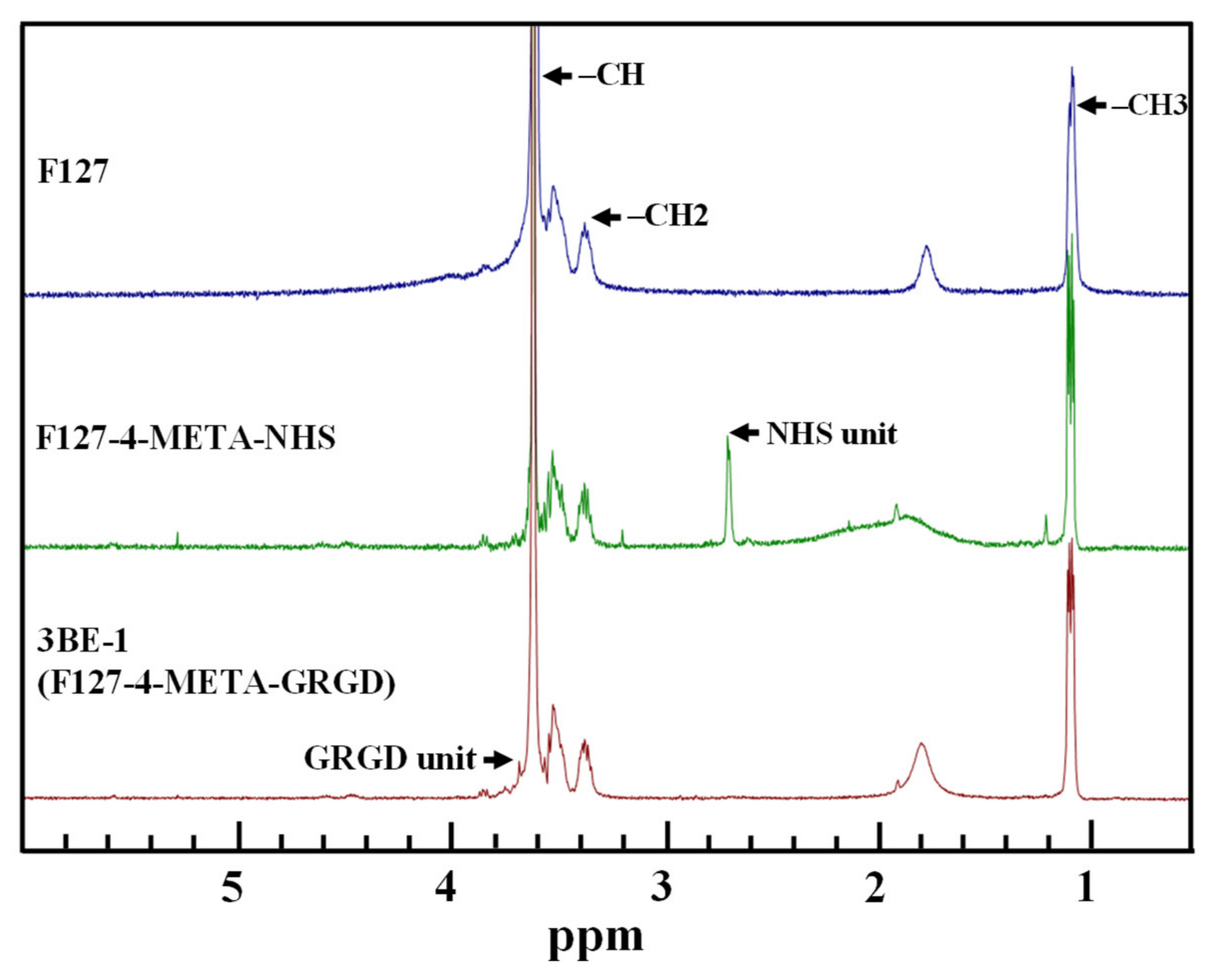
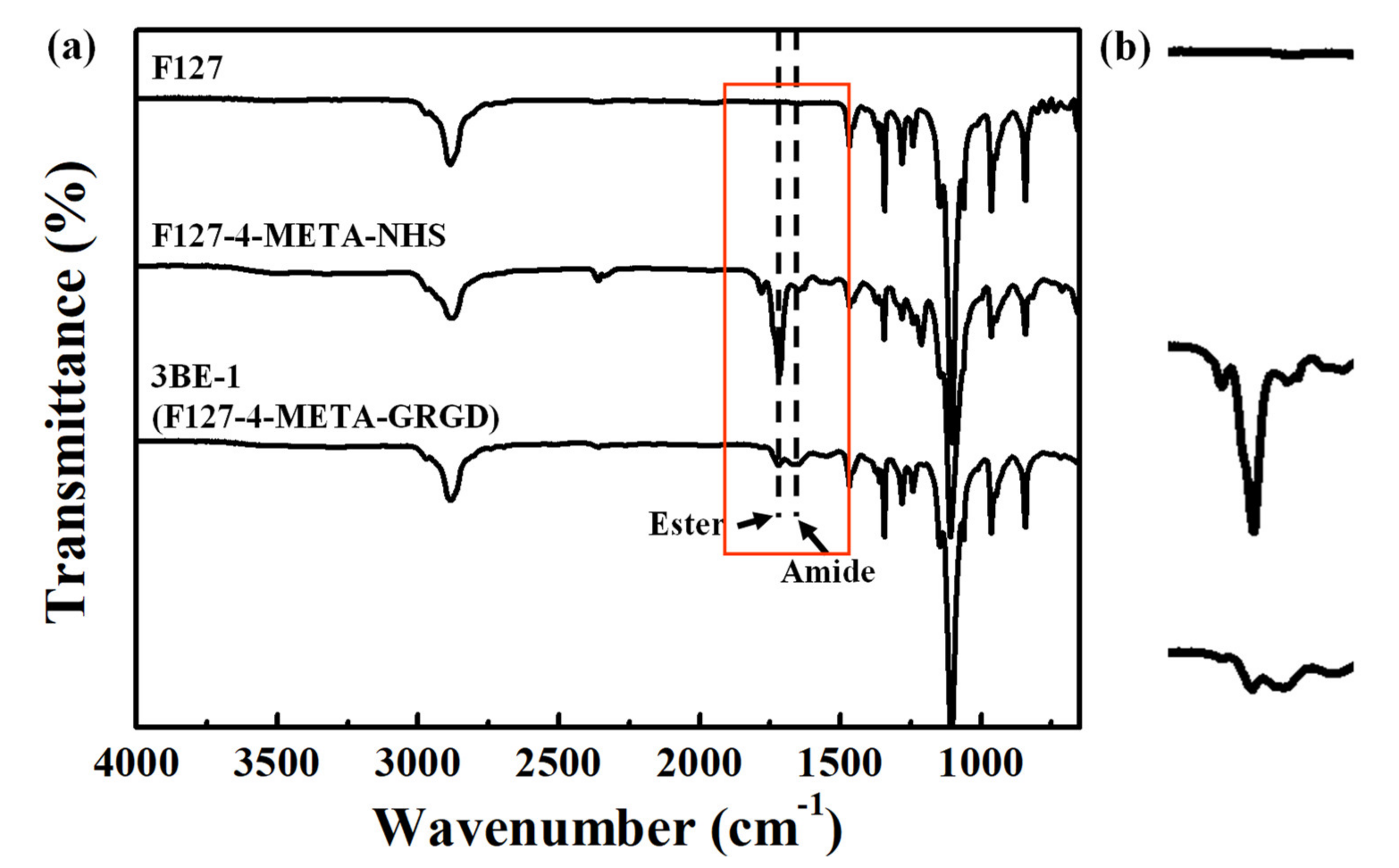
3.1. Characteristics of the Chemical Bonding State
3.2. Multilineage Differentiation Potential of HFMSCs
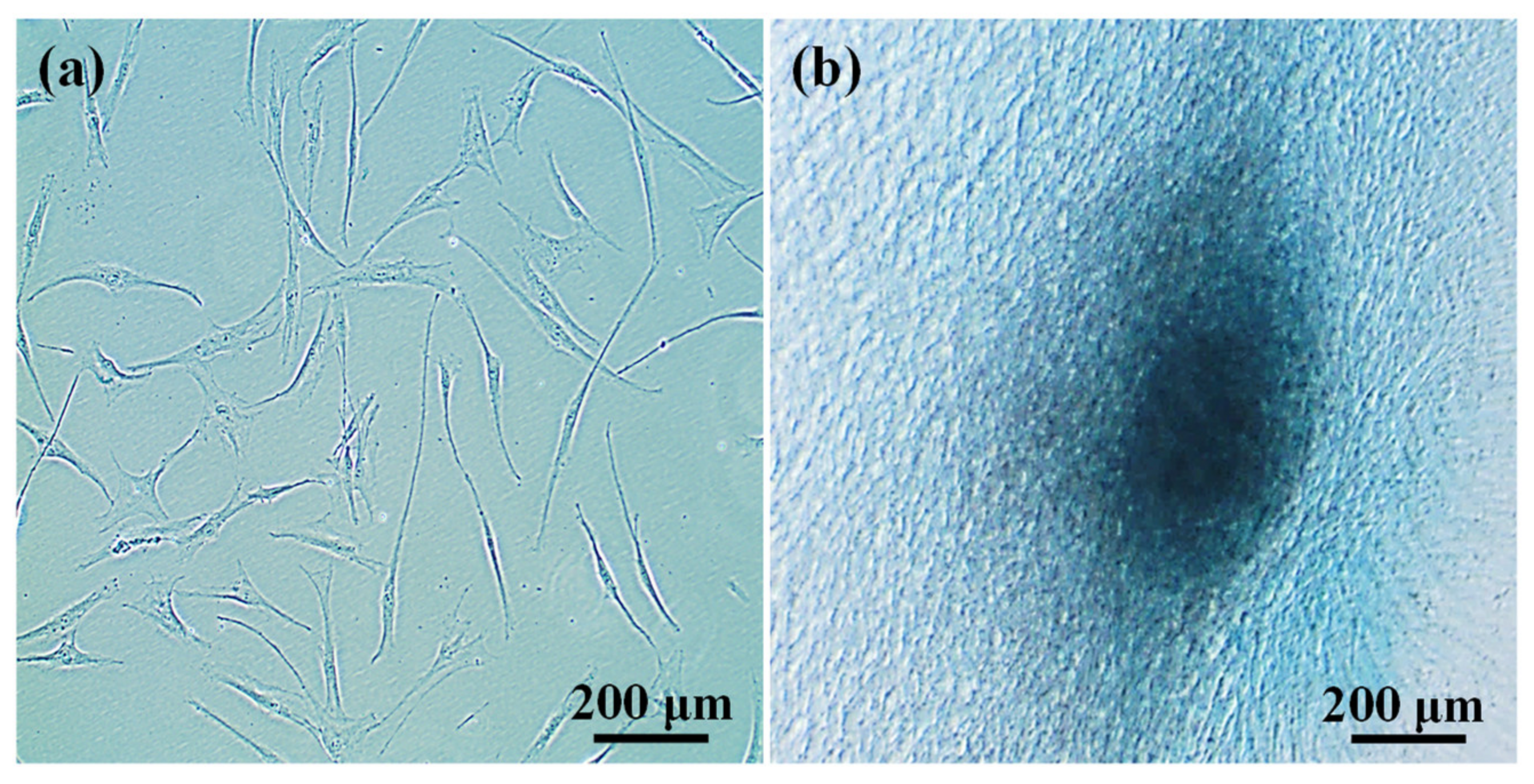
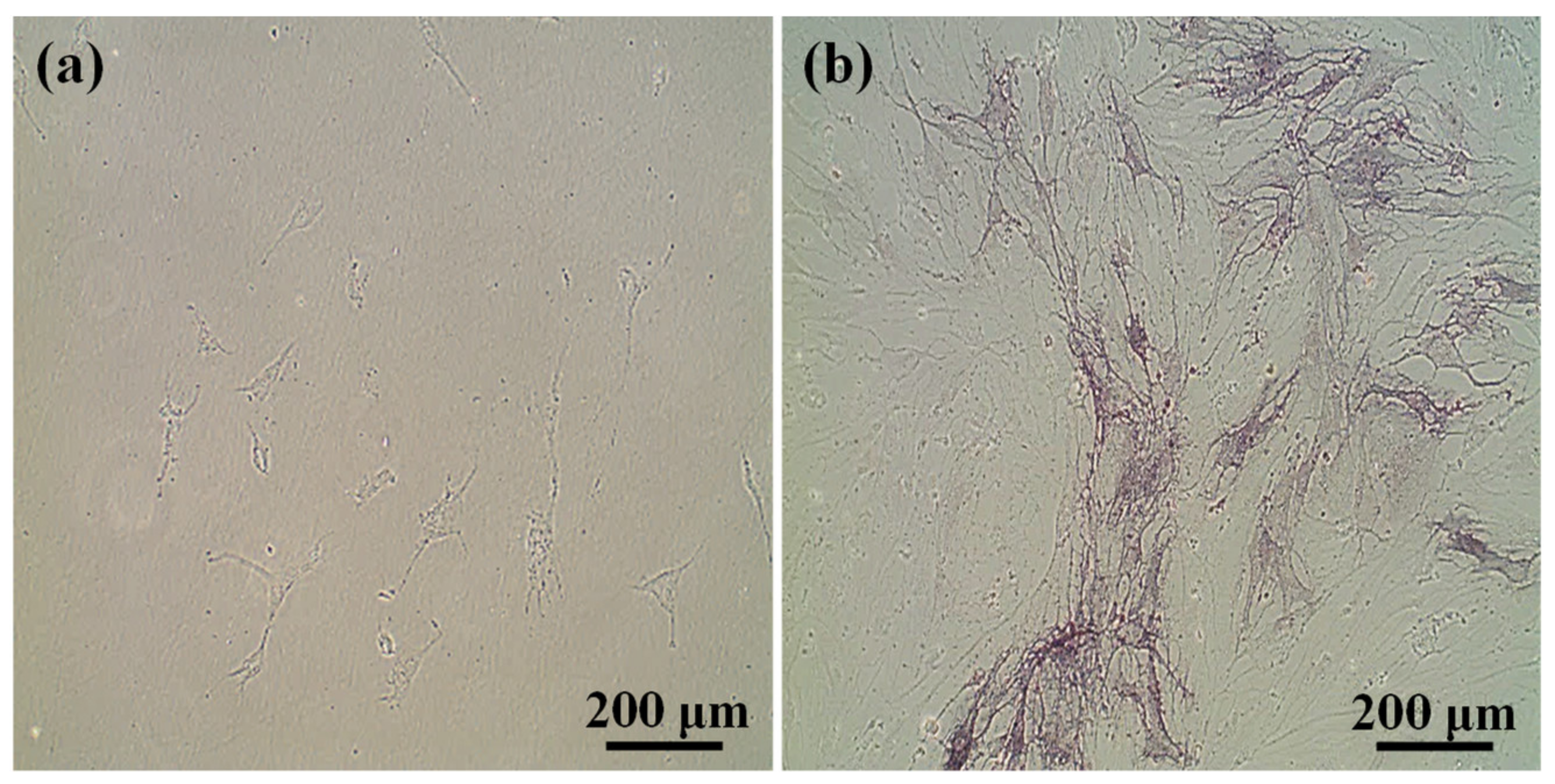
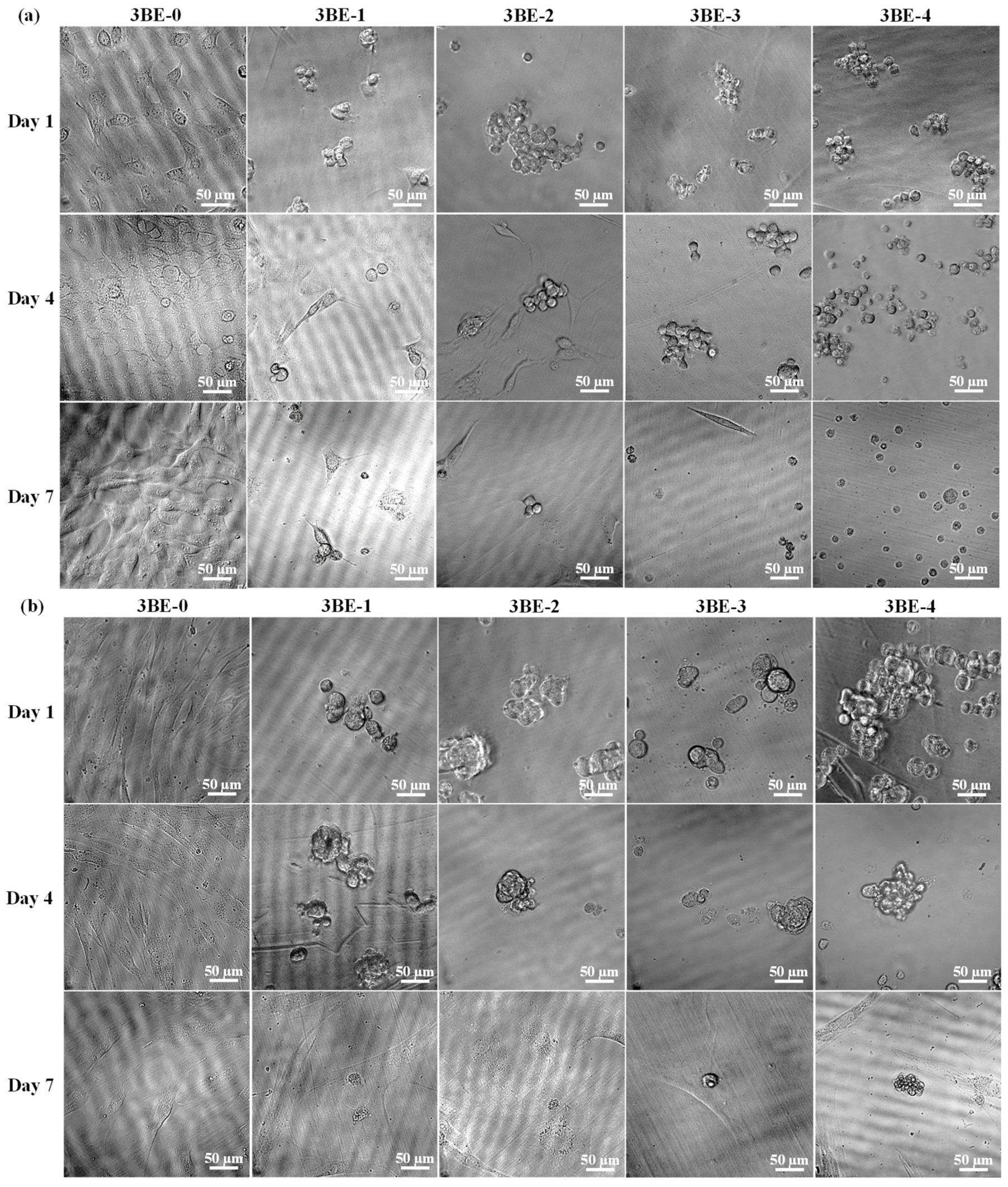
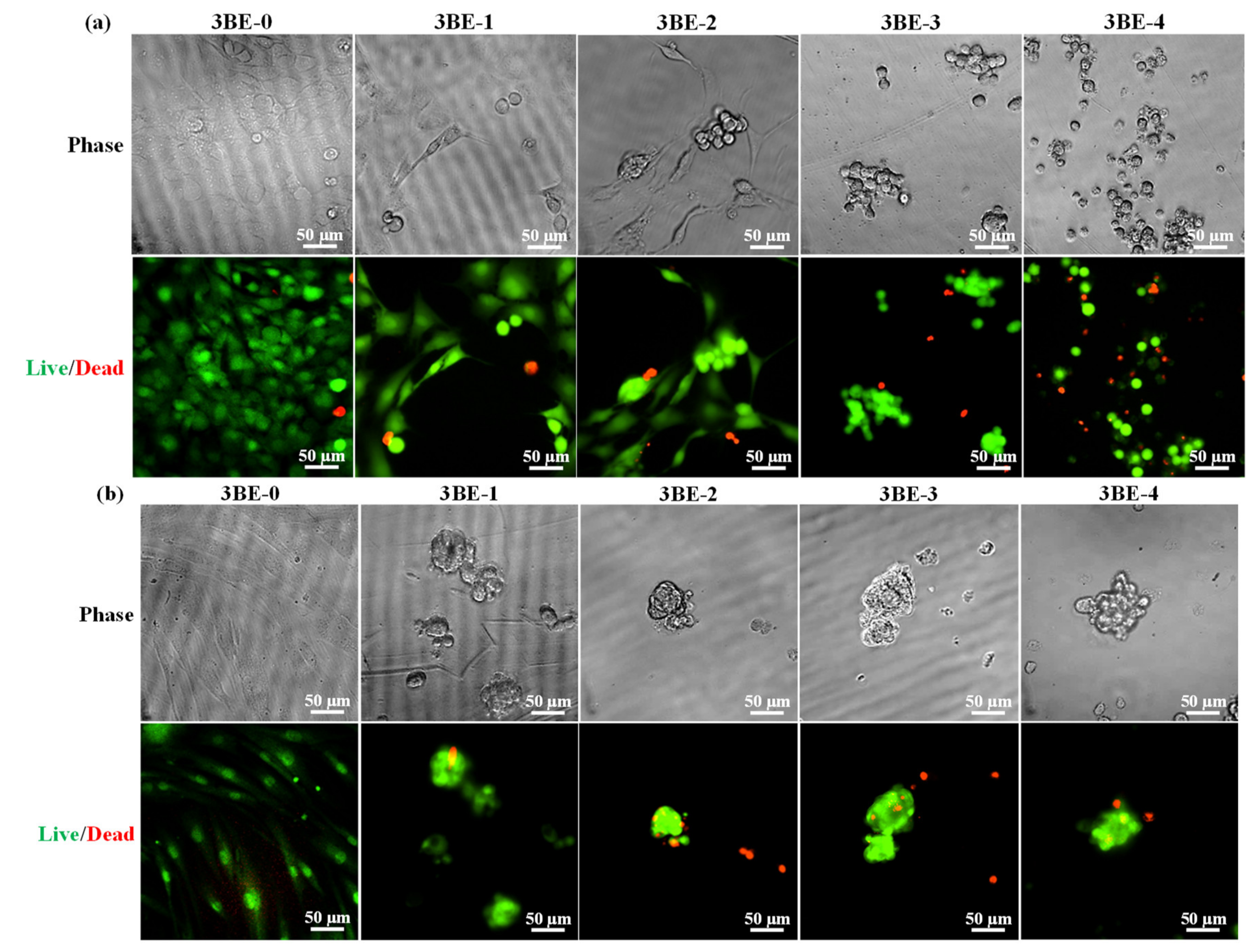
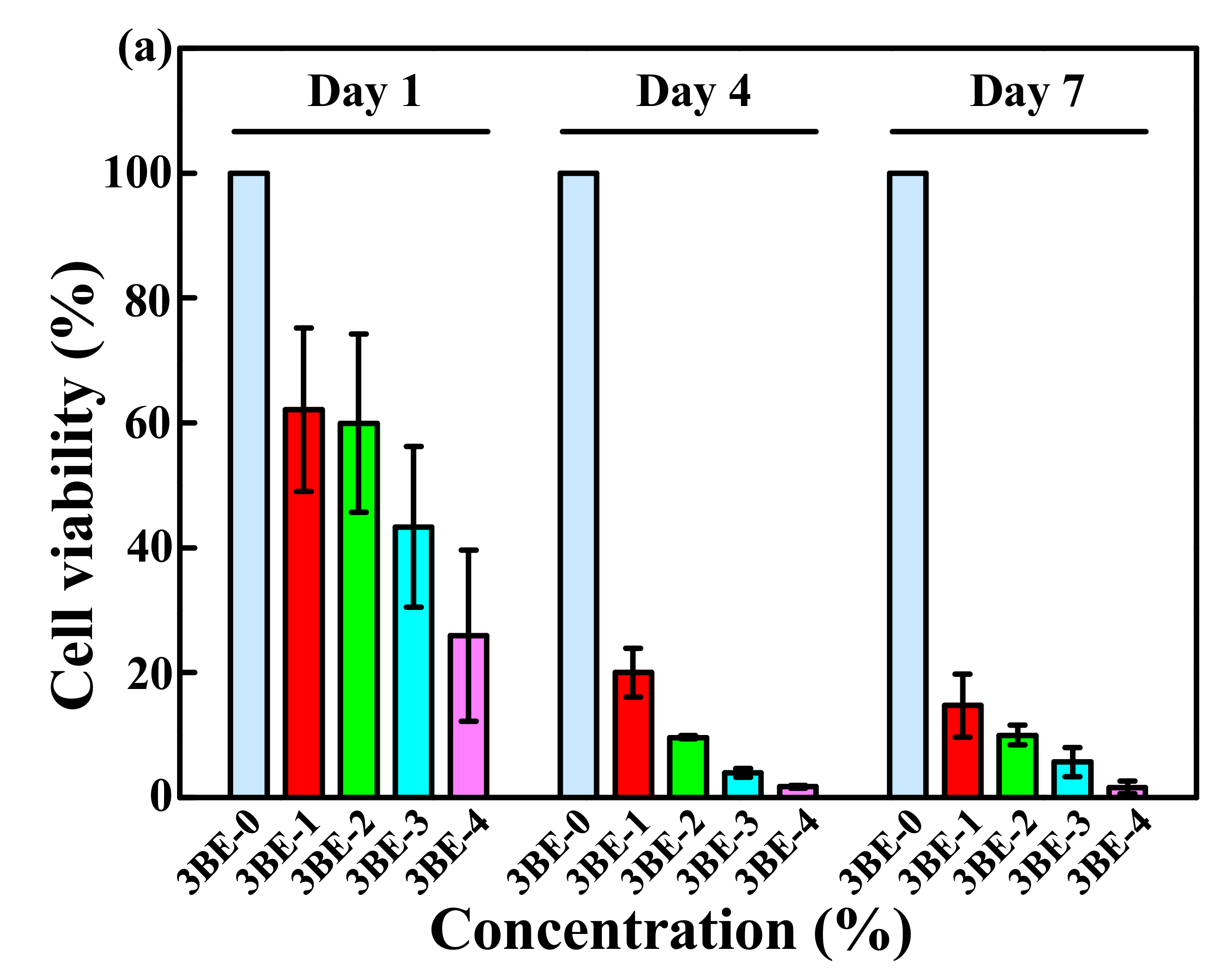
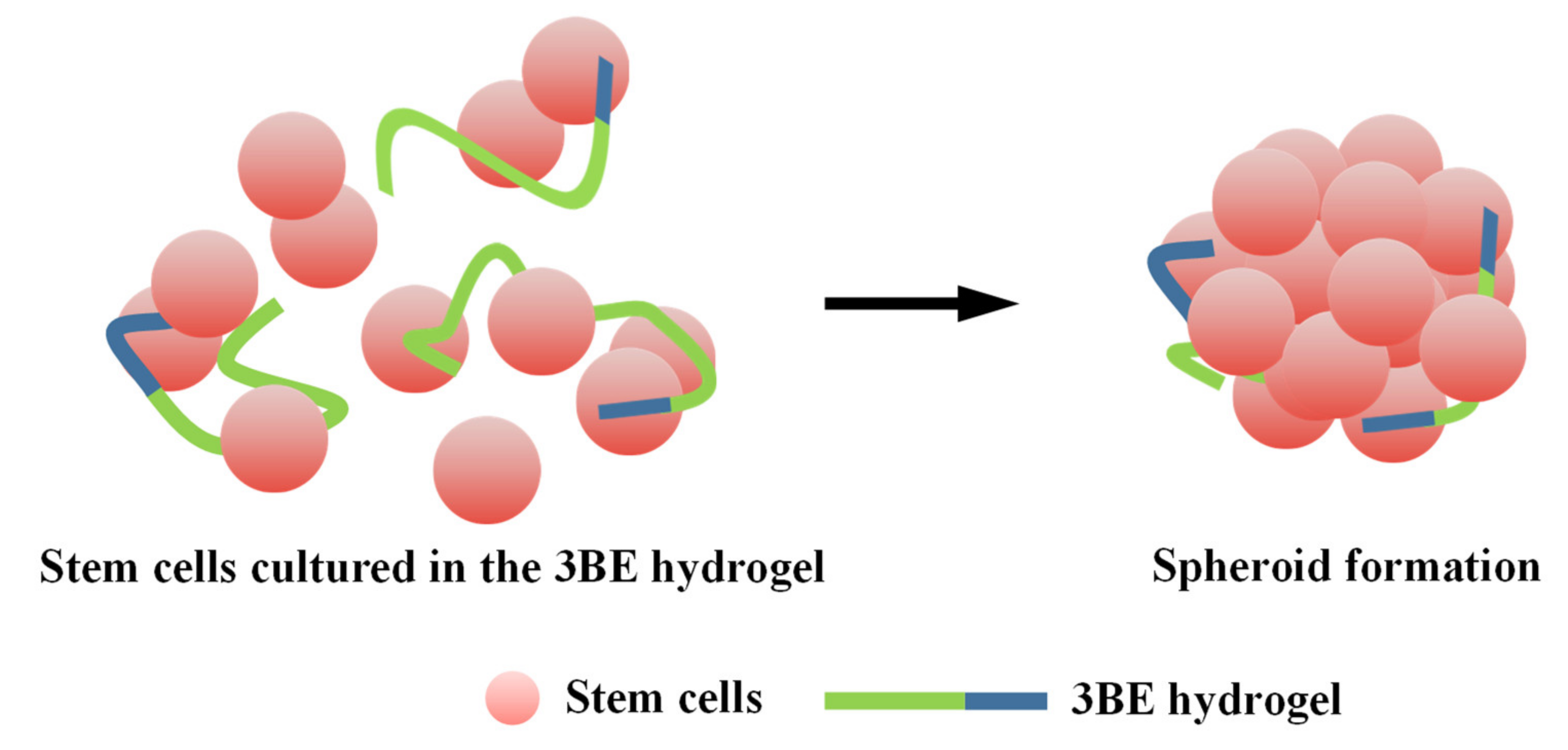
3.3. Potential of 3BE Hydrogel to Enhance Cell-to-Cell Interaction of Swiss 3T3 and HFMSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hashemi, M.; Kalalinia, F. Application of encapsulation technology in stem cell therapy. Life Sci. 2015, 143, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues—State of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xueqing, Z. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Moschella, J.; Kreiner, D.; Pena, J.; Zapantis, G.; Bolkas, M. Cumulus cell co-culture in the treatment of IVF patients with poor prognosis due to advanced maternal age or two or more failed previous cycles. Fertil. Steril. 2007, 88, S323. [Google Scholar] [CrossRef]
- Torras, N.; García-Díaz, M.; Fernández-Majada, V.; Martínez, E. Mimicking Epithelial Tissues in Three-Dimensional Cell Culture Models. Front. Bioeng. Biotechnol. 2018, 6. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Luzhansky, I.D.; Schwartz, A.D.; Cohen, J.D.; MacMunn, J.P.; Barney, L.E.; Jansen, L.E.; Peyton, S.R. Anomalously diffusing and persistently migrating cells in 2D and 3D culture environments. APL Bioeng. 2018, 2, 026112. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.; Anuradha, E.; Solomon, F.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Chaudhuri, O. Viscoelastic hydrogels for 3D cell culture. Biomater. Sci. 2017, 5, 1480–1490. [Google Scholar] [CrossRef]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloids Surfaces B Biointerface. 2017, 159, 62–77. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, X.-Y.; Wang, X.; Wang, C.; Tang, B. Microenvironment of alginate-based microcapsules for cell culture and tissue engineering. J. Biosci. Bioeng. 2012, 114, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Pluronic F127 as a Cell Encapsulation Material: Utilization of Membrane-Stabilizing Agents. Tissue Eng. 2005, 11, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Park, T.G. Temperature-responsive and degradable hyaluronic acid/Pluronic composite hydrogels for controlled release of human growth hormone. J. Control. Release 2002, 80, 69–77. [Google Scholar] [CrossRef]
- Chen, W.-J.; Huang, J.-W.; Niu, C.-C.; Chen, L.-H.; Yuan, L.-J.; Lai, P.-L.; Yang, C.-Y.; Lin, S.-S. Use of fluorescence labeled mesenchymal stem cells in pluronic F127 and porous hydroxyapatite as a bone substitute for posterolateral spinal fusion. J. Orthop. Res. 2009, 27, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Diniz, I.M.A.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 1–10. [Google Scholar] [CrossRef]
- Re’em, T.; Tsur-Gang, O.; Cohen, S. The effect of immobilized RGD peptide in macroporous alginate scaffolds on TGF beta 1-induced chondrogenesis of human mesenchymal stem cells. Biomaterials 2010, 31, 6746–6755. [Google Scholar] [CrossRef]
- LeCarpentier, Y.; Kindler, V.; Bochaton-Piallat, M.-L.; Sakic, A.; Claes, V.; Hébert, J.-L.; Vallée, A.; Schussler, O. Tripeptide Arg-Gly-Asp (RGD) modifies the molecular mechanical properties of the non-muscle myosin IIA in human bone marrow-derived myofibroblasts seeded in a collagen scaffold. PLoS ONE 2019, 14, e0222683. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Mistriotis, P.; Andreadis, S.T. Clonal multipotency and effect of long-term in vitro expansion on differentiation potential of human hair follicle derived mesenchymal stem cells. Stem Cell Res. 2012, 8, 74–84. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; Gorjup, E.; Genever, P.G. Comparative Characterization of Hair Follicle Dermal Stem Cells and Bone Marrow Mesenchymal Stem Cells. Stem Cells Dev. 2006, 15, 49–60. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Gao, Y.; Liu, X.; Bai, T.; Li, M.; Li, L.; Chi, G.; Xu, H.; Liu, F.; et al. Maintenance of high proliferation and multipotent potential of human hair follicle-derived mesenchymal stem cells by growth factors. Int. J. Mol. Med. 2013, 31, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Peng, H.F.; Gopinath, S.; Tian, J.; Andreadis, S.T. Derivation of Functional Smooth Muscle Cells from Multipotent Human Hair Follicle Mesenchymal Stem Cells. Tissue Eng. Part. A 2010, 16, 2553–2564. [Google Scholar] [CrossRef] [PubMed]
- Danti, S.; D’Acunto, M.; Trombi, L.; Berrettini, S.; Pietrabissa, A. A Micro/Nanoscale Surface Mechanical Study on Morpho-Functional Changes in Multilineage-Differentiated Human Mesenchymal Stem Cells. Macromol. Biosci. 2007, 7, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Dumbleton, J.; Agarwal, P.; Huang, H.; Hogrebe, N.J.; Han, R.; Gooch, K.J.; He, X. The effect of RGD peptide on 2D and miniaturized 3D culture of HEPM cells, MSCs, and ADSCs with alginate hydrogel. Cell. Mol. Bioeng. 2016, 9, 277–288. [Google Scholar] [CrossRef]
- Richards, D.; Jia, J.; Yost, M.; Markwald, R.; Mei, Y. 3D Bioprinting for Vascularized Tissue Fabrication. Ann. Biomed. Eng. 2017, 45, 132–147. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Tan, X.; Li, G.; Gao, Y.; Liu, X.; Zhang, L.; Li, Y. Induced Pluripotent Stem Cells from Human Hair Follicle Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2013, 9, 451–460. [Google Scholar] [CrossRef]
- Yue, K.; Santiago, G.T.-D.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Keskar, V.; Marion, N.W.; Mao, J.J.; Gemeinhart, R.A. In Vitro Evaluation of Macroporous Hydrogels to Facilitate Stem Cell Infiltration, Growth, and Mineralization. Tissue Eng. Part A 2009, 15, 1695–1707. [Google Scholar] [CrossRef]
- Lippens, E.; Swennen, I.; Girones, J.; Declercq, H.; Vertenten, G.; Vlaminck, L.; Gasthuys, F.; Schacht, E.; Cornelissen, R. Cell survival and proliferation after encapsulation in a chemically modified pluronic(r) f127 hydrogel. J. Biomater. Appl. 2013, 27, 828–839. [Google Scholar] [CrossRef]
- Lee, H.; Park, T.G. Photo-crosslinkable, biomimetic, and thermo-sensitive pluronic grafted hyaluronic acid copolymers for injectable delivery of chondrocytes. J. Biomed. Mater. Res. Part. A 2009, 88, 797–806. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Lin, W.-J.; Hong, W.-H.; Hung, W.-C.; Nowotarski, S.H.; Gouveia, S.M.; Cristo, I.; Lin, K.-H. Morphology and organization of tissue cells in 3D microenvironment of monodisperse foam scaffolds. Soft Matter 2011, 7, 10010–10016. [Google Scholar] [CrossRef]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Hartgerink, J.D.; Cavender, A.C.; Schmalz, G.; D’Souza, R.N. A Customized Self-Assembling Peptide Hydrogel for Dental Pulp Tissue Engineering. Tissue Eng. Part. A 2012, 18, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.-C.; Huang, H.-T.; Lan, W.-C.; Huang, M.-S.; Saito, T.; Huang, B.-H.; Tsai, C.-H.; Fan, F.-Y.; Ou, K.-L. The Potential of a Tailored Biomimetic Hydrogel for In Vitro Cell Culture Applications: Characterization and Biocompatibility. Appl. Sci. 2020, 10, 9035. https://doi.org/10.3390/app10249035
Cho Y-C, Huang H-T, Lan W-C, Huang M-S, Saito T, Huang B-H, Tsai C-H, Fan F-Y, Ou K-L. The Potential of a Tailored Biomimetic Hydrogel for In Vitro Cell Culture Applications: Characterization and Biocompatibility. Applied Sciences. 2020; 10(24):9035. https://doi.org/10.3390/app10249035
Chicago/Turabian StyleCho, Yung-Chieh, Hsiao-Ting Huang, Wen-Chien Lan, Mao-Suan Huang, Takashi Saito, Bai-Hung Huang, Chi-Hsun Tsai, Fang-Yu Fan, and Keng-Liang Ou. 2020. "The Potential of a Tailored Biomimetic Hydrogel for In Vitro Cell Culture Applications: Characterization and Biocompatibility" Applied Sciences 10, no. 24: 9035. https://doi.org/10.3390/app10249035
APA StyleCho, Y.-C., Huang, H.-T., Lan, W.-C., Huang, M.-S., Saito, T., Huang, B.-H., Tsai, C.-H., Fan, F.-Y., & Ou, K.-L. (2020). The Potential of a Tailored Biomimetic Hydrogel for In Vitro Cell Culture Applications: Characterization and Biocompatibility. Applied Sciences, 10(24), 9035. https://doi.org/10.3390/app10249035





