Hydrogen Storage in Propane-Hydrate: Theoretical and Experimental Study
Abstract
:1. Introduction
2. Simulation Methodology
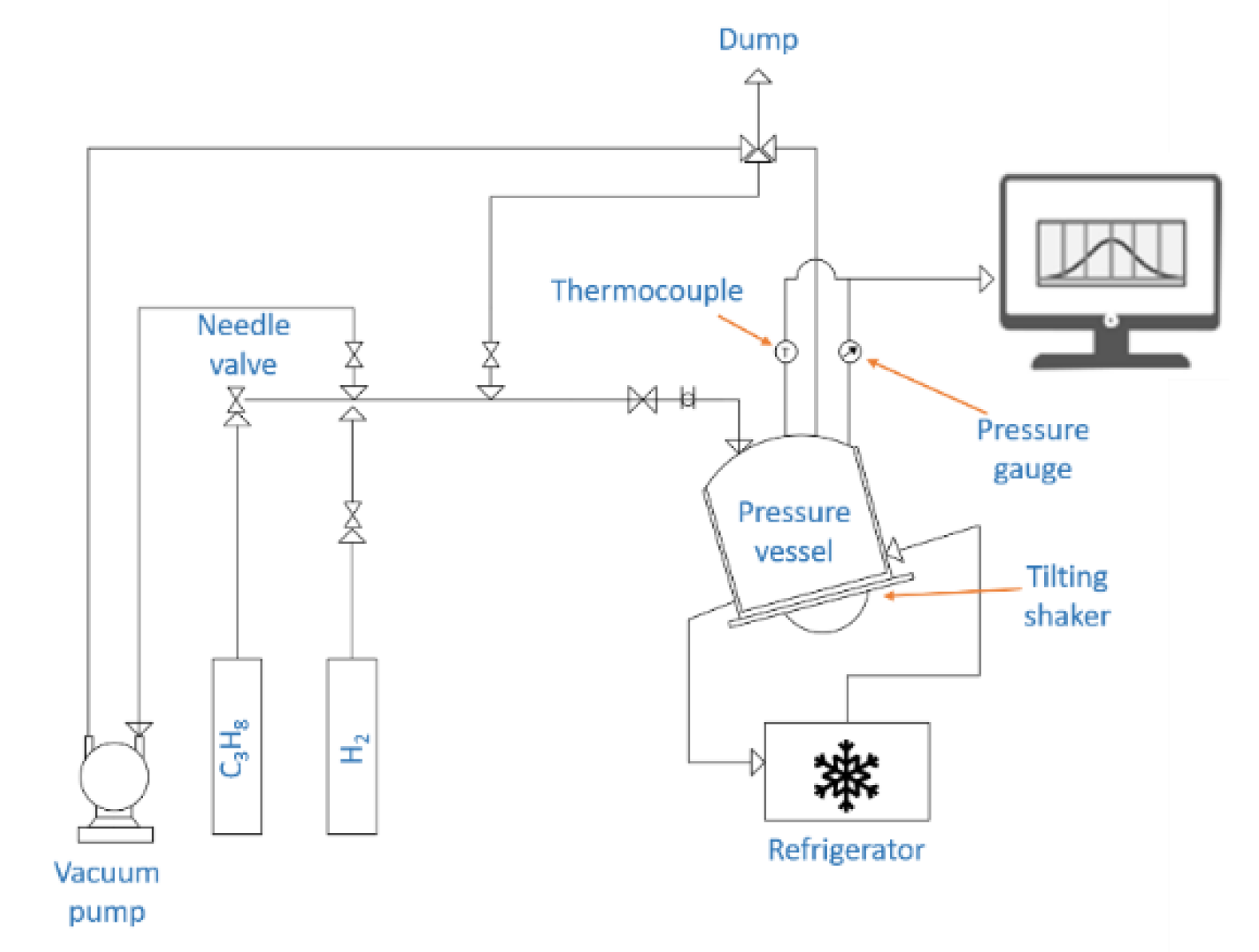
3. Experimental Methodology
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Makogon, I.F. Hydrates of Hydrocarbons; Pennwell Books: Tulsa, OK, USA, 1997; ISBN 0878147187. [Google Scholar]
- Veluswamy, H.P.; Kumar, R.; Linga, P. Hydrogen storage in clathrate hydrates: Current state of the art and future directions. Appl. Energy 2014, 122, 112–132. [Google Scholar] [CrossRef]
- Mao, W.L.; Mao, H.-K. Hydrogen storage in molecular compounds. Proc. Natl. Acad. Sci. USA 2004, 101, 708–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandford, S.; Allamandola, L.; Geballe, T.; Mao, H.-K.; Hemley, R.J.; Hu, J.; Shu, J.; Hemley, R.J.; Somayazulu, M.; Zhao, Y. Spectroscopic detection of molecular hydrogen frozen in interstellar ices. Science 1993, 262, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, P.; Arca, S.; Rossi, F.; Filipponi, M. Comparison of hydrogen hydrates with existing hydrogen storage technologies: Energetic and economic evaluations. Int. J. Hydrogen Energy 2009, 34, 9173–9180. [Google Scholar] [CrossRef]
- Shibata, T.; Yamachi, H.; Ohmura, R.; Mori, Y.H. Engineering investigation of hydrogen storage in the form of a clathrate hydrate: Conceptual designs of underground hydrate-storage silos. Int. J. Hydrogen Energy 2012, 37, 7612–7623. [Google Scholar] [CrossRef]
- Nakayama, T.; Tomura, S.; Ozaki, M.; Ohmura, R.; Mori, Y.H. Engineering Investigation of Hydrogen Storage in the Form of Clathrate Hydrates: Conceptual Design of Hydrate Production Plants. Energy Fuels 2010, 24, 2576–2588. [Google Scholar] [CrossRef]
- Florusse, L.J.; Peters, C.J.; Schoonman, J.; Hester, K.C.; Koh, C.A.; Dec, S.F.; Marsh, K.N.; Sloan, E.D. Stable low-pressure hydrogen clusters stored in a binary clathrate hydrate. Science 2004, 306, 469–471. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Kim, D.Y.; Park, J.; Seo, Y.-T.; Zeng, H.; Moudrakovski, I.L.; Ratcliffe, C.I.; Ripmeester, J.A. Tuning clathrate hydrates for hydrogen storage. Nature 2005, 434, 743–746. [Google Scholar] [CrossRef]
- Mao, W.L.; Mao, H.; Goncharov, A.F.; Struzhkin, V.V.; Guo, Q.; Hu, J.; Shu, J.; Hemley, R.J.; Somayazulu, M.; Zhao, Y. Hydrogen Clusters in Clathrate Hydrate. Science 2002, 297, 2247–2249. [Google Scholar] [CrossRef]
- Hasegawa, T.; Brumby, P.E.; Yasuoka, K.; Sum, A.K. Mechanism for H 2 diffusion in sII hydrates by molecular dynamics simulations. J. Chem. Phys. 2020, 153, 054706. [Google Scholar] [CrossRef]
- Skiba, S.S.; Larionov, E.G.; Manakov, A.Y.; Kolesov, B.A.; Ancharov, A.I.; Aladko, E.Y. Double clathrate hydrate of propane and hydrogen. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 383–386. [Google Scholar] [CrossRef]
- English, N.J.; MacElroy, J.M.D. Perspectives on molecular simulation of clathrate hydrates: Progress, prospects and challenges. Chem. Eng. Sci. 2015, 121, 133–156. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Wang, L.; Cao, X.; Su, Y.; Jiang, X.; Meng, S.; Zhao, J.; Zeng, X.C. A new phase diagram of water under negative pressure: The rise of the lowest-density clathrate s-III. Sci. Adv. 2016, 2, e1501010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaani, M.R.; English, N.J. Molecular-dynamics study of propane-hydrate dissociation: Fluctuation-dissipation and non-equilibrium analysis. J. Chem. Phys. 2018, 148, 114504. [Google Scholar] [CrossRef]
- Ghaani, M.R.; English, N.J. Molecular Dynamics Study of Propane Hydrate Dissociation: Nonequilibrium Analysis in Externally Applied Electric Fields. J. Phys. Chem. C 2018, 122, 7504–7515. [Google Scholar] [CrossRef]
- Ghaani, M.R.; English, N.J. Hydrogen-/propane-hydrate decomposition: Thermodynamic and kinetic analysis. Mol. Phys. 2019, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Kollman, P.A. Application of RESP charges to calculate conformational energies, hydrogen bond energies, and free energies of solvation. J. Am. Chem. Soc. 1993, 115, 9620–9631. [Google Scholar] [CrossRef]
- Alavi, S.; Ripmeester, J.A.; Klug, D.D. Molecular-dynamics study of structure II hydrogen clathrates. J. Chem. Phys. 2005, 123, 024507. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 2017; ISBN 0192524704. [Google Scholar]
- Udachin, K.A.; Lu, H.; Enright, G.D.; Ratcliffe, C.I.; Ripmeester, J.A.; Chapman, N.R.; Riedel, M.; Spence, G. Single crystals of naturally occurring gas hydrates: The structures of methane and mixed hydrocarbon hydrates. Angew. Chem. Int. Ed. 2007, 46, 8220–8222. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Stillinger, F.H. Proton Distribution in Ice and the Kirkwood Correlation Factor. J. Chem. Phys. 1972, 57, 4009–4017. [Google Scholar] [CrossRef] [Green Version]
- Bernal, J.D.; Fowler, R.H. A Theory of Water and Ionic Solution, with Particular Reference to Hydrogen and Hydroxyl Ions. J. Chem. Phys. 1933, 1, 515–548. [Google Scholar] [CrossRef]
- Ghaani, M.R.; English, N.J.; Allen, C.C.R. Magnetic-Field Manipulation of Naturally Occurring Microbial Chiral Peptides to Regulate Gas-Hydrate Formation. J. Phys. Chem. Lett. 2020, 9079–9085. [Google Scholar] [CrossRef] [PubMed]
- Burnham, C.J.; English, N.J. Free-Energy Calculations of the Intercage Hopping Barriers of Hydrogen Molecules in Clathrate Hydrates. J. Phys. Chem. C 2016, 120, 16561–16567. [Google Scholar] [CrossRef]
- Burnham, C.J.; Futera, Z.; English, N.J. Quantum and classical inter-cage hopping of hydrogen molecules in clathrate hydrate: Temperature and cage-occupation effects. Phys. Chem. Chem. Phys. 2017, 19, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, F.; Yang, L.; Fang, X.W.; Zhou, S.H.; Kramer, M.J.; Wang, C.Z.; Ho, K.M.; Napolitano, R.E. A computational study of diffusion in a glass-forming metallic liquid. Sci. Rep. 2015, 5, 10956. [Google Scholar] [CrossRef] [Green Version]
- Aman, Z.M.; Koh, C.A. Interfacial phenomena in gas hydrate systems. Chem. Soc. Rev. 2016, 45, 1678–1690. [Google Scholar] [CrossRef]
- Cao, H.; English, N.J.; MacElroy, J.M.D. Diffusive hydrogen inter-cage migration in hydrogen and hydrogen-tetrahydrofuran clathrate hydrates. J. Chem. Phys. 2013, 138, 094507. [Google Scholar] [CrossRef]
- Ohmura, R.; Kashiwazaki, S.; Shiota, S.; Tsuji, H.; Mori, Y.H. Structure-I and Structure-H Hydrate Formation Using Water Spraying. Energy Fuels 2002, 16, 1141–1147. [Google Scholar] [CrossRef]
- Tsuji, H.; Ohmura, R.; Mori, Y.H. Forming Structure-H Hydrates Using Water Spraying in Methane Gas: Effects of Chemical Species of Large-Molecule Guest Substances. Energy Fuels 2004, 18, 418–424. [Google Scholar] [CrossRef]
- Li, G.; Liu, D.; Xie, Y.; Xiao, Y. Study on Effect Factors for CO 2 Hydrate Rapid Formation in a Water-Spraying Apparatus. Energy Fuels 2010, 24, 4590–4597. [Google Scholar] [CrossRef]
- Takahashi, M.; Kawamura, T.; Yamamoto, Y.; Ohnari, H.; Himuro, S.; Shakutsui, H. Effect of Shrinking Microbubble on Gas Hydrate Formation. J. Phys. Chem. B 2003, 107, 2171–2173. [Google Scholar] [CrossRef]
- Havelka, P.; Linek, V.; Sinkule, J.; Zahradník, J.; Fialova, M. Effect of the ejector configuration on the gas suction rate and gas hold-up in ejector loop reactors. Chem. Eng. Sci. 1997, 52, 1701–1713. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, J.; He, Y.; Wang, C. Modelling and experimental study of hydrate formation kinetics of natural gas-water-surfactant system in a multi-tube bubble column reactor. Can. J. Chem. Eng. 2019, 97, 2765–2776. [Google Scholar] [CrossRef]
- Hould, N.; Elanany, M.S.; Aleisa, R.M.; Al-Majnouni, K.A.; Al-Malki, A.; Abba, I. Evaluating polymeric inhibitors of ethane clathrate hydrates. J. Nat. Gas Sci. Eng. 2015, 24, 543–549. [Google Scholar] [CrossRef]
- Vysniauskas, A.; Bishnoi, P.R. A kinetic study of methane hydrate formation. Chem. Eng. Sci. 1983, 38, 1061–1072. [Google Scholar] [CrossRef]
- Tang, L.-G.; Li, X.-S.; Feng, Z.-P.; Lin, Y.-L.; Fan, S.-S. Natural Gas Hydrate Formation in an Ejector Loop Reactor: Preliminary Study. Ind. Eng. Chem. Res. 2006, 45, 7934–7940. [Google Scholar] [CrossRef]
- Park, J.; Lee, H. Spectroscopic evidences of the double hydrogen hydrates stabilized with ethane and propane. Korean J. Chem. Eng. 2007, 24, 624–627. [Google Scholar] [CrossRef]
- Abbondondola, J.A.; Fleischer, E.B.; Janda, K.C. Comparative study of hydrogen, argon, and xenon uptake into a propane hydrate. AIChE J. 2010, 56, 2734–2741. [Google Scholar] [CrossRef]





| Temp (K) | Diffusion Coefficient (10−5 cm2/s) | |
|---|---|---|
| Uptake | Release | |
| 180 | 0.0169 | 0.00002 |
| 200 | 0.0368 | 0.00003 |
| 220 | 0.0165 | 0.00066 |
| 240 | 0.0281 | 0.01226 |
| 260 | 0.0441 | 0.02897 |
| 273 | 0.0694 | 0.03813 |
| Activation Energy (kJ/mol) | 13.11 ± 1.11 | 44.80 ± 5.35 |
| Large Cages Occupancy (%) | Water Molecules | Propane Molecules | Hydrogen Molecules | Storage Capacity (wt%) |
|---|---|---|---|---|
| 100 | 1360 | 80 | 160 | 1.13% |
| 95 | 1360 | 76 | 172 | 1.22% |
| 90 | 1360 | 72 | 184 | 1.31% |
| 85 | 1360 | 68 | 196 | 1.41% |
| 80 | 1360 | 64 | 208 | 1.50% |
| 75 | 1360 | 60 | 220 | 1.60% |
| 70 | 1360 | 56 | 232 | 1.69% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaani, M.R.; Takeya, S.; English, N.J. Hydrogen Storage in Propane-Hydrate: Theoretical and Experimental Study. Appl. Sci. 2020, 10, 8962. https://doi.org/10.3390/app10248962
Ghaani MR, Takeya S, English NJ. Hydrogen Storage in Propane-Hydrate: Theoretical and Experimental Study. Applied Sciences. 2020; 10(24):8962. https://doi.org/10.3390/app10248962
Chicago/Turabian StyleGhaani, Mohammad Reza, Satoshi Takeya, and Niall J. English. 2020. "Hydrogen Storage in Propane-Hydrate: Theoretical and Experimental Study" Applied Sciences 10, no. 24: 8962. https://doi.org/10.3390/app10248962
APA StyleGhaani, M. R., Takeya, S., & English, N. J. (2020). Hydrogen Storage in Propane-Hydrate: Theoretical and Experimental Study. Applied Sciences, 10(24), 8962. https://doi.org/10.3390/app10248962







