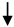Featured Application
The application and limits of advanced oxidation, reduction, and oxidation/reduction processes to water and wastewater treatment are discussed.
Abstract
Emerging contaminants’ presence in water, wastewater, and aquatic environments has been widely reported. Their environmental and health-related effects, and the increasing tendency towards wastewater reuse require technology that could remove to a greater degree, or even mineralize, all these contaminants. Currently, the most commonly used process technologies for their removal are advanced oxidation processes (AOPs); however, recent advances have highlighted other advanced treatment processes (ATPs) as possible alternatives, such as advanced reduction processes (ARPs) and advanced oxidation-reduction processes (AORPs). Although they are not yet widely diffused, they may remove contaminants that are not readily treatable by AOPs, or offer better performance than the former. This paper presents an overview of some of the most common or promising ATPs for the removal of contaminants from water and wastewater, and their application, with discussion of their limitations and merits. Issues about technologies’ costs and future perspectives in the water sector are discussed.
1. Introduction
The detection of emerging contaminants in the environment and in waste and supply water is increasing due to the ever-growing role of chemistry in industrial production and to advancements in analytical technology [1,2,3]. These, commonly addressed as a group under the term of ‘contaminants of emerging concern’ (CECs), are diverse and ubiquitous, frequently lumped into categories that describe their purpose, use, or other characteristic (Table 1) [4]. These contaminants could negatively affect water uses, human health, and ecosystem integrity, and cause the spread of antibiotic-resistant bacteria due to low persistent doses of residual pharmacological principles in human and animal excreta [5]. It was estimated that, just in Germany, about 16,000 tons/year of discarded pharmaceutical and personal care products (PPCPs) are flushed down toilets, or disposed of in household waste, eventually finding their way into natural waters [6]. Online measurement of these pollutants in the environment, and especially in water supply systems, is a very sensitive issue, which has not yet been satisfactorily addressed [7]. The increasing tendency towards wastewater reuse, both for non-potable and drinking uses [8], is prompting the development of more robust water and wastewater treatment through increasingly sophisticated multiple barrier treatment (MBT) schemes [9]. However, even in current state-of-the-art wastewater treatment plants (WWTPs), these contaminants may be removed but not degraded to satisfactory levels: Many current advanced processes, for example, adsorption, ion exchange, reverse osmosis, and membrane nano/ultra-filtration, only concentrate contaminants, without degrading or ultimately eliminating them, leaving this step to a subsequent phase. Even with the use of advanced degradation technologies, these compounds may only be partly transformed, possibly into hazardous byproducts or intermediates. Furthermore, their transformation pathways may often still be undetermined [10]. Several studies have shown that many compounds subject to biological, chemical, or surface processes are not completely degraded (mineralized) but may end up as transformation byproducts in the effluent, or accumulated within the residual solid phase (i.e., excess sludge) [11,12,13]. These residuals, then, require additional processing to avoid byproducts’ environmental dispersion. Incomplete removal (even in µg/L-ng/L concentration ranges) of these contaminants has been related to potential long-term adverse impacts on the environment and human health.

Table 1.
Common CEC classes (elaborated from [4]).
Most currently used water and wastewater process technologies, including state-of-the-art advanced oxidation processes (AOPs), have some, and occasionally significant, technological drawbacks: Almost all require extended process time (up to several hours) to achieve destruction of contaminants and often they require costly addition of chemicals or catalyzers to enhance treatment performance. This paper presents a review of some of the most common AOP process technologies used for the removal of emerging contaminants from water and wastewater, and introduces discussion about two lesser-known classes of processes, advanced reduction processes (ARPs) and advanced oxidation-reduction processes (AORPs), that may offer a similar or better performance than the former.
2. Advanced Treatment Processes for Emerging Contaminants
The classes of processes examined below are termed advanced treatment processes (ATPs), since they are capable of achieving degradation of specific constituents in solution, not normally achieved by other treatment options. This class covers all those unit operations that do not act on mechanical or biological principles: Coagulation-flocculation-precipitation, stripping, filtration (all types), ion exchange, absorption, electroflotation, biological and bioelectrochemical methods, etc. They may, however, be combined with these and other treatment units to improve overall pollutants’ removal efficiency, i.e., in MBT schemes [9]. ATP in combination with common or modified biotreatment technologies can be the key for successful emission control for many CECs, as in many cases, they significantly increase the biodegradability of recalcitrant or inhibitory pollutants, as discussed later.
One crucial factor for assessing the feasibility of a particular treatment process is represented by the kinetic rates of the reactions involved in the degradation of target compounds. Advance treatment technologies make use of highly reactive minimally selective reagents that will achieve transformation of contaminants in solution into simpler (e.g., partial decomposition of non-biodegradable organic pollutants into biodegradable intermediates) or innocuous (e.g., mineralization to CO2, water, and residual elements) products, even though the formation of hazardous byproducts cannot be excluded a priori.
Advanced oxidation processes are aqueous phase oxidation methods, investigated and developed since the 1970s, and well established in the water sector, as they are currently considered the state-of-the-art in commercially available advanced treatment processes for many contaminants [14]. AOPs are based on the generation of highly reactive radicals (predominantly OH, hydroxyl radical) for the oxidation of organic pollutants within a solution treated by means of different activation processes (ultraviolet photolysis, Fenton processes, electrochemical oxidation, sonochemical processes, microwave, supercritical water and wet air oxidation, and others), often combined with chemicals (e.g., O3, H2O2) and/or catalysts (e.g., TiO2) [15,16]. OH is among the strongest oxidants applicable in water processes, and can unselectively oxidize virtually any susceptible compound present through the following basic pathways: Radical addition, hydrogen abstraction, electron transfer, and radical combination. The chain of reaction that is generated leads to further generation of reactive species, such as H2O2 and super oxide (·O2−). The mechanisms of radical generation in the most common AOPs for wastewater treatment were summarized in other works [14,17]. Figure 1 summarizes the various technologies available in the AOP and in other ATP classes. AOPs have been and are still widely researched: A non-comprehensive bibliography search counted over 20,000 published works on their application for the removal of pharmaceutical residues, endocrine disruptors, natural organic matter, bio-recalcitrant organics, generic and specific pollutants, and pathogens from water and wastewater [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32].
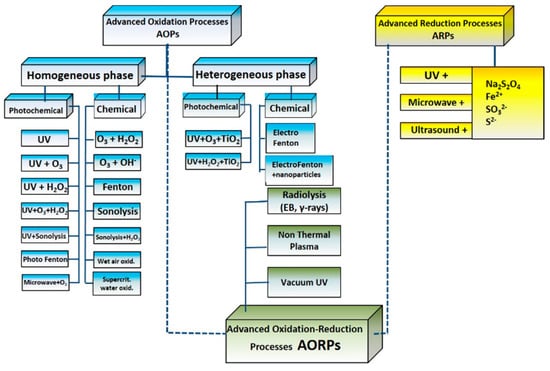
Figure 1.
Various process technologies in the ATP realm.
AOPs promote the degradation of organic compounds into simpler molecules: Partial decomposition of non-biodegradable organics may lead to more biodegradable intermediates or, more rarely, to their mineralization and to the destruction of pathogenic organisms, by the effect of strong oxidants. The oxidation potential is hence the strength and, at the same time, the limit of AOPs: Some contaminants, such as, for example, chlorinated and brominated compounds, are poorly or not degraded at all by oxidation.
While AOPs and their applications in the water sector are well known, advanced reduction processes (ARPs) are a recently emerged class of processes combining activation methods similar to those used by AOPs (UV, ultrasound, microwave) and reductive agents (sulfite, ferrous iron, sulfide, dithionite) to generate reactive radicals capable of strong reducing reactions (hydrated electrons, e−aq, hydrogen ·H, and sulfite radicals ·SO3−). These, unlike those generated by AOPs, can easily degrade oxidized contaminants in solution [33,34]. Reductants are selected for ARPs based on the applied activation method’s capacity to produce reducing radicals, or other effective reducing agents.
Dithionite (i.e., sodium hydrosulfite, Na2S2O4), for example, is a water-soluble salt used as reducing agent in aqueous solutions. By cleaving the S-S bond upon absorption of 315-nm UV radiation, dithionite can be broken into two sulfur dioxide radical anions (·SO2−), a strong reductant. Additionally, sulfite (SO32−), upon irradiation, will react to create a sulfite radical anion (·SO3−) and e−aq, also a strong reductant, while the sulfite radical could act indifferently as an oxidant or reductant, accepting an electron to form sulfite or donating one to form sulfate. Sulfide, S2−, adsorbs UV light at 230 nm, promoting the formation of hydrogen in solution, similarly to ferrous iron.
While the chemical mechanisms of reducing radical formation are fairly well known, little research has been conducted so far on the application of these processes to water treatment and contaminant degradation. Preliminary results [33] have demonstrated that ARPs can degrade a wide variety of contaminants, including: Perchlorate (a highly oxidized chlorine form normally difficult to reduce) [35], 1,2 dichloroethane [36], perfluorooctanoic acid (PFOA, a synthetic, difficult to treat, fluorinated organic acid) [37], 2,4-dichlorophenol (2,4-DCP), a chlorinated highly toxic phenol derivative used as herbicide preparation intermediate [38], several halogenated compounds [39], nitrate (a ubiquitous groundwater contaminant that can be removed by chemical or electrochemical methods) [40,41], and metals (e.g., arsenic, selenium) [42]. Despite these encouraging results, however, this technology has not reported real-scale applications so far. Considering the fact that some CECs can only be removed by strong reduction reactions, these processes will likely gain greater consideration in the near future.
Advanced oxidation-reduction processes (AORPs), a third class of ATPs, combine both types of radical reactions to directly degrade contaminants. They are perhaps the most interesting development in advanced treatment technology. Concurrently generated radicals carry out simultaneous oxidation and reduction reactions, achieving accelerated destruction of a wide range of contaminants [43]. AORPs are chemical-less high-rate energy-efficient processes, with a wide applicability range. AORPs may be activated by high-energy inputs, such as photoionization (Vacuum UV -VUV- with irradiation at 185 nm and 254 nm) [44], non-thermal plasma electric discharges [45], or ionizing radiations [46].
In the VUV process, photoionization and homolysis of water molecules occur with the absorption of high-energy photons in water, according to the following reactions:
H2O + VUV185nm → ∙OH + H++ e− aq,
H2O + VUV185nm → ∙OH + ∙H,
H2O +∙H → H3O+ + e− aq,
O2 + VUV185nm → 2 ∙O,
O2 + ∙O → O3,
O3 + H2O + UV254nm → 2∙OH + O2.
In non-thermal plasma processes (NTPs), high-voltage electrical pulses generate a corona discharge, which in turn excites electrons in the air above a liquid solution with an ionization effect, producing singlet oxygen atoms, which generate ozone and hydroxyl radicals in the underlying water layer [47]. In other alternative NTP configurations, dielectric barrier discharges, corona discharges in aerosols or in the water matrix itself, are applied without substantial differences in the products of the reactions sequence previously indicated (Equations (1)–(6)). NTP technology is confined to thin-layer low-flow applications, due to the limited water penetration of excited electrons. An example of a pilot-scale NTP unit is schematized in Figure 2. This ‘‘electrode-to-plate’’ scheme consists of two units: In the first, water flowing in a thin film (~5 mm) over a ground electrode (anode) is exposed to high-voltage pulses from a carbon fiber electrode (cathode). Electric pulses with a frequency in the range 500–1000 Hz, at maximum voltage of 8.0 kV, 100 A current, are released by a generator. Upon leaving this reactor, water enters an ozone contactor, where it is mixed with ozone-rich air (generated in the previous reactor’s headspace). Other configurations, such as wetted-wall or falling film reactors, have been used experimentally [48,49].
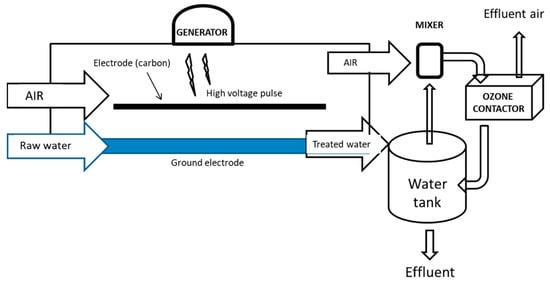
Figure 2.
NTP ‘‘electrode-to-plate’’ reactor scheme described in [45].
The most common AORP technology, however, operates through the use of ionizing radiations (Figure 3). Ionizing radiation is defined as any electromagnetic wave (at a frequency higher than 1017 Hz) carrying sufficient energy to ionize or remove electrons from an atom. Unlike non-ionizing radiation (e.g., radiofrequency, infrared, and UV), with the kinetic energy of particles (photons, electrons, etc.) too small to generate charged ions when passing through matter, the energy of ionizing radiation particles can form ions by losing electrons (i.e., ionize) from target atoms. Two types of electromagnetic waves can do that: X-rays, and γ-rays, emitted by radionuclides, such as 60Co and 137Cs. The same effect may be achieved by particulate radiation, consisting of subatomic particles (electrons, protons, etc.) carrying energy in kinetic form, such as those emitted by electron accelerators (electron beam, EB). These types of ionizing sources do not have the capability of making their targets radioactive, although they can break chemical bonds and damage DNA. Neutron radiation, such as that emitted from nuclear fission, on the other hand, is not able to directly ionize atoms (due to its lack of charge) but can make them unstable, turning materials radioactive, and must not be confused with the former.
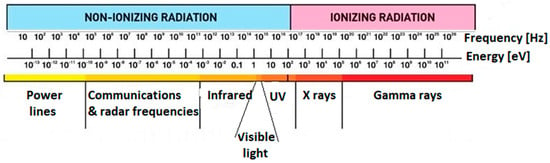
Figure 3.
Non-ionizing and ionizing radiation ranges: frequency and energy.
Ionizing irradiation therefore results in the cleavage of one or more molecular bonds, dissociating the exposed molecules (radiolysis) without radioactivity induction. The process differs from “conventional” AOPs, for instance, photolysis, where lower energy UV sources are used in conjunction with chemicals or catalyzers. Irradiation of a dilute (total solute concentration lower than 10%) solution with ionizing radiation instantaneously (t ≤ 10−12 s) splits water molecules into strongly oxidative hydroxyl radicals (∙OH), and strongly reductive species (e−aq, ∙H), in addition to other species, such as H+, H2, H2O2, H3O+, and ∙O, without chemical or catalyst addition [50].
The yields of reactive species formed in this process are expressed by the G-value, a measure of the number of molecules formed/consumed per unit of absorbed energy. For example, in the simplest water radiolysis reaction:
the G-Values of the generated species are 0.28, 0.28, and 0.06, respectively, much higher than in other conventional AOPs [51]. However, the possible reaction mechanisms of hydroxyl radicals are the same as those in AOPs, i.e.,:
H2O irradiation> ∙OH + e−aq +∙H,
Additive OH + C6H6 → C6H6-OH,
Hydrogen abstraction OH + CHCl3 → CCl3 + H2,
Electron transfer ∙OH + [Fe(CN)6]4− → [Fe(CN)6]3− + OH−,
Radical combination OH +∙OH → H2O.
Gamma rays are still used in many experimental radiolysis studies, but, due to their high organic matter penetration, they require strong precautions and shielding protection to avoid harm to living cells. Furthermore, γ-ray sources are highly radioactive isotopes requiring periodical replacement and special (and costly) high-risk handling procedures. The efficiency of a γ-irradiation process depends on the “age” of the radiation sources, which determines the deliverable dose. EB technology, instead, does not rely on constantly decaying isotopes but on electromagnetic particle generation, and can therefore be turned off when the process is not needed, much like a television set. Old cathodic tube television sets were actually quite similar, in principle, but at lower energy, to modern electron accelerators (Figure 4). Safety and practical considerations have therefore contributed to widespread industrial adoption of EB, commercially used in many manufacturing processes requiring an improvement of the material properties by molecular modification (e.g., submarine data cables, membranes grafting, medical hydrogels), sterilization or disinfection (including food processing), and environmental areas (industrial flue gas treatment, sludge processing, textile effluents processing) [52]. However, despite the advantages of this practical process, few full-scale water and wastewater treatment applications have been reported to date [53].
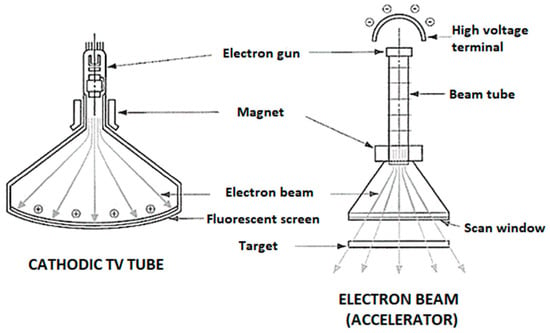
Figure 4.
Cathodic tube television set (left) vs. EB (right).
Studies on radiolytic processes for water treatment date back to the 1950s, when this technology was initially tested on domestic and industrial wastewater [51]. Since at that time CECs had not yet been addressed nor identified, treatment of organic matter solely in terms of COD, though successful, immediately appeared anti-economical, given the availability of cheaper established biological processes, and the high cost of irradiation equipment at the time. Some applications were also attempted with the use of X-rays [54], which have not been followed up further, due to the low specific efficiency of this source. While γ-rays are still often used in laboratory studies, optimal irradiation dose-rates can be achieved more easily, in practice, with EB systems. The dose-rate parameter, one of the main indicators for process applicability, and the process time are in fact much more favorable for EB irradiation than for γ or X-rays, as reported in Table 2. Dose-rate determines the process contact time for an established irradiation dose, as indicated in the table: A dose of 1 kGy represents a relatively low value used, for example, to achieve wastewater disinfection, and is in the low range of those commonly used in the pretreatment of foods in industrial distribution applications. It should also be noted that although 1 Curie (37 GBq in SI units) corresponds to a very high value of radioactivity, it still generates a low dose-rate even compared to small EB units.

Table 2.
Achievable dose-rates and process contact times of different radiation sources.
3. Applications and Limits of ATPs in Water and Wastewater Treatment
While oxidation is considered a key removal mechanism for many compounds, especially when achieved by strong oxidants, such as chlorine, ozone, or AOPs, as discussed in the previous section, not all CECs are susceptible to destruction by it. Table 3 summarizes selected organic compounds’ removal rates in solution by different radicals, and the relative importance of each reactive species in the process. It can be seen that several compounds (e.g., halogenated substances) respond poorly to oxidation but are well degraded by reduction. Others, such as, for example, trihalomethanes, are not degraded at all by oxidation, and seem to be poorly degraded also by reduction, although recent reports claimed an 82% total THMs removal by means of an AORP VUV/UV-C process [55] and complete degradation by a UV-C/free chlorine process [56].

Table 3.
Organic compounds’ removal rates by different radicals, and the relative importance of each species in the process (modified from [50]).
The effectiveness of an ATP depends on the reactivity of the radical species generated. This is indicated by the redox potential, a measure of a species’ tendency to reduce or oxidize, measured in volts (V). The standard oxidation-reduction potential, E0, is defined relative to a standard reference hydrogen electrode (SHE), which is arbitrarily given a potential of 0 V. A high positive value of E0, therefore, indicates a high capacity to oxidate; a high negative value, on the contrary, a high capacity to reduce. Table 4 compares the E0 values of different radical and commonly used chemical reagents.

Table 4.
Oxidation and reduction potential of different radical and commonly used reagents.
In addition to the E0 potential, reaction rates are paramount for the efficiency of process development: Literature-reported reaction rate constants determined for different organic compounds reacting with ozone and hydroxyl radicals show that the latter originates rates eight to nine orders of magnitude (108−109) faster than the former. Hence, a process generating large amounts of OH radicals will be much faster than one based on ozone, or on lower concentrations of hydroxyl radicals, although the mere oxidative potential of the two basic species is not that different, as seen in Table 4.
In radiolytic processes, the kinetics is determined by the reaction order and the values of rate constants for specific reactions. The irradiation process yield is commonly expressed by G values, i.e., the number of molecules of reactant consumed per 100 eV of energy absorbed, the value of the dose constant, d, and the dose magnitude required for 50% (D0.5) or 90% (D0.9) decomposition of chemical solutes [57]. G-values, commonly reported in the applied radiolysis literature, however, are not useful in practice to predict the required decomposition dose for a compound, since this depends on its actual concentration in solution, while G values are often calculated according to a linear kinetics approximation, not fully representative of the actual reaction. The dose constant, d, represents decomposition rates as a function of the absorbed radiation dose, calculated as the slope of the line obtained from plotting ln(CD/C0) against the absorbed dose. This is considered a more reliable parameter than the former, because it represents the entire irradiation process. Large values of G0 and d indicate high pollutant decomposition yields; their increase corresponds to a lower required dose for pollutant decomposition. Experimental dose-constants serve as a first approximation of the irradiation required to remove a major component compound from a multi-component solution; however, for these and other considerations exposed below, the actual required dose depends on the overall matrix composition, and on compounds’ decomposition pathway(s). Dose estimates can also be used for a preliminary evaluation of treatment costs (equipment size and energy requirements).
An important factor affecting the efficiency of all ATPs is the composition of the original water matrix, which may contain natural compounds acting as radical scavengers. Reaction rates involving scavengers may be of the same magnitude as those involving target pollutants; as a result, the pollutant decomposition yield in real conditions may significantly differ from that assessed in a pure solution. Some examples of scavenger species, typically occurring in natural waters and wastewater, are summarized in Table 5, together with the rate constants of their reaction with the main radical species. In addition to those reported in the table, the presence of other scavengers in wastewater may include both organic (e.g., humic and fulvic acids, natural organic matter—NOM, aminoacids, proteins, carbohydrates) and inorganic species (e.g., sulfide, bromide).

Table 5.
Scavenger molecules’ removal rates by different radicals (data from [50]).
As a consequence of the introduction of ATPs in water and wastewater treatment, the generation of by-products from CECs transformation, water or wastewater original matrix oxidation, or reactions with disinfecting chemicals may be observed. Some of these by-products may be proven or potential carcinogenic, or carcinogenic precursor compounds, or induce otherwise undesired contamination if released into the environment. This may occur when insufficient applied oxidation or reductants doses do not result in complete mineralization of the target pollutants. For example, UV irradiation alone is considered the least effective AOP for organic contaminants’ removal. An indication that this process cannot be considered as a feasible standalone process was shown in a study proving that, while it could remove 50–80% of targeted antibiotics, it required doses 100 times greater than those required for disinfection alone to achieve this target. This would be highly expensive, and such high doses unpractical to deliver [58]. Preference should therefore be given to processes with a high radical yield and reaction rates, with reduced process contact time, and in which doses could be rapidly adjusted, when needed. In addition to the greater radical generation capacity, the achievable EB dose-rates are about 100 times higher than a gamma’s source, requiring very short irradiation exposure.
The literature on radical reactions in water solutions is established [34], with works on the kinetics of decomposition of various types of environmental pollutants. However, studies have mostly been carried out on single-component synthetic solutions. Prediction (modeling) of specific pollutant’s degradation in pure monocomponent solutions can be achieved in most cases by using reaction rate constants as a function of the solute concentration, pH, and operational conditions, but the influence of the actual solution matrix on the process can be determined, to date, only experimentally. Hence, ATP application cannot be based solely on reaction yield calculations but must be empirically tested in real conditions. While most AOPs and ARPs require the addition of chemical substances and/or catalyzers in order to increase the intensity of the reactions, in radiolytic processes (especially with EB technology), this is not strictly necessary. However, in the presence of external additives, which interact with radiolytic reaction products, increased radical generation may occur. For example, N2O added to an irradiated solution exhibits fast reactivity with both e−aq and ∙H, converting the former to ∙OH.
Studies showed that oxidants, such as hypochlorite (NaOCl) [59] or ozone [60], added to irradiated solutions could significantly enhance process yields. When irradiation is carried out in air-saturated solutions, ∙O2− and ∙HO2 radicals are present, as a result of the oxygen reaction with e−aq and ∙H. H2O2’s presence, which reacts with those radicals, could be an additional source of hydroxyl radicals, although excessive peroxide concentrations may act as scavengers. Hydrated electrons predominate when irradiated neutral solutions are saturated with N2 or Ar gas, while at pH < 2, ∙H radicals predominate. Due to technological reasons, the most practical process modification to increase the concentration of hydroxyl radicals in irradiated solutions is the moderate addition of O3 or H2O2.
3.1. Applications of ATPs in Water and Wastewater Treatment
During the last decade, a dramatic rise in ATPs research, development, and applications was observed, due to increasing concerns about CECs’ presence in water and wastewater, and to the increasing diffusion of water reuse projects for high-quality use [8]. While ozonation, activated carbon adsorption, and membrane filtration processes are, at the moment, the most common consolidated processes for advanced wastewater treatment [61], none of them are technically defined as a proper ATP. In fact, ozone alone in pure water does not generate radicals, unless combined with UV or peroxide, although studies on the side reactions of ozone with phenols or amine groups show its ∙OH generation potential in the presence of dissolved organic matter (DOC). Ozone doses ≥ 0.25 g O3/g DOC were shown to be sufficient to remove at least 80% of some CECs with high ozone reactivity in different wastewater treatment plant effluents [62]. For this reason, wastewater effluent ozonation could be classified as an AOP under specific circumstances [63]. Ozone-resistant compounds include pesticides (e.g., prometon, aldicarb), aromatic compounds, and chlorinated solvents [64].
Since UV irradiation alone is, as mentioned, the least effective AOP process in CECs removal, its combination with photocatalysis was tested, using TiO2 as the catalyst at a concentration between 0.2 and 2 g TiO2/L, to enhance free hydroxyl radical formation. This approach removed endocrine-disrupting compounds between 12 and 99%, requiring contact times between 0.5 and 8 h [65]. Photocatalysis also showed bisphenol A (BPA) removal up to 99% with a process time up to 140 min, with a mineralization ratio of just 40% (the other 60% consisting of intermediate by-products of an undetermined nature). Reducing the contact time to 20 min, conversion was reduced to 35%, with a 10% mineralization fraction only [66]. UV can also be combined with other oxidants, such as O3 and H2O2, to increase the process effectiveness. Wang and Xu carried out a wide review of AOP applications in the treatment of water from various sources, as summarized in Table 6 [18]. An updated review was recently published by Cuerda-Correa et al. [67].

Table 6.
Application of AOPs for the removal of different types of compounds (data from [18]).
As it can be seen from Table 6, most conventional AOPs may require extended process times for contaminants’ degradation, ranging from a fraction to several hours to achieve at least a 1-log reduction of the original contaminants’ mass. It may also be noticed that, when reporting the results of their studies, researchers refer to the fate of the primary target contaminant with the words “removed”, “degraded”, or “decomposed” without specifying (save for rare occasions) if the actual contaminant’s “end-of-life” (i.e., mineralization) was reached, or the nature of the degradation products. It is conventionally agreed that CEC compounds’ complete destruction (i.e., mineralization) should be required to eliminate any possible residual risk; however, this could require treatment processes more advanced than those currently available, even in facilities of last-generation design.
The application of ARPs was initially tested for the removal of bromate (a DBP considered a possible human carcinogen [68,69]), and in recent laboratory studies, it showed its potential towards the degradation of chlorinated organic compounds like vinyl chloride, mono-chloroacetic acid, and 1,2-DCA [39]. Due to the fact that ARPs have received attention as a water treatment process only recently, little is known about the effect of the matrix composition on the specific degradation processes taking place. Table 7 summarizes the reported experimental ARPs applications in water treatment.

Table 7.
Application of ARPs for removal of different types of compounds. Adapted from [33,35,38,69,70,71,72,73].
Although, as mentioned, few full-scale applications of AORPs were reported to date, many lab-scale or pilot studies on the application of this class of processes are found in the literature, as summarized in Table 8.

Table 8.
Application of AORPs for the removal of different types of compounds. Data from [6,74,75,76,77].
In addition to the performance reported in the removal of emerging contaminants, irradiation-based AORPs have proven highly effective in the removal of natural dyes from food industry wastewater. Observed efficiencies were up to 48% for pure tincture and up to 100% for dilute solutions at a 32 kGy irradiation dose [78]. In municipal wastewater effluent disinfection, 95% removal of coliforms at a 0.2 kGy dose and 99% removal at a 0.5 kGy dose were measured [79].
As previously mentioned, the efficiency of a treatment should not only be evaluated on its degradation efficiency of specific compounds but also on the generation of intermediates of a lesser or non-toxic nature. A study on E-beam irradiation on real pharmaceutical industrial wastewater showed that when EB was applied as pre-treatment technology before biological treatment, almost complete detoxification of wastewater was achieved, compared to non-irradiated wastewater. The cytotoxicity of un-irradiated and irradiated wastewater was tested against E. coli, P. aeruginosa, and B. subtilis, indicating the high potential of irradiation processes to eliminate cytotoxicity [80].
Among AORPs, radiation processes have been recognized as a more environmentally friendly technology compared to chemical processes, with the greatest progress in the latter years in the EB accelerator technology sector in terms of a larger capacity, wider range of applications, reliability, and cost reduction, substantially gaining researchers’ appreciation in the mitigation of many pollution problems. Furthermore, among the studied AORPs, EB irradiation may be the most suitable in industrial-scale operations due to the ease of implementation and achievable reaction yield.
3.2. ATP Efficiency Enhancement and Process Combination
While conventional AOPs and ARPs rely heavily on chemicals or catalysts for the generation of reaction-driving radicals, radiolysis-based AORPs do not necessarily require such additions, due to the high intensity of the energy transfer occurring in these processes. However, even their efficiency may be further increased by appropriate process management, with possible synergetic effects of irradiation and the addition of nanoparticles (NPs) of various nature on solutes’ decomposition, enhancing radical production and the reaction yield [81].
Most ATPs’ performance may be enhanced by the application of advanced materials of different nature and/or NPs, including semiconductors, nanoclays, nanocatalysts, nanoclusters, nanorods, and nanocomposites. Nanotechnology consists of the manipulation of materials, including TiO2, palladium, Fe3O4, cerium oxide, graphene oxides, magnetic chitosan, and others, at a scale of <100 nm, taking advantage of unique phenomena that may occur realized at that size scale due to their high surface area to volume ratios, greatly improving adsorption properties. Research recently addressed wastewater treatment also with the use of complex nanocomposites, such as CoxFe3−xO4, CoFe2O4 magnetic nanoparticles, and bismuth silver oxide [82]. Polymeric dendrimers are randomly hyper-branched polymers consisting of spherical macromolecules with a dense shell morphology between 2 and 20 nm size. They are very efficient adsorbents for the removal of toxic metal ions, radionuclide, and organic solutes from water [83]. Metal/metal oxide nanoparticles, including silver, gold, and palladium, have also been widely investigated for wastewater treatment: Noble metal-structured photocatalysts can significantly improve the photoactivity of the semiconductor NPs, depending on the substrate to be degraded [84]. Nanosized silver showed strong antimicrobial properties; gold-impregnated palladium NPs have been used to destroy TCE from groundwater, with a 2200 times better performance than palladium catalyst alone; other metal oxide nanoparticles, such as TiO2, ZnO, and CeO2, have demonstrated a capacity for the degradation of organic pollutants in solutions [82]. Carbon-based nanomaterials, such as fullerenes, carbon nanotubes (CNTs), nanosize diamonds and nanosize wires, graphene oxide [85], and chitosan [86], have been used as sorbents for organic compounds in aqueous solutions. In particular, graphene, a two-dimensional recently identified form of carbon, is the thinnest and the strongest material ever measured, and has received much attention in various scientific applications due to its chemical stability, higher mobility as a charge carrier, and large surface area. One major drawback of pure graphene in water treatment applications is its hydrophobic nature; however, its oxide and reduced oxide (also known as functionalized graphene) are derived hydrophilic materials, suitable for such applications that were tested extensively in combination with AOPs. Studies have shown that graphene-based materials can improve the generation of hydroxyl and sulfate radicals in solutions treated with Fenton processes (enhancing the ferrous ion regeneration rate in conventional Fenton, hydrogen peroxide generation capacity in electro-Fenton processes, and hydroxyl radical generation in photo-Fenton processes), significantly improve the performance of catalytic ozonation, and enhance the activation of sulfate radicals from persulfate and peroxymonosulfate in ARPs [85].
Gamma radiation was observed to induce catalytic degradation of p-nitrophenol (PNP) in the presence of titanium dioxide (TiO2) nanoparticles in aqueous solution. In the presence of low TiO2 doses (0–2.0 g/L), the catalytic effect of NPs on PNP decomposition was not obvious, since this compound could be removed well by irradiation alone; however, the removal of total organic carbon (TOC) and total nitrogen (TN) was significantly accelerated in the presence of TiO2. TOC removal increased from 16% to 42%, and PNP mineralization was dramatically enhanced [87]. An innovative Fe/C nanomaterial fabricated with Fe-based metal organic frameworks (MOFs) was shown to catalyze γ-induced degradation of the antibiotics, cephalosporin C (CEP-C) and sulfamethazine (SMT), in aqueous solutions [88]. An increase of the H2O2 yield (at pH < 4 or > than 11) was observed in radiolysis of aerated solutions with added Al2O3 NPs. It was also observed that the addition of Al2O3 could inhibit scavenging of ∙OH radical precursors by solute NO3− [89]. Growing research interest in the field of radiation-induced heterogeneous chemical transformations in solutions with nanocatalyst addition could provide significant progress to irradiation-based processes in the future.
The potential of ATPs and in particular EB technology to remove toxic organic substances was widely documented, as shown in Table 6, Table 7 and Table 8. The possibility of combining these and other conventional processes to maximize efficiency and cost-effectiveness was also demonstrated. Kim et al. showed that the EB irradiation and activated sludge process combination was a highly effective method for enhancing the biodegradability of textile wastewater [90]. The effect of ionizing radiation on the biodegradability and toxicity of individual drugs was also studied: Changes in the biodegradability and toxicity of aqueous solutions containing sulfamethoxazole (SMX) by ionizing radiation showed that SMX biodegradability was improved by applying small (0.4 Kgy) irradiation doses. At the 2.5 Kgy dose, full SMX conversion to biologically treatable substances was noted [91]. The combination of radiolytic treatment with conventional processes was studied in a significant number of applications to wastewaters of different origins. The application of γ-irradiation to landfill leachates showed considerable biodegradability improvement [92]; cellulosic wastewater showed accelerated enzymatic hydrolysis [93]; pulp mill effluents registered increased removal of adsorbable organic halogen (AOX) in biological treatment (from 50% to 96%) following γ-irradiation [94], and significant improvement of COD removal after EB pre-treatment [95]. The combined EB irradiation and activated sludge treatment of textile dyeing effluents achieved equal treatment levels at more than halved biological process HRTs [53]. The biodegradability of textile dyeing wastewater was reported to increase from the 0.34–0.61 range, to between 0.87 and 0.96 after irradiation [96]. Studies highlighted that other intermediate processes (i.e., coagulation prior to irradiation) could be appropriate for some (e.g., textile) wastewaters [97].
The combination of EB irradiation and ozone (3 ppm) resulted in an eightfold reduction of the required dose for 95% tricholoroethylene removal from contaminated groundwater, increasing the equipment irradiation capacity from 146 to 1200 m3/h while reducing treatment costs threefold from 0.25 to 0.075 US$/m3 [98].
A hybrid process combining microfiltration membrane separation with EB irradiation was tested for the production of safe water from secondary effluents. The EB’s high effectiveness in modifying the effluent organic matter structure significantly reduced membrane fouling and improved permeate quality at an irradiation dose of 1 kGy, without significantly changing the overall process energetic consumption [99]. Hybrid radiolytic approaches (coagulation, biological treatment, and γ-irradiation) were tested for the decomposition of real pharmaceutical wastewater. The sequence of coagulation, irradiation, and biological treatment accounted for synergistic degradation and detoxification of recalcitrant pharmaceutical wastewater, with an overall improved COD removal at over 90% [100]. The study confirmed that irradiation pre-treatment is a realistic approach for the treatment of recalcitrant wastewaters. In particular, due to the very short process contact time, EB-based AORPs do not require large additional units, and can therefore be easily inserted in existing facilities’ treatment trains with minimal disruption, to improve treatment efficiency.
3.3. Energy Demand and Cost-Effectiveness of ATPs
The sustainability of a treatment technology is determined by its competitive performance, economic feasibility, and reliable operational practice. While these factors have been deemed acceptable so far for many conventional AOPs currently in use, the high capital investment and energy demand by innovative AORPs, in particular EB, still raise doubts about their sustainability among water practitioners, although several studies have demonstrated the economic feasibility of these processes.
Direct cost comparison of different technologies is not immediate, as it may depend on local site conditions and market factors. The cost of ozone and UV technology, for example, can roughly be estimated as indicated in Table 9, based on a commercial survey of primary suppliers for the EU market today. These figures include only the generation equipment for typical disinfection uses (dedicated AOP applications will require different doses) and not the structural costs necessary for installation, such as buildings and process tanks. For UV systems, significant differences may arise depending on the specific system configuration (e.g., horizontal vs. inclined lamp installation). Process contact volumes may increase significantly the capital cost (CAPEX), depending on the required contact time, which may range from 10–15 min for O3 disinfection, to several hours for AOP removal of specific compounds.

Table 9.
Investment and operational cost of ozone and UV facilities for water treatment.
Given the scarce diffusion of EB systems in water applications, it is difficult to provide an upfront reliable cost figure for this technology. The cost of an accelerator (CEB) is generally proportional to the installed electron energy (E in MeV), power (P, in kW) through a factor (f) that depends on site-specific conditions, accelerator type, and manufacturer according to the following relationship:
as suggested by Zimek and Kaluska [101]. Available cost figures from 2012 [53] suggested that the specific price tag (US$/W) of electron accelerators (at 1 MeV electron energy) decreases with the installed power according to the approximate relationship:
that would make the cost of this technology more affordable with increasing treatment capacity (both in treated flow and irradiation dose).
cEB = 220 P0.665,
This is confirmed by preliminary comparative calculations estimating the total cost of 3-log disinfection of municipal effluents for irrigation water reuse, summarized in Figure 5 [102]. It can be seen that at increasing capacity, the irradiation technology-specific cost becomes similar or lower than that of conventional AOP processes, save for chlorination. Since all these processes (except chlorination) are based on energy conversion (electric to radiation, or to oxidants), it is safe to assume that similar economies of scale would be seen also in uses different from simple disinfection. From the OPEX point of view, it was noted that, generally, EB is energetically more efficient than other AOPs, such as ozonation and UV irradiation [50].
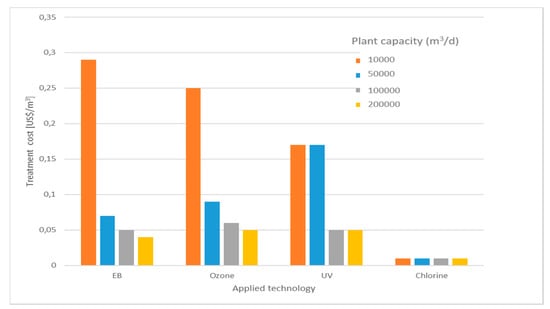
Figure 5.
Specific total disinfection costs (US$/m3 treated) of urban effluents for irrigation reuse according to different technologies (data from [102]).
O3 production from liquid oxygen generally requires 8–9 kWh/kg (may decrease to 7 kWh/kg in large facilities with capacity > 25 kg/h). Production from ambient air requires the circulation of higher air flows (about fivefold, due to O2 air content); therefore, the overall specific consumption is higher in this case. UV irradiation normally absorbs about one third of the total installed nominal power. It has been shown that process efficiency does not depend solely on the conversion from electric current to UV-C radiation but also on the system configuration: Even if last-generation horizontal lamps are individually more efficient (42% electric to UV-C conversion) than inclined ones (35% conversion), due to the more advantageous installation geometry, the latter result in a lower overall specific energy demand. In this respect, an important feature of EB equipment is the high conversion efficiency of electric power to irradiation, in excess of 95% for modern accelerators. This results in high radicals’ generation efficiency of over 1.0 M∙OH/kW.
The energy demand and efficiency of water treatment facilities is a currently an issue of great relevance, associated with process sustainability and their environmental impact [103].
A method to estimate the energy efficiency of different ATPs was suggested as the determination of the electrical energy per order (EE/O) of a process for a specific contaminant [104]. Defined as the amount of kWhs required to reduce a pollutant concentration by an order of magnitude in 1 m3 of solution, it is easily applicable to any energy-driven process, and could also include the embedded energy contained in chemical additives or catalysts (i.e., the energy required for their production and supply to the process).
Although EE/O is not directly related to the total process costs (it only reflects operational costs, it could provide an indication about the feasibility of alternative process approaches, with rapid determination of their operating costs, known as the expected reduction in contaminant concentration (in orders of magnitude) and the local cost of electricity. Of course, there are other factors (chemical needs, operation/maintenance costs, capital amortization, etc.) that go into a complete cost analysis. EE/O values may vary widely (even more than one order of magnitude) when considering common ATPs, and largely depend on a process efficiency in radicals’ generation but are also affected by matrix interference (scavengers), specific process equipment (e.g., lamp efficiency for UV systems), and operating conditions. As an example, Table 10 reports the calculated EE/O values for the decomposition of antibiotics (sulfamethazole and chlortetracycline) and other contaminants by different ATPs [104,105]. Lower values indicate higher treatment efficiency.

Table 10.
EE/O values for the decomposition of solute contaminants by different ATPs.
A study examining different wastewaters, two textile and one municipal effluent, evaluated different treatment options (primary and activated sludge, low-dose EB, and chlorination) to establish the reliability and optimum conditions for different treatment processes. EB was used for disinfection (municipal effluent) and treatment (textile effluent). The evidence of the study indicated that as far as cost estimates, EB at doses between 1 and 3 kGy compared to conventional methods was less expensive than activated sludge for treatment (but more expensive than chlorination for disinfection, as also shown in Figure 4) [97].
As noted earlier, the appropriate combination of different conventional (e.g., biological) and advanced processes (e.g., EB and O3) may result in more efficient and economic treatment of specific pollutants. Klein et al. conducted a pilot study on the treatment of landfill leachate containing significant concentrations of recalcitrant organic substances with a scheme consisting of an activated sludge pre-treatment combined with the Fenton process and biological post-oxidation. The results indicated that the combined treatment according to the suggested scheme proved more efficient, both in efficacy and cost, than individual biological or chemical processes [106]. Pikaev et al. compared pilot plant results obtained by combined EB and ozone treatment and conventional (biological plus disinfection) treatment of municipal wastewater from the town of Raduzhnyi (Russia). The combined EB+O3 treatment cost was estimated at 17–30% of the cost of conventional treatment, depending on the influent COD concentration, to achieve comparable effluent quality [107]. Other authors compared the cost of using EB and γ radiation under different conditions. In general, the cost of running an EB unit was estimated at approximately half of that of a γ unit, and up to 60% lower than that of conventional technology [108]. A comparative treatment study of low (COD = 11,940 mg/L) and high (COD = 52,856 mg/L) real pharmaceutical wastewater indicated a treatment cost of EB radiolysis at 0.50 US$/m3, activated sludge treatment at 2.35 US$/m, and EB/biological combination at 2.85 US$/m3 for low-strength wastewater, whereas for the high-strength effluent, costs were 0.67, 0.7, and 1.37 US$/m3, respectively. Activated sludge HRT was 2 days, and the EB treatment contact time was 3 and 4 min, respectively, for low- and high-strength wastewater but decreased to 2 and 1 min, respectively, with the addition of H2O2 to the process. Peroxide addition, however, increased the combined treatment cost to 7.87 and 11.48 US$/m3, respectively, in the low- and high-strength cases [80].
Waite et al. compared the cost of effluent disinfection in a 1.5 MeV EB facility to that of a UV peroxidation process. The reported total operating cost of the latter was US$ 0.68/m3, and the calculated costs for EB treatment (including capital amortization and running costs) ranged between US$ 0.66 and 0.066 depending on the applied treated flow (36–480 m3/h) [109]. It should be noted that the previous estimate was based on an EB installation cost of US$ 2.35 million (in 1998). Although a direct estimate of the current cost of an equivalent facility is quite difficult, the industrial diffusion of this technology and new accelerator construction technology can now offer better economic and technical characteristics than those available in the late 1990s.
An analysis of EB irradiation of municipal sewage sludge showed that operational irradiation costs were poorly sensitive to delivered doses: At 6.7 kGy irradiation, the energy cost for treatment was estimated at 1.10 US$/kg, increasing to 1.26 US$/kg (+14.5%) when raising the irradiation dose to 25.7 kGy (+283.6%) [110]. This indicates that once the accelerator is in place, increasing the irradiation doses (in response to new contaminants’ presence or increased removal requirements) has minimal impact on the cost of treatment, resulting in an unparalleled adaptation flexibility and operability of this specific process. Other AOPs’ (e.g., ozone and UV) cost are, on the contrary, directly proportional to the oxidant or irradiation doses, as shown in Table 9.
4. Discussion
Recent development of highly performant advanced treatment processes, in addition to traditional AOPs, has opened new horizons in the field of water purification and production. Although not all the proposed processes will turn out to be industrially applicable in the end, some are well beyond the stage of laboratory testing and have already seen real-world application. Table 11 summarizes the pros and cons of some of the processes examined in this paper.

Table 11.
Pros and cons of ATP technologies.
Despite many real -scale applications of some of these processes with real wastewaters, there is a need for a deeper understanding of their reaction kinetics in complex systems, to enable better design of combination treatment schemes. For all processes examined, an understanding of chemical reaction mechanism (in the form of reliable chemical models) is needed to address issues about contaminant decomposition and byproduct formation in complex solutions. It was shown, for example, that ionizing radiation together with oxidants, such as ozone or hydrogen peroxide, may further improve an individual process removal efficiency but may also induce secondary scavenging effects in solution.
There appears to be a general lack of comparative investigations between consolidated AOP processes and innovative ARPs/AORPs, and of their combinations with other conventional processes. This prevents reaching conclusive evaluations about the most suitable and cost-effective solution for advanced treatment of water/wastewater for specific uses. It should also be considered that site-specific conditions may lead to different conclusions for different sites.
5. Conclusions
Despite the absence, so far, of explicit regulations concerning the elimination of CECs from water and wastewater, the recognized need for highly efficient non-selective purification processes and ongoing related research has generated increased knowledge about the degradation of numerous classes of industrial and emerging environmental pollutants.
In particular, a significant understanding and development of advanced treatment processes based on free radicals’ efficient reactions was achieved recently. One of the most efficient ways for the production of such radicals consists of ionizing radiation-based processes, showing the highest free radical yield per unit energy input, and a wider range of reacting compounds to tackle a larger spectrum of contaminants. Controversial perception about radiation technology, however, has so far slowed down its application in the water sector, notwithstanding the many notable application examples in other industrial sectors, including the food and medical industry. Compared to other technologies, ionizing radiations could provide economical, reliable, and safer operations that would not be affected by seasonal variations in effluent composition, and could reduce the generation of secondary toxic intermediates.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- US EPA. Contaminants of Emerging Concern Including Pharmaceuticals and Personal Care Products. 2016. Available online: https://www.epa.gov/wqc/contaminants-emerging-concern-including-pharmaceuticals-and-.personal-care-products (accessed on 15 May 2017).
- Daughton, C.G.; Jones-Lepp, T. Pharmaceuticals and Personal Care Products in the Environment—Scientific and Regulatory Issues; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2001; Volume 791. [Google Scholar]
- Copetti, D.; Marziali, L.; Viviano, G.; Valsecchi, L.; Guzzella, L.; Capodaglio, A.G.; Tartari, G.; Polesello, S.; Valsecchi, S.; Mezzanotte, V.; et al. Intensive monitoring of conventional and surrogate quality parameters in a highly urbanized river affected by multiple combined sewer overflows. Water Sci. Technol. Water Supply 2018, 19, 953–966. [Google Scholar] [CrossRef]
- WRRC. Contaminants of Emerging Concern in Water. Water Resources Research Center, College of 1-Agriculture and Life Sciences, The University of Arizona. 2013. Available online: wrrc.arizona.edu/sites/wrrc.arizona.edu/files/Arroyo2013LR_0 (accessed on 15 January 2020).
- Lee Ventola, C. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Capodaglio, A.G.; Bojanowska-Czajka, A.; Trojanowicz, M. Comparison of different advanced degradation processes for the removal of the pharmaceutical compounds diclofenac and carbamazepine from liquid solutions. Environ. Sci. Pollut. Res. 2018, 25, 27704–27723. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G. In-stream detection of waterborne priority pollutants, and applications in drinking water contaminant warning systems. Water Sci. Technol. Water Supply 2017, 17, 707–725. [Google Scholar] [CrossRef]
- UN-WATER. Wastewater: The Untapped Resource; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2017. [Google Scholar]
- Capodaglio, A.G. Fit-for-Purpose Urban Wastewater Reuse: Analysis of Issues and Available Technologies for Sustainable Multiple Barrier Approaches. Crit. Rev. Environ. Sci. Technol. 2020. Published online: 15 May 2020. [Google Scholar] [CrossRef]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef]
- Wu, Q.; Shi, H.; Adams, C.D.; Timmons, T.; Ma, Y. Oxidative removal of selected endocrine-disruptors and pharmaceuticals in drinking water treatment systems, and identification of degradation products of triclosan. Sci. Total Environ. 2012, 439, 18–25. [Google Scholar] [CrossRef]
- Esplugas, S.; Bila, D.M.; Krause, L.G.T.; Dezotti, M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Biological combination processes for efficient removal of pharmaceutically active compounds from wastewater: A review and future perspectives. J. Environ. Chem. Eng. 2017, 5, 3590–3603. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Ikehata, K.; Gamal, E.D.M.; Snyder, S.A. Ozonation and advanced oxidation treatment of emerging organic pollutants in water and wastewater. Ozone Sci. Eng. 2008, 30, 21–26. [Google Scholar] [CrossRef]
- Parsons, S. Advanced Oxidation Processes for Water and Wastewater; IWA Publishing: London, UK, 2004; p. 368. [Google Scholar]
- Klein, K.; Kattel, E.; Goi, A.; Kivi, A.; Dulova, N.; Saluste, A.; Zekker, I.; Trapido, M.; Tenno, T. Combined treatment of pyrogenic wastewater from oil shale retorting. Oil Shale 2017, 34, 82–96. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of Hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2016. [Google Scholar]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, A.; Sillanpää, M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 2010, 80, 351–0365. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, H.R. Advanced oxidation processes for the treatment of biorecalcitrant organics in wastewater. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1167–1219. [Google Scholar] [CrossRef]
- Tsydenova, O.; Batoev, V.; Batoeva, A. Solar-enhanced advanced oxidation processes for water treatment: Simultaneous removal of pathogens and chemical pollutants. Int. J. Environ. Res. Public Health 2015, 12, 9542–9561. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the Advanced Oxidation Processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Buthiyappan, A.; Aziz, A.R.A.; Daud, W.M.A.W. Recent advances and prospects of catalytic Advanced Oxidation Process in treating textile effluents. Rev. Chem. Eng. 2016, 32, 1–47. [Google Scholar] [CrossRef]
- Biń, A.K.; Sobera-Madej, S. Comparison of the Advanced Oxidation Processes (UV, UV/H2O2 and O3) for the removal of antibiotic substances during wastewater treatment, ozone: Science & Engineering. J. Int. Ozone Assoc. 2012, 34, 136–139. [Google Scholar]
- Laghrib, F.; Bakasse, M.; Lahrich, S.; El-Mhammedi, M.A. Advanced oxidation processes: Photo-electro-Fenton remediation process for wastewater contaminated by organic azo dyes. Int. J. Environ. Anal. Chem. 2020, in press. [Google Scholar] [CrossRef]
- Gil, A.; Galeano, L.A.; Vicente, M.A. Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment; The Handbook of Environmental Chemistry (HEC) book series; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Arzate, S.; Pfister, S.; Oberschelp, C.; Sánchez-Pérez, J.A. Environmental impacts of an advanced oxidation process as tertiary treatment in a wastewater treatment plant. Sci. Total Environ. 2019, 694, 133572. [Google Scholar] [CrossRef] [PubMed]
- Serna-Galvis, E.A.; Botero-Coy, A.M.; Martínez-Pachón, D.; Moncayo-Lasso, A.; Ibáñez, M.; Hernández, F.; Torres-Palma, R.A. Degradation of seventeen contaminants of emerging concern in municipal wastewater effluents by sonochemical advanced oxidation processes. Water Res. 2019, 154, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Syam Babu, D.; Srivastava, V.; Nidheesh, P.V.; Suresh Kumar, M. Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Pešoutová, R.; Stříteský, L.; Hlavínek, P. A pilot scale comparison of advanced oxidation processes for estrogenic hormone removal from municipal wastewater effluent. Water Sci. Technol. 2014, 70, 70–75. [Google Scholar] [CrossRef]
- Vellanki, B.P.; Batchelor, B.; Abdel-Wahab, A. Advanced reduction processes: A new class of treatment processes. Environ. Eng. Sci. 2013, 30, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O-) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513. [Google Scholar] [CrossRef]
- Vellanki, B.P.; Batchelor, B. Perchlorate reduction by the sulfite/ultraviolet light advanced reduction process. J. Hazard. Mater. 2013, 262, 348–356. [Google Scholar] [CrossRef]
- Liu, X.; Vellanki, B.P.; Batchelor, B.; Abdel-Wahab, A. Degradation of 1,2-dichloroethane with advanced reduction processes (ARPs): Effects of process variables and mechanisms. Chem. Eng. J. 2014, 237, 300–307. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Front. Environ. Sci. Eng. China 2009, 3, 129. [Google Scholar]
- Yu, X.; Cabooter, D.; Dewil, R. Effects of process variables and kinetics on the degradation of 2,4-dichlorophenol using advanced reduction processes (ARP). J. Hazard. Mater. 2018, 357, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Yu, S.; Li, L.; Wang, T.; Liao, X.; Ye, Y. An overview of advanced reduction processes for bromate removal from drinking water: Reducing agents, activation methods, applications and mechanisms. J. Hazard. Mater. 2017, 324, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Fanning, J.C. The chemical reduction of nitrate in aqueous solution. Coord. Chem. Rev. 2000, 199, 159–179. [Google Scholar] [CrossRef]
- Cecconet, D.; Bolognesi, S.; Callegari, A.; Capodaglio, A.G. Controlled sequential biocathodic denitrification for contaminated groundwater bioremediation. Sci. Total Environ. 2019, 651, 3107–3116. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Luo, G.; Jung, B.; Kaushik, V.; Batchelor, B.; Abdel-Wahab, A. Photochemical degradation of arsenic and selenium with Advanced Reduction Processes—Effects of reagents. Environ. Eng. Sci. 2017, 34, 481–488. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Could eb irradiation be the simplest solution for removing emerging contaminants from water and wastewater? Water Pract. Technol. 2018, 13, 172–183. [Google Scholar] [CrossRef]
- Moussavi, G.; Rezaei, M. Exploring the advanced oxidation/reduction processes in the VUV photoreactor for dechlorination and mineralization of trichloroacetic acid: Parametric experiments, degradation pathway and bioassessment. Chem. Eng. J. 2017, 328, 331–342. [Google Scholar] [CrossRef]
- Locke, B.R.; Sato, M.; Sunka, P.; Hoffmann, M.R.; Chang, J.S. Electrohydraulic discharge and nonthermal plasma for water treatment. Ind. Eng. Chem. Res. 2006, 45, 882–905. [Google Scholar] [CrossRef]
- Capodaglio, A.G. High-energy oxidation process: An efficient alternative for wastewater organic contaminants removal. Clean Technol. Environ. Policy 2017, 19, 1995–2006. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Contaminants of emerging concern removal by High-Energy Oxidation-Reduction Processes: State of the art. Appl. Sci. 2019, 9, 4562. [Google Scholar] [CrossRef]
- Krause, H.; Schweiger, B.; Prinz, E.; Kim, J.; Steinfeld, U. Degradation of persistent pharmaceuticals in aqueous solutions by a positive dielectric barrier discharge treatment. J. Electrost. 2011, 69, 333–338. [Google Scholar] [CrossRef]
- Magureanu, M.; Piroi, D.; Mandache, N.B.; David, V.; Medvedovici, A.; Bradu, C.; Parvulescu, V.I. Degradation of antibiotics in water by non-thermal plasma treatment. Water Res. 2011, 45, 3407–3416. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Capodaglio, A.G. Can radiation chemistry supply a highly efficient AO(R)P process for organics removal from drinking and waste water? A review. Environ. Sci. Pollut. Res. 2017, 24, 20187–20208. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.G. Treatment of water and sewage by ionizing radiations. Sew. Ind. Waste 1953, 25, 1277–1281. [Google Scholar]
- IAEA. Nuclear technology review. In Proceedings of the International Atomic Energy Agency, Vienna, Austria, 26–29 May 2014. [Google Scholar]
- Han, B.; Kim, J.K.; Kim, Y.; Choi, J.S.; Jeong, K.Y. Operation of industrial-scale electron beam wastewater treatment plant. Radiat. Phys. Chem. 2012, 81, 1475–1478. [Google Scholar] [CrossRef]
- Trebše, P.; Arčon, I. Degradation of organophosphorus compounds by X-ray irradiation. Radiat. Phys. Chem. 2003, 67, 527–530. [Google Scholar] [CrossRef]
- Zhang, K. Degradation of Trihalomethanes and Chloramines by UVC and VUS Irradiation. Master’s Thesis, Purdue University Graduate School, Lafayette, IN, USA, 2018. [Google Scholar]
- Cerreta GRoccamante, M.A.; Plaza-Bolaño, P.; Oller, I.; Aguera, A.; Malato, S.; Rizzo, L. Advanced treatment of urban wastewater by UV-C/free chlorine process: Micro-pollutants removal and effect of UV-C radiation on trihalomethanes formation. Water Res. 2020, 169, 115220. [Google Scholar] [CrossRef] [PubMed]
- Kurucz, C.N.; Waite, T.D.; Cooper, W.J.; Nickelsen, M.G. High energy electron beam irradiation of water, wastewater and sludge. In Advances in Nuclear Science and Technology; Plenum Press: New York, NY, USA, 1991; Volume 23, pp. 1–43. [Google Scholar]
- Adams, C.; YWang KLoftin Meyer, M. Removal of antibiotics from surface and distilled water in conventional water treatment process. J. Environ. Eng. 2002, 128, 253–260. [Google Scholar] [CrossRef]
- Craft, T.F.; Eichholz, G.G. Synergistic treatment of textile dye wastes by irradiation and oxidation. Int. J. Appl. Radiat. Isot. 1971, 22, 543–547. [Google Scholar] [CrossRef]
- Sakumoto, A.; Miyata, T. Treatment of waste water by a combined technique of irradiation and conventional method. Radiat. Phys. Chem. 1977, 24, 99–115. [Google Scholar]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.L.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Gerrity, D.; Lee, M.; Bogeat, A.E.; Salhi, E.; Gamage, S.; Trenholm, R.A.; Wert, E.C.; Snyder, S.A.; von Gunten, U. Prediction of micropollutant elimination during ozonation of municipal wastewater effluents: Use of kinetic and water specific information. Environ. Sci. Technol. 2013, 47, 5872–5881. [Google Scholar] [CrossRef] [PubMed]
- Buffle, M.O.; Schumacher, J.; Meylan, S.; Jekel, M.; von Gunten, U. Ozonation and advanced oxidation of wastewater: Effect of O3 dose, pH, DOM and OH-radical scavengers on ozone decomposition and OH-radical generation. Ozone Sci. Eng. 2006, 28, 247–259. [Google Scholar] [CrossRef]
- Liu, C.; Qiang, Z.M.; Tian, F.; Zhang, T. Reactivity of several classes of pesticides with UV, ozone and permanganate. Huan Jing Ke Xue 2009, 30, 127–133. (In Chinese) [Google Scholar]
- Belgiorno, V.; Rizzo, L.; Fatta, D.; Della Rocca, C.; Lofrano, G.; Nikol aou, A.; Naddeo, V.; Meric, S. Review on endocrine disrupting emerging compounds in urban wastewater: Occurrence and removal by photocatalysis and ultrasonic irradiation for wastewater reuse. Desalination 2007, 215, 166–176. [Google Scholar] [CrossRef]
- Mboula, V.M.; Hequet, V.; Andres, Y.; Pastrana-Martınez, L.M.; Dona-Rodrıguez, J.M.; Silva, A.M.T.; Falaras, P. Photocatalytic degradation of endocrine disruptor compounds under simulated solar light. Water Res. 2013, 47, 3997–4005. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced oxidation processes for the removal of antibiotics from water. An overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- IARC. Potassium Bromate; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer: Lyon, France, 1999. [Google Scholar]
- Botlaguduru, V.S.V.; Batchelorm, B.; Abdel-Wahab, A. Application of UV–sulfite advanced reduction process to bromate removal. J. Water Proc. Eng. 2015, 5, 76–82. [Google Scholar] [CrossRef]
- Schoutteten, K. Advanced Reduction and Adsorption for Trace Organic Contaminant Removal from Water. Ph.D. Thesis, Ghent University, Belgium, Belgian, 2017. [Google Scholar]
- Liu, X.; Zhang, T.; Wang, L.; Shao, Y.; Fang, L. Hydrated electron-based degradation of atenolol in aqueous solution. Chem. Eng. J. 2015, 260, 740–748. [Google Scholar] [CrossRef]
- Liu, X.; Yoon, S.; Batchelor, B.; Abdel-Wahab, A. Degradation of vinyl chloride (VC) by the sulfite/UV advanced reduction process (ARP): Effects of process variables and a kinetic model. Sci. Total Environ. 2013, 454–455, 578–583. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Liu, G.; Fang, J.; Yue, S.; Guan, Y.; Chen, L.; Liu, X. Efficient reductive dechlorination of monochloroacetic acid by Sulfite/UV process. Environ. Sci. Technol. 2012, 46, 7342–7349. [Google Scholar] [CrossRef]
- Basfar, A.; Mohamed, K.A.; Al-Abduly, A.J.; Al-Kuraiji, T.S.; Al-Shahrani, A.A. Degradation of diazinon contaminated waters by ionizing radiation. Radiat. Phys. Chem. 2007, 76, 1474–1479. [Google Scholar] [CrossRef]
- Abdel Rahman, O.; Hung, Y.T. Application of ionizing radiation in wastewater treatment: An overview. Water 2020, 12, 19. [Google Scholar] [CrossRef]
- Jan, S.; Nahaid Kamili, A.; Parween, T.; Hamid, R.; Parray, J.A.; Siddiqi, T.O.; Mahmooduzzafar Ahmad, P. Feasibility of radiation technology for wastewater treatment. Desalin. Water Treat. 2014, 55, 2053–2068. [Google Scholar] [CrossRef]
- Cooper, W.J.; Nickelsen, M.G.; Mezyk, S.P.; Leslie, G.; Tornatore, P.M.; Hardison, W.; Hajali, P.A. MTBE and priority contaminant treatment with high energy electron beam injection. Radiat. Phys. Chem. 2002, 65, 451–460. [Google Scholar] [CrossRef]
- Cosentino, H.M.; Takinami, P.Y.I.; del Mastro, N.L. Comparison of the ionizing radiation effects on cochineal, annatto and turmeric natural dyes. Radiat. Phys. Chem. 2016, 124, 208–211. [Google Scholar] [CrossRef]
- Han, B.; Kim, J.; Kang, W.; Choi, J.S.; Jeong, K.W. Development of mobile electron beam plant for environmental applications. Radiat. Phys. Chem. 2016, 124, 174–178. [Google Scholar] [CrossRef]
- Changotra, R.; Rajput, H.; Guin, J.P.; Khader, S.A.; Dhir, A. Techno-economical evaluation of coupling ionizing radiation and biological treatment process for the remediation of real pharmaceutical wastewater. J. Clean Prod. 2020, 242, 118544. [Google Scholar] [CrossRef]
- Baldacchino, G.; Brun, E.; Denden, I.; Bouhadoun, S.; Roux, R.; Khodja, H.; Sicard-Roselli, C. Importance of radiolytic reactions during high-LET irradiation modalities: LET effect, role of O2 and radiosensitization by nanoparticles. Cancer Nanotechnol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.R. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Proc. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas DPaleos, C.M. Functional dendrimeric “nanosponges” for the removal of polycyclic aromatic hydrocarbons from water. Chem. Mater. 2003, 15, 2844–2847. [Google Scholar] [CrossRef]
- Murcia, J.J. Visible active noble metals–structured photocatalysts for the removal of emerging contaminants. In Visible Light Active Structured Photocatalysts for the Removal of Emerging Contaminants; Sacco, O., Vaiano, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 27–40. [Google Scholar]
- Nidheesh, P.V. Graphene-based materials supported advanced oxidation processes for water and wastewater treatment: A review. Environ. Sci. Pollut. Res. 2017, 24, 27047–27069. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Enhancing the adsorption capacities of acid dyes by chitosan nano particles. Bioresour. Technol. 2009, 100, 1143–1148. [Google Scholar] [CrossRef]
- Shaoqing, Y.; Jun, H.; Jianlong, W. Radiation-induced catalytic degradation of p-nitrophenol (PNP) in the presence of TiO2 nanoparticles. Radiat. Phys. Chem. 2010, 79, 1039–1046. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, D.; Chu, L.; Wang, J. Enhancement of ionizing radiation-induced catalytic degradation of antibiotics using Fe/C nanomaterials derived from Fe-based MOFs. J. Hazard. Mater. 2020, 389, 122148. [Google Scholar] [CrossRef] [PubMed]
- Roth, O.; Hiroki, A.; LaVerne, J.A. Effect of Al2O3 Nanoparticles on Radiolytic H2O2 Production in Water. J. Phys. Chem. C 2011, 115, 8144–8149. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.K.; Lee, M.J. Biodegradability enhancement of textile wastewater by electron beam irradiation. Radiat. Phys. Chem. 2006, 76, 1037–1041. [Google Scholar] [CrossRef]
- Sági, G.; Kovács, K.; Bezsenyi, A.; Csay, T.; Takács, E.; Wojnárovits, L. Enhancing the biological degradability of sulfamethoxazole by ionizing radiation treatment in aqueous solution. Radiat. Phys. Chem. 2016, 124, 179–183. [Google Scholar] [CrossRef]
- Sawai, T.; Sawai, T.; Yamazaki, M. The effect of γ-irradiation on the biodegradability of landfill leachate. Bull. Chem. Soc. Jpn. 1981, 54, 313–314. [Google Scholar] [CrossRef]
- Kumakura, M.; Kaetsu, I. Effect of electron beam current on radiation pretreatment of cellulosic wastes with electron beam accelerator. Radiat. Phys. Chem. 1984, 23, 523–527. [Google Scholar] [CrossRef]
- Taghipour, F.; Evans, G.J. Radiolytic elimination of organochlorine in pulp mill effluent. Environ. Sci. Technol. 1986, 30, 1558–1564. [Google Scholar] [CrossRef]
- Shin, H.; Kim, Y.; Han, B.; Makarov, I.E.; Ponomarev, A.V.; Pikaev, A.K. Application of electron beam to treatment of wastewater from papermill. Radiat. Phys. Chem. 2002, 65, 539–547. [Google Scholar] [CrossRef]
- Nasir, N.M.; Ming, T.T.; Ahmadun, F.R.; Sobri, S. Decomposition and biodegradability enhancement of textile wastewater using a combination of electron beam irradiation and activated sludge process. Water Sci. Technol. 2010, 62, 42–47. [Google Scholar] [CrossRef]
- Emami-Meibodi, M.; Parsaeian, M.R.; Amraei, R.; Banaei, M.; Anvari, F.; Tahami, S.M.R.; Vakhshoor, B.; Mehdizadeh, A.; Fallah Nejad, A.; Shirmardi, S.P.; et al. An experimental investigation of wastewater treatment using electron beam irradiation. Radiat. Phys. Chem. 2016, 125, 82–87. [Google Scholar] [CrossRef]
- Gehringer, P.; Eschweiler, H.; Fiedler, H. Ozone-electron beam treatment for groundwater remediation. Radiat. Phys. Chem. 1995, 46, 1075–1078. [Google Scholar] [CrossRef]
- Meng, M.; Pellizzari, F.; Boukari, S.O.B.; Karpel, N.; Leitner, V.; Teychene, B. Impact of e-beam irradiation of municipal secondary effluent on MF and RO membranes performances. J. Membr. Sci. 2014, 471, 1–8. [Google Scholar] [CrossRef]
- Changotra, R.; Rajput, H.; Guin, J.P.; Varshney, L.; Dhir, A. Hybrid coagulation, gamma irradiation and biological treatment of real pharmaceutical wastewater. Chem. Eng. J. 2019, 370, 595–605. [Google Scholar] [CrossRef]
- Zimek, Z.; Kaluska, I. Economical aspects of radiation sterilization with electron beam. In Proceedings of the International Atomic Energy Agency, Vienna, Austria, 28 May–1 June 1998. [Google Scholar]
- Maruthi, A.Y.; Das, N.L.; Hossain, K.; Rawat, K.P.; Sarma, K.S.S.; Sabharwal, S. Disinfection and reduction of organic load of sewage water by electron beam radiation. Appl. Water Sci. 2011, 1, 49–56. [Google Scholar] [CrossRef][Green Version]
- Capodaglio, A.G.; Olsson, G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the urban water cycle. Sustainability 2020, 12, 266. [Google Scholar] [CrossRef]
- Bolton, J.R.; Valladares, J.E.; Zanin, J.P.; Cooper, W.J.; Nickelson, M.G.; Kajdi, D.C.; Waite, T.D.; Kurucz, C.N. Figures-of-merit for advanced oxidation technologies: A comparison of homogeneous UV/H2O2, heterogeneous UV/TiO2 and electron beam processes. J. Adv. Oxid. Technol. 1998, 3, 174–181. [Google Scholar] [CrossRef]
- Kim, T.; Kim, S.D.; Kim, H.Y.; Lim, S.J.; Lee, M.; Yu, S. Degradation and toxicity assessment of sulfamethoxazole and chlorteteracycline using electron beam, ozone and UV. J. Hazard. Mater. 2012, 227–228, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Kivi, A.; Dulova, N.; Zekker, I.; Molder, E.; Tenno To Trapido, M.; Tenno, T. A pilot study of three-stage biological–chemical treatment of landfill leachate applying continuous ferric sludge reuse in Fenton-like process. Clean Technol. Environ. Policy 2017, 19, 541–551. [Google Scholar] [CrossRef]
- Pikaev, A.K.; Podrozova, E.V.; Bakhtiu, O.M.; Lysenko, S.L.; Belyshev, V.A. Electron beam technology for purification of municipal wastewater in the aerosol flow. IAEA-TECDOC 1225. In Proceedings of the International Atomic Energy Agency, Vienna, Austria, June 2001. [Google Scholar]
- Meeroff, D.; Waite, T.; Kazumi, J.; Kurucz, C. Radiation-assisted process enhancement in wastewater treatment. J. Environ. Eng. 2004, 130, 155–166. [Google Scholar] [CrossRef]
- Waite, T.D.; Kurucz, C.N.; Cooper, W.J.; Brown, D. Full scale electron beam systems for treatment of water, wastewater and medical waste. IAEA-TECDOC-1023. In Proceedings of the International Atomic Energy Agency (IAEA), Vienna, Austria, June 1998. [Google Scholar]
- Engohang-Ndong, J.; Uribe, R.M.; Gregory, R.; Gangoda, M.; Nickelsen, M.G.; Loar, P. Effect of electron beam irradiation on bacterial and Ascaris ova loads and volatile organic compounds in municipal sewage sludge. Radiat. Phys. Chem. 2015, 112, 6–12. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).