Photo-Transformation of Effluent Organic Matter by ZnO-Based Sunlight Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Sample Collection and Preparation
2.2. Photocatalytic Degradation Experiments
2.3. Analytical Methods
2.4. Statistical Analyses
3. Results and Discussion
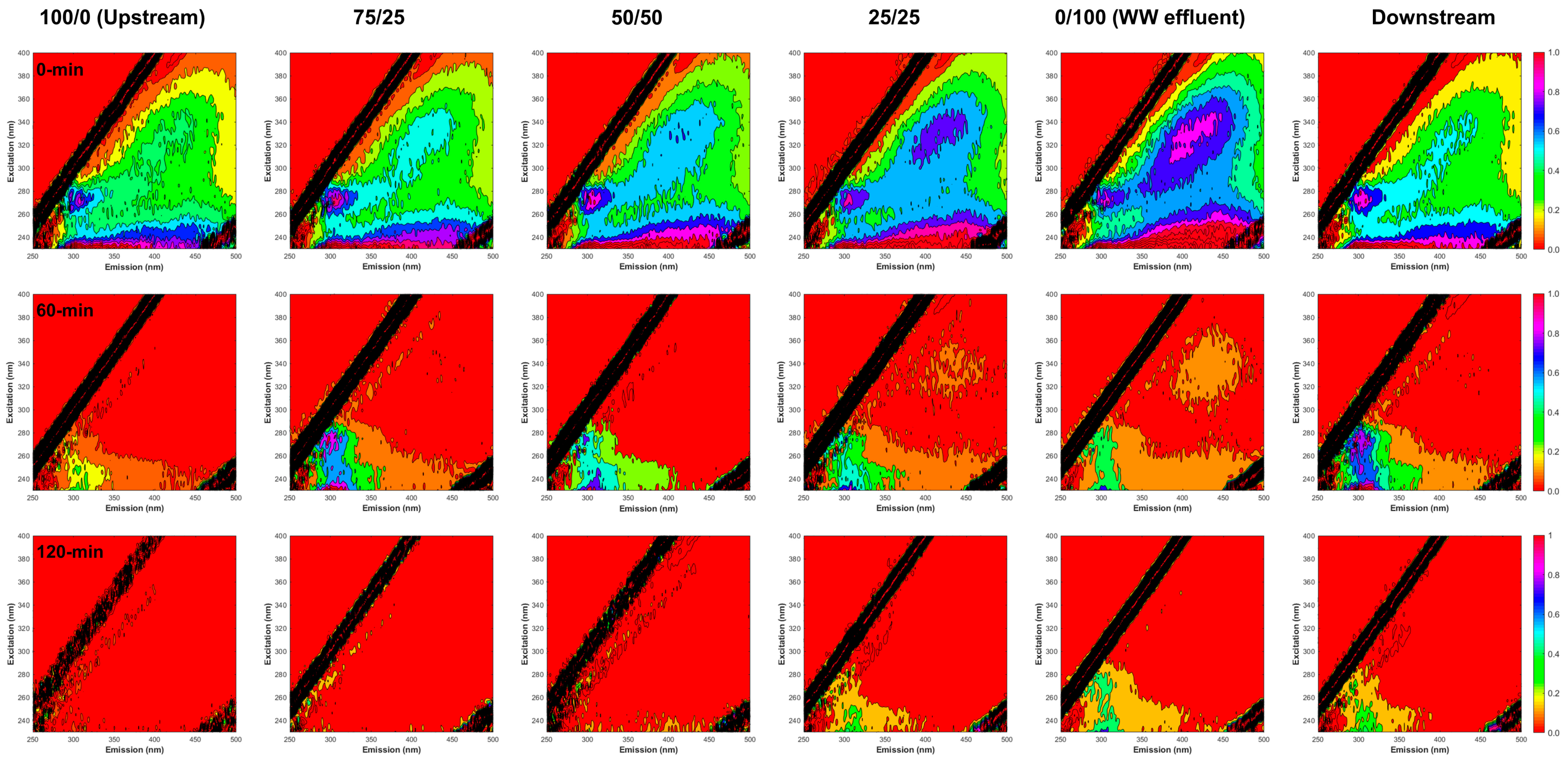
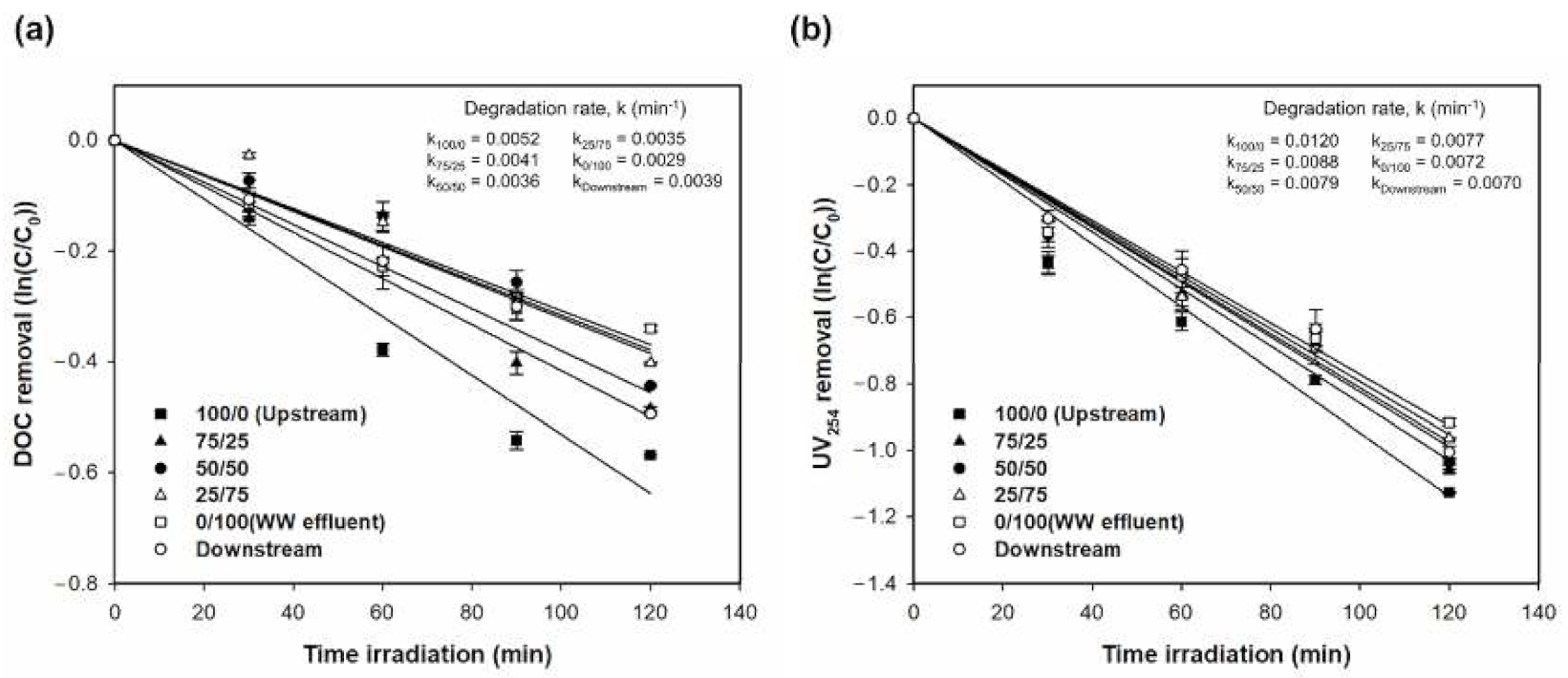
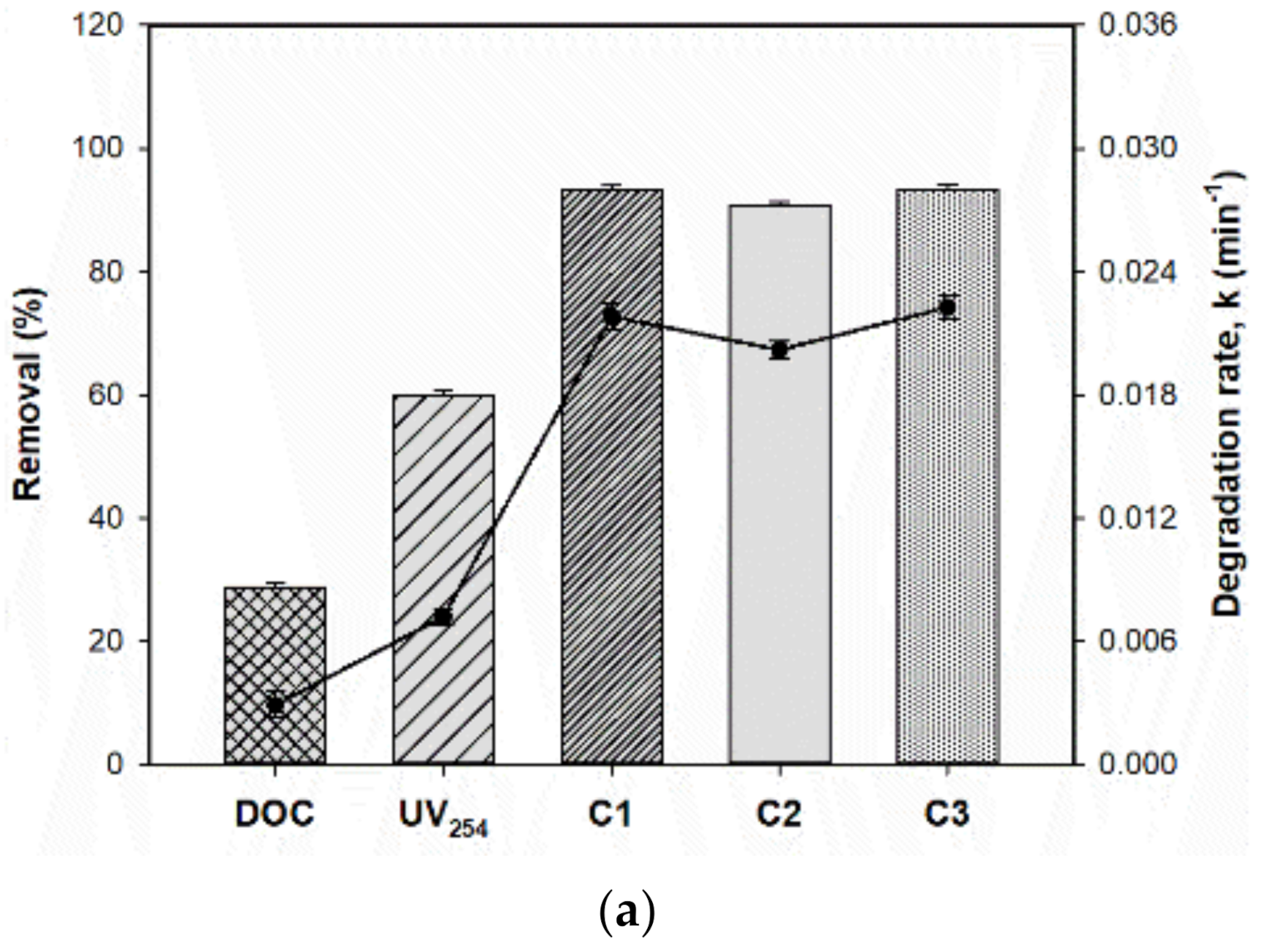
3.1. DOC and Optical Parameters
3.1.1. Characterization of the DOM, EfOM, and EfOM-Impacted
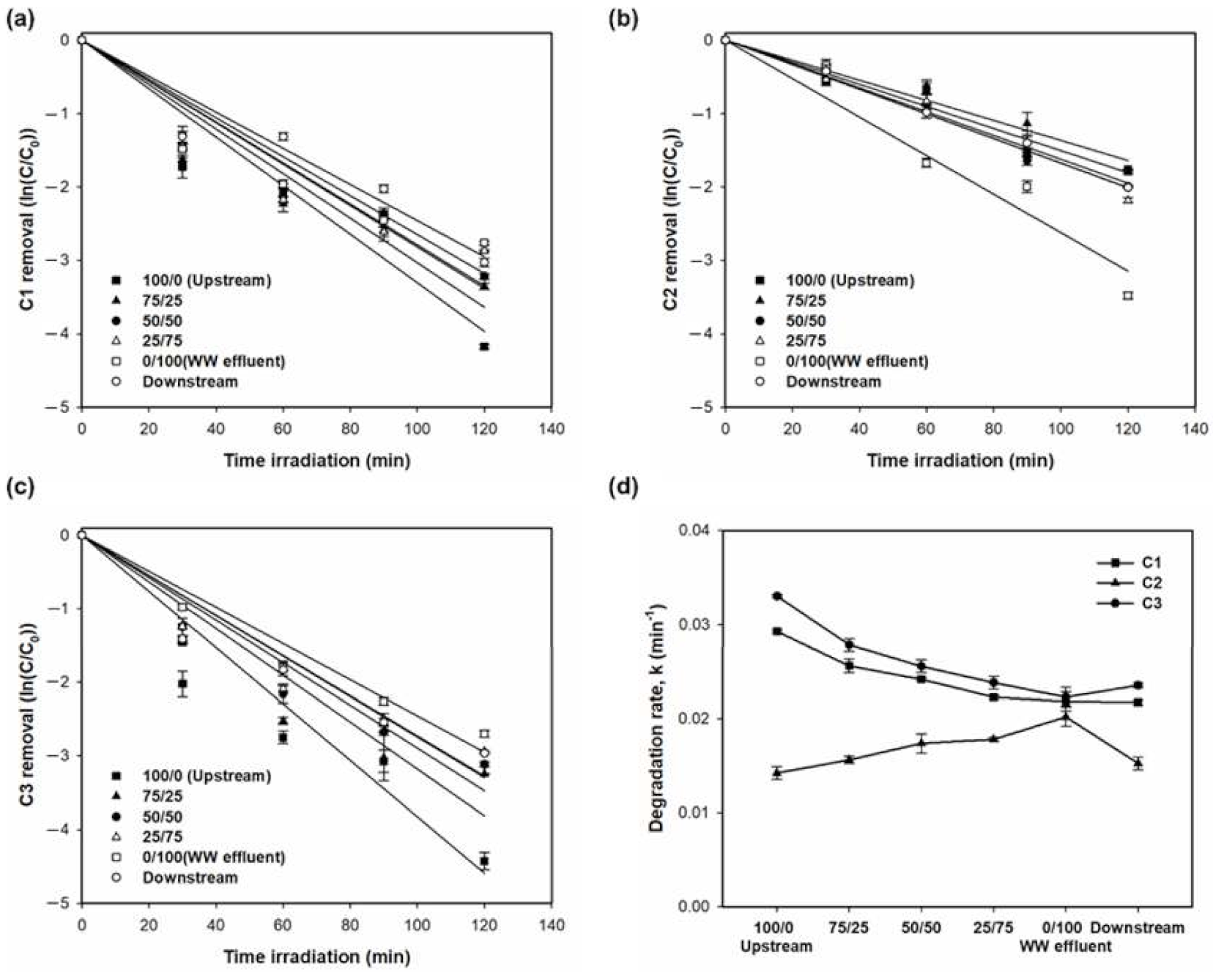
3.1.2. Photocatalytic Degradation of DOM, EfOM, and EfOM-Impacted
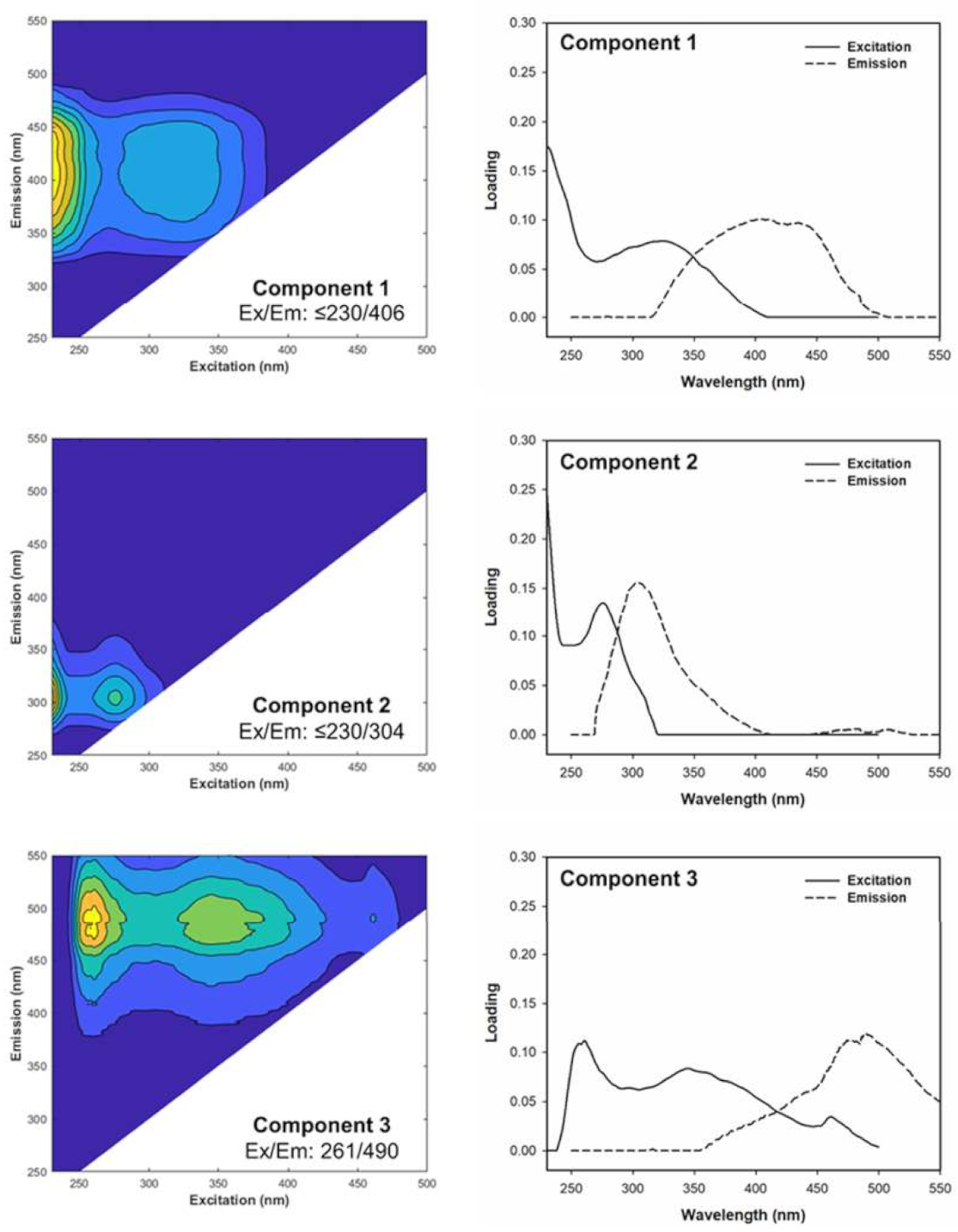
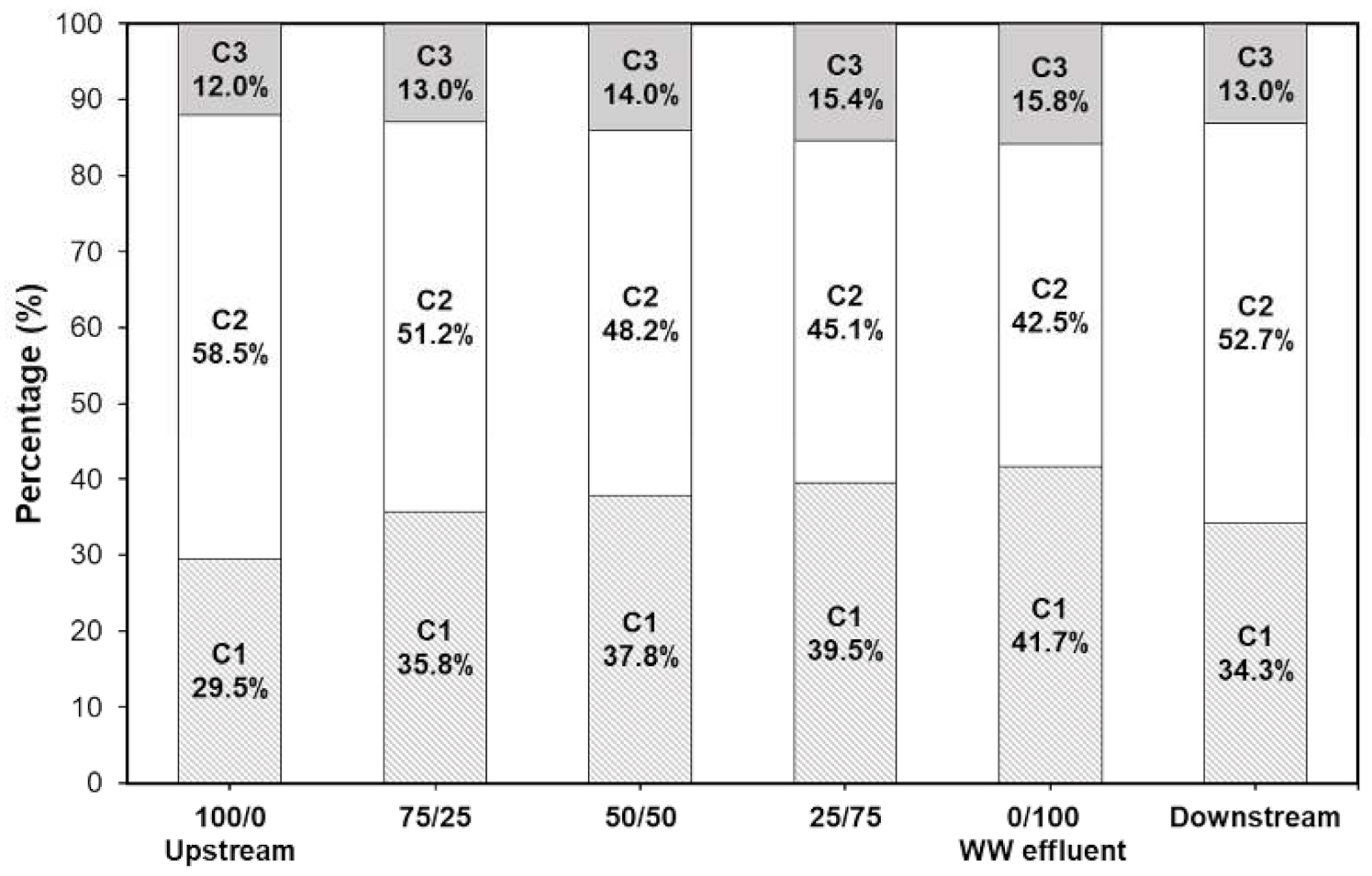
3.2. EEM-PARAFAC Components
3.2.1. Spectral Characteristics of PARAFAC Components
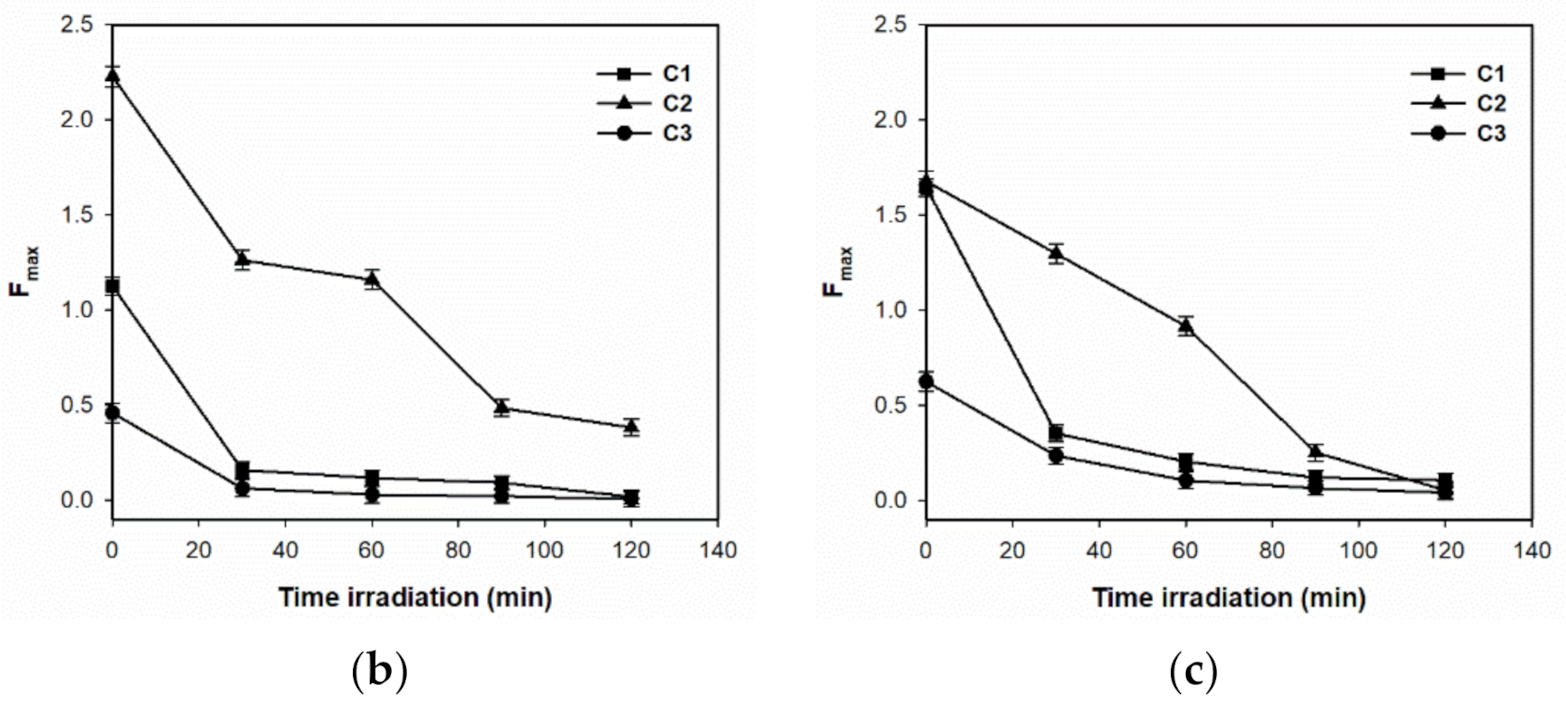
3.2.2. Changes in PARAFAC Components during Photocatalysis
3.3. Estimating the Impact of EfOM on Receiving Waters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shon, H.K.; Vigneswaran, S.; Snyder, S.A. Effluent organic matter (EfOM) in wastewater: Constituents, effects, and treatment. Crit. Rev. Environ. Sci. Technol. 2006, 36, 327–374. [Google Scholar] [CrossRef]
- Michael-Kordatou, I.; Michael, C.; Duan, X.; He, X.; Dionysiou, D.D.; Mills, M.A.; Fatta-Kassinos, D. Dissolved effluent organic matter: Characteristics and potential implications in wastewater treatment and reuse applications. Water Res. 2015, 77, 213–248. [Google Scholar] [CrossRef]
- Nam, S.-N.; Amy, G.L. Differentiation of wastewater effluent organic matter (EfOM) from natural organic matter (NOM) using multiple analytical techniques. Water Sci. Technol. 2008, 57, 1009–1015. [Google Scholar] [CrossRef]
- Vakondios, N.; Koukouraki, E.E.; Diamadopoulos, E. Effluent organic matter (EfOM) characterization by simultaneous measurement of proteins and humic matter. Water Res. 2014, 63, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Coble, P.G.; Lead, J.; Baker, A.; Reynolds, D.M.; Spencer, R.G. Aquatic Organic Matter Fluorescence, 1st ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Nam, S.-N.; Krasner, S.W.; Amy, G.L. Differentiating Effluent Organic Matter (EfOM) from Natural Organic Matter (NOM): Impact of EfOM on Drinking Water Sources. In Advanced Environmental Monitoring; Springer: Dordrecht, The Netherland, 2008; pp. 259–270. [Google Scholar] [CrossRef]
- Bodhipaksha, L.C.; Sharpless, C.M.; Chin, Y.P.; Sander, M.; Langston, W.K.; Mackay, A.A. Triplet Photochemistry of Effluent and Natural Organic Matter in Whole Water and Isolates from Effluent-Receiving Rivers. Environ. Sci. Technol. 2015, 49, 3453–3463. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Meng, F.; Huang, G.; Sun, L.; Lin, Z. Sunlight-induced changes in chromophores and fluorophores of wastewater-derived organic matter in receiving waters—The role of salinity. Water Res. 2014, 62, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manage. 2018, 208, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms, and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nam, S.-N.; Kim, J.; Oh, J. Photocatalytic degradation of dissolved organic matter under ZnO-catalyzed artificial sunlight irradiation system. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Phong, D.D.; Hur, J. Non-catalytic and catalytic degradation of effluent dissolved organic matter under UVA-and UVC-irradiation tracked by advanced spectroscopic tools. Water Res. 2016, 105, 199–208. [Google Scholar] [CrossRef]
- Phong, D.D.; Hur, J. Insight into photocatalytic degradation of dissolved organic matter in UVA/TiO2 systems revealed by fluorescence EEM-PARAFAC. Water Res. 2015, 87, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Lee, D.; Kwon, M.; Choi, I.-H.; Nam, S.-N.; Kang, J.W. Characteristics and fate of natural organic matter during UV oxidation processes. Chemosphere 2017, 184, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Korshin, G.; Chow, C.W.K.; Fabris, R.; Drikas, M. Absorbance spectroscopy-based examination of effects of coagulation on the reactivity of fractions of natural organic matter with varying apparent molecular weights. Water Res. 2009, 43, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Li, P.; Hur, J. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 131–154. [Google Scholar] [CrossRef]
- John, R.H.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2009, 54, 1023. [Google Scholar] [CrossRef]
- Leenheer, J.A.; Croué, J.-P. Characterizing aquatic dissolved organic matter. Environ. Sci. Technol. 2003, 37, 18A–26A. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence Inner-Filtering Correction for Determining the Humification Index of Dissolved Organic Matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Waite, T.D.; Ruiz, G.M. Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar. Chem. 2008, 108, 40–58. [Google Scholar] [CrossRef]
- Murphy, K.R.; Hambly, A.; Singh, S.; Henderson, R.K.; Baker, A.; Stuetz, R.; Khan, S.J. Organic Matter Fluorescence in Municipal Water Recycling Schemes: Toward a Unified PARAFAC Model. Environ. Sci. Technol. 2011, 45, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Baghoth, S.A.; Sharma, S.K.; Amy, G.L. Tracking natural organic matter (NOM) in a drinking water treatment plant using fluorescence excitation-emission matrices and PARAFAC. Water Res. 2011, 45, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Sharp, E.L.; Parsons, S.A.; Jefferson, B. Seasonal variations in natural organic matter and its impact on coagulation in water treatment. Sci. Total Environ. 2006, 363, 183–194. [Google Scholar] [CrossRef]
- Bose, P.; Reckhow, D.A. The effect of ozonation on natural organic matter removal by alum coagulation. Water Res. 2007, 41, 1516–1524. [Google Scholar] [CrossRef]
- Henderson, R.K.; Baker, A.; Murphy, K.R.; Hambly, A.; Stuetz, R.M.; Khan, S.J. Fluorescence as a potential monitoring tool for recycled water systems: A review. Water Res. 2009, 43, 863–881. [Google Scholar] [CrossRef]
- Musikavong, C.; Wattanachira, S. Reduction of dissolved organic matter in terms of DOC, UV−254, SUVA and THMFP in industrial estate wastewater treated by stabilization ponds. Environ. Monit. Assess. 2007, 134, 489–497. [Google Scholar] [CrossRef]
- Hansen, A.M.; Kraus, T.E.C.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnol. Oceanogr. 2016, 61, 1015–1032. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, M.L.Q.; Ren, N. Evolution of molecular weight and fluorescence of effluent organic matter (EfOM) during oxidation processes revealed by advanced spectrographic and chromatographic tools. Water Res. 2017, 124, 566–575. [Google Scholar] [CrossRef]
- Pickard, A.E.; Heal, K.V.; McLeod, A.R.; Dinsmore, K.J. Temporal changes in photoreactivity of dissolved organic carbon and implications for aquatic carbon fluxes from peatlands. Biogeosciences 2017, 14, 1793–1809. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Tranvik, L.; Kronberg, L.; Slätis, T.; Martinsen, W. Photochemical production of ammonium and transformation of dissolved organic matter in the Baltic Sea. Mar. Chem. 2007, 104, 227–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Qin, B.; Feng, S. Photochemical degradation of chromophoric-dissolved organic matter exposed to simulated UV-B and natural solar radiation. Hydrobiologia 2009, 627, 159–168. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Y.; Feng, L.; Zhu, G.; Shi, Z.; Liu, X.; Zhang, Y. Characterizing chromophoric dissolved organic matter in Lake Tianmuhu and its catchment basin using excitation-emission matrix fluorescence and parallel factor analysis. Water Res. 2011, 45, 5110–5122. [Google Scholar] [CrossRef] [PubMed]
| Parameters | 100/0 (Upstream) | 75/25 | 50/50 | 25/75 | 0/100 (WW Effluent) | Downstream | |
|---|---|---|---|---|---|---|---|
| DOC (mg/L) | Initial | 6.51 ± 0.03 | 7.05 ± 0.07 | 7.62 ± 0.03 | 7.79 ± 0.06 | 8.54 ± 0.01 | 8.04 ± 0.07 |
| Photocatalysis | 3.68 ± 0.03 | 4.34 ± 0.03 | 4.89 ± 0.05 | 5.21 ± 0.02 | 6.08 ± 0.06 | 4.90 ± 0.05 | |
| UV254 (A.U.) | Initial | 0.073 | 0.079 | 0.089 ± 0.001 | 0.092 | 0.103 ± 0.001 | 0.099 |
| Photocatalysis | 0.024 | 0.027 | 0.031 | 0.035 | 0.041 ± 0.001 | 0.036 ± 0.001 | |
| SUVA254 (L mg−1 m−1) | Initial | 1.124 ± 0.008 | 1.116 ± 0.080 | 1.163 ± 0.020 | 1.177 ± 0.012 | 1.205 ± 0.018 | 1.234 ± 0.013 |
| Photocatalysis | 0.643 ± 0.011 | 0.627 ± 0.009 | 0.647 | 0.671 ± 0.006 | 0.677 ± 0.012 | 0.740 ± 0.029 | |
| E2/E3 | Initial | 7.06 ± 1.17 | 7.60 ± 0.01 | 7.57 ± 1.24 | 6.86 ± 0.57 | 6.42 ± 0.72 | 7.12 ± 0.06 |
| Photocatalysis | 28.96 ± 1.30 | 23.30 ± 1.02 | 26.19 ± 1.99 | 24.45 ± 2.54 | 21.50 ± 1.13 | 35.08 ± 1.18 | |
(L mol−1 m−1) | Initial | 21.25 | 20.42 | 20.99 | 22.57 | 22.87 | 23.16 |
| Photocatalysis | 7.92 ± 0.27 | 7.45 ± 0.56 | 7.87 ± 0.08 | 8.16 ± 0.29 | 8.27 ± 1.06 | 7.92 ± 0.45 | |
| S275−295 (nm−1) | Initial | −0.017 ± 0.002 | −0.016 | −0.016 ± 0.003 | −0.014 ± 0.002 | −0.013 ± 0.001 | −0.014 |
| Photocatalysis | −0.025 ± 0.005 | −0.019 | −0.018 ± 0.001 | −0.016 | −0.020 ± 0.002 | −0.025 ± 0.005 | |
| S350–400 (nm−1) | Initial | −0.016 ± 0.002 | −0.019 | −0.017 ± 0.002 | −0.018 | −0.017 | −0.018 |
| Photocatalysis | −0.024 ± 0.003 | −0.021 ± 0.001 | −0.030 ± 0.003 | −0.023 ± 0.001 | −0.022 ± 0.005 | −0.024 ± 0.003 | |
| SR | Initial | 1.022 ± 0.012 | 0.872 ± 0.002 | 0.909 ± 0.121 | 0.768 ± 0.111 | 0.744 ± 0.027 | 0.783 ± 0.010 |
| Photocatalysis | 1.051 ± 0.091 | 0.905 ± 0.045 | 0.620 ± 0.070 | 0.706 ± 0.012 | 0.952 ± 0.263 | 1.051 ± 0.091 | |
| FI | Initial | 1.26 ± 0.03 | 1.31 ± 0.01 | 1.45 ± 0.05 | 1.49 ± 0.07 | 1.66 ± 0.04 | 1.50 ± 0.01 |
| Photocatalysis | 0.33 ± 0.01 | 0.37 | 0.50 | 0.66 ± 0.05 | 0.87 ± 0.03 | 0.51 ± 0.01 | |
| HIX | Initial | 1.01 | 1.14 | 1.19 | 1.37 | 1.54 | 1.18 |
| Photocatalysis | 0.150 | 0.16 | 0.18 | 0.20 | 0.22 ± 0.01 | 0.20 | |
| BIX | Initial | 0.96 | 0.97 | 0.99 | 1.01 | 1.04 | 1.00 |
| Photocatalysis | 0.03 | 0.27 | 0.37 | 0.41 | 0.52 | 0.31 | |
| Parameters | Sources of Variation | Sum of Squares | Degree of Freedom | Mean Square | F | Significance |
|---|---|---|---|---|---|---|
| DOC | Between Groups | 7.069 | 4 | 1.767 | 992.871 | 0.000 |
| Within Groups | 0.018 | 10 | 0.002 | |||
| Total | 7.087 | 14 | ||||
| UV254 | Between Groups | 0.002 | 4 | 0.000 | 612.306 | 0.000 |
| Within Groups | 0.000 | 10 | 0.000 | |||
| Total | 0.002 | 14 | ||||
| SUVA254 | Between Groups | 0.017 | 4 | 0.004 | 26.324 | 0.000 |
| Within Groups | 0.002 | 10 | 0.000 | |||
| Total | 0.018 | 14 | ||||
| Between Groups | 13.254 | 4 | 3.313 | 5.664 | 0.012 | |
| Within Groups | 5.850 | 10 | 0.585 | |||
| Total | 19.104 | 14 | ||||
| S275–295 | Between Groups | 0.000 | 4 | 0.000 | 11.866 | 0.001 |
| Within Groups | 0.000 | 10 | 0.000 | |||
| Total | 0.000 | 14 | ||||
| S350–400 | Between Groups | 0.000 | 4 | 0.000 | 1.840 | 0.198 |
| Within Groups | 0.000 | 10 | 0.000 | |||
| Total | 0.000 | 14 | ||||
| SR | Between Groups | 0.248 | 4 | 0.062 | 16.305 | 0.000 |
| Within Groups | 0.038 | 10 | 0.004 | |||
| Total | 0.286 | 14 | ||||
| E2/E3 | Between Groups | 2.980 | 4 | 0.745 | 1.266 | 0.346 |
| Within Groups | 5.886 | 10 | 0.589 | |||
| Total | 8.865 | 14 | ||||
| FI | Between Groups | 0.303 | 4 | 0.076 | 47.810 | 0.000 |
| Within Groups | 0.016 | 10 | 0.002 | |||
| Total | 0.318 | 14 | ||||
| HIX | Between Groups | 0.524 | 4 | 0.131 | 313.496 | 0.000 |
| Within Groups | 0.004 | 10 | 0.000 | |||
| Total | 0.528 | 14 | ||||
| BIX | Between Groups | 0.011 | 4 | 0.003 | 10.298 | 0.001 |
| Within Groups | 0.003 | 10 | 0.000 | |||
| Total | 0.013 | 14 | ||||
| C1_Fmax | Between Groups | 0.638 | 4 | 0.159 | 174.686 | 0.000 |
| Within Groups | 0.009 | 10 | 0.001 | |||
| Total | 0.647 | 14 | ||||
| C2_Fmax | Between Groups | 0.850 | 4 | 0.212 | 186.690 | 0.000 |
| Within Groups | 0.011 | 10 | 0.001 | |||
| Total | 0.861 | 14 | ||||
| C3_Fmax | Between Groups | 0.096 | 4 | 0.024 | 263.678 | 0.000 |
| Within Groups | 0.001 | 10 | 0.000 | |||
| Total | 0.097 | 14 |
| Samples | Removal (%) | Degradation Rate, k (min−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DOC | UV254 | C1 | C2 | C3 | DOC | UV254 | C1 | C2 | C3 | |
| 100/0 (upstream) | 43.4 | 67.6 | 98.4 | 82.8 | 98.7 | 0.0052 | 0.0102 | 0.0292 | 0.0142 | 0.0330 |
| 75/25 | 38.4 | 65.4 | 96.1 | 84.0 | 96.5 | 0.0041 | 0.0088 | 0.0256 | 0.0156 | 0.0278 |
| 50/50 | 35.9 | 64.6 | 95.6 | 86.4 | 95.7 | 0.0036 | 0.0079 | 0.0242 | 0.0174 | 0.0255 |
| 25/75 | 33.1 | 61.9 | 94.2 | 88.7 | 94.7 | 0.0035 | 0.0077 | 0.0223 | 0.0178 | 0.0238 |
| 0/100 (WW effluent) | 28.8 | 60.0 | 93.3 | 90.8 | 93.5 | 0.0029 | 0.0072 | 0.0218 | 0.0202 | 0.0223 |
| Downstream | 39.0 | 63.4 | 94.6 | 86.5 | 94.8 | 0.0039 | 0.0070 | 0.0217 | 0.0152 | 0.0235 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Nam, S.N.; Oh, J. Photo-Transformation of Effluent Organic Matter by ZnO-Based Sunlight Irradiation. Appl. Sci. 2020, 10, 9002. https://doi.org/10.3390/app10249002
Nguyen TT, Nam SN, Oh J. Photo-Transformation of Effluent Organic Matter by ZnO-Based Sunlight Irradiation. Applied Sciences. 2020; 10(24):9002. https://doi.org/10.3390/app10249002
Chicago/Turabian StyleNguyen, Thao Thi, Seong Nam Nam, and Jeill Oh. 2020. "Photo-Transformation of Effluent Organic Matter by ZnO-Based Sunlight Irradiation" Applied Sciences 10, no. 24: 9002. https://doi.org/10.3390/app10249002
APA StyleNguyen, T. T., Nam, S. N., & Oh, J. (2020). Photo-Transformation of Effluent Organic Matter by ZnO-Based Sunlight Irradiation. Applied Sciences, 10(24), 9002. https://doi.org/10.3390/app10249002






