Hydrophobic Fluorinated Porous Organic Frameworks for Enhanced Adsorption of Nerve Agents
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of C-1 and C-1-F
2.3. Synthesis of C-1 and C-1-F
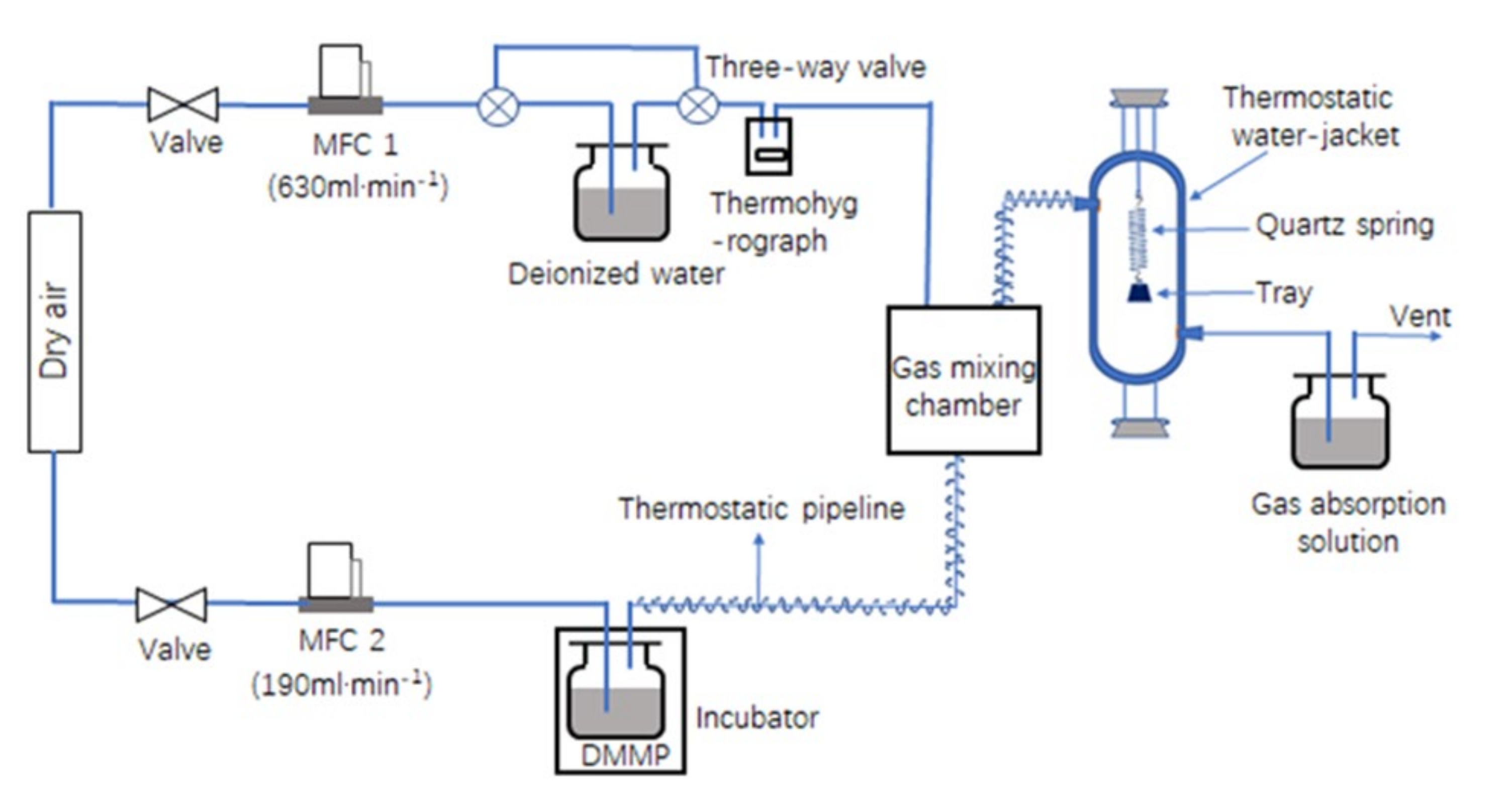
2.4. DMMP Dynamic Adsorption Performance Measurements
3. Results and Discussion
3.1. Structure and Morphology
3.2. Stability
3.3. Surface and Pore Hydrophobicity
3.4. Dynamic Adsorption of DMMP
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, R.C. Handbook of Chemical Warfare Agents; Academic Press LTD-Elsevier science LTD: London, UK, 2009; ISBN 978–0–12–374484–5. [Google Scholar]
- Zou, R.Q.; Zhong, R.Q.; Han, S.B.; Xu, H.W.; Burrell, A.K.; Henson, N.; Cape, J.L.; Hickmott, D.D.; Timofeeva, T.V.; Larson, T.E.; et al. A porous metal-organic replica of α-PbO2 for capture of nerve agent surrogate. J. Am. Chem. Soc. 2010, 132, 17996–17999. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Update 1 of: Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2015, 115, PR1–PR76. [Google Scholar] [CrossRef]
- Navarro, J.A.R.; Montoro, C.; Navarro, J.A.R. Toxic gas removal—Metal–organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef]
- Rosseinsky, M.J.; Smith, M.W.; Timperley, C.M. Breaking bad chemicals down. Nat. Mater. 2015, 14, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.-J.; Liu, S.-X.; Sun, C.-Y.; Liang, D.-D.; Ren, G.-J.; Wei, F.; Chen, Y.-G.; Su, Z.-M. A Sodalite-Type Porous Metal−Organic Framework with Polyoxometalate Templates: Adsorption and Decomposition of Dimethyl Methylphosphonate. J. Am. Chem. Soc. 2011, 133, 4178–4181. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, L.; Klichko, Y.; Chang, E.P.; Speakman, S.; Straut, C.M.; Wilusz, E.; Hatton, T.A. Alkylaminopyridine-Modified Aluminum Aminoterephthalate Metal-Organic Frameworks As Components of Reactive Self-Detoxifying Materials. ACS Appl. Mater. Interfaces 2012, 4, 4595–4602. [Google Scholar] [CrossRef]
- Roy, A.; Srivastava, A.K.; Singh, B.; Shah, D.; Mahato, T.H.; Gutch, P.K.; Halve, A.K. Degradation of sarin, DEClP and DECNP over Cu-BTC metal organic framework. J. Porous Mater. 2013, 20, 1103–1109. [Google Scholar] [CrossRef]
- Katz, M.J.; Moon, S.-Y.; Mondloch, J.E.; Beyzavi, M.H.; Stephenson, C.J.; Hupp, J.T.; Farha, O.K. Exploiting parameter space in MOFs: A 20-fold enhancement of phosphate-ester hydrolysis with UiO-66-NH2. Chem. Sci. 2015, 6, 2286–2291. [Google Scholar] [CrossRef]
- Roy, A.; Srivastava, A.K.; Singh, B.; Shah, D.; Mahato, T.H.; Srivastava, A. Kinetics of degradation of sulfur mustard and sarin simulants on HKUST-1 metal organic framework. Dalton Trans. 2012, 41, 12346–12348. [Google Scholar] [CrossRef]
- Ni, Z.; Jerrell, J.P.; Cadwallader, K.R.; Masel, R.I. Metal−Organic Frameworks as Adsorbents for Trapping and Preconcentration of Organic Phosphonates. Anal. Chem. 2007, 79, 1290–1293. [Google Scholar] [CrossRef]
- Katz, M.J.; Klet, R.C.; Moon, S.-Y.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. One Step Backward Is Two Steps Forward: Enhancing the Hydrolysis Rate of UiO-66 by Decreasing [OH–]. ACS Catal. 2015, 5, 4637–4642. [Google Scholar] [CrossRef]
- Yu, H.; Son, Y.R.; Yoo, H.; Gil Cha, H.; Lee, H.; Lee, H. Chitosan-Derived Porous Activated Carbon for the Removal of the Chemical Warfare Agent Simulant Dimethyl Methylphosphonate. Nanomater 2019, 9, 1703. [Google Scholar] [CrossRef] [PubMed]
- Osovsky, R.; Kaplan, D.; Nir, I.; Rotter, H.; Elisha, S.; Columbus, I. Decontamination of Adsorbed Chemical Warfare Agents on Activated Carbon Using Hydrogen Peroxide Solutions. Environ. Sci. Technol. 2014, 48, 10912–10918. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, B.; Neaţu, Ş.; Pârvulescu, V.I.; ¸Somoghi, V.; Petrea, N.; Epure, G.; Alvaro, M.; Garcia, H. Synergism of activated carbon and undoped and nitrogen-doped TiO2 in the photocatalytic degradation of the chemical warfare agents Soman, VX, and Yperite. ChemSusChem 2009, 2, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Cha, G.-Y.; Chun, H.; Hong, D.-Y.; Kim, J.; Cho, K.-H.; Lee, U.-H.; Chang, J.-S.; Ryu, S.G.; Lee, H.W.; Kim, J.Y.; et al. Unique design of superior metal-organic framework for removal of toxic chemicals in humid environment via direct functionalization of the metal nodes. J. Hazard. Mater. 2020, 398, 122857. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.J.; Isley, W.C., 3rd; Ghosh, P.; Liao, P.; Bury, W.; Wagner, G.W.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W.; et al. Destruction of chemical warfare agents using metal-organic frameworks. Nat. Mater. 2015, 14, 512–516. [Google Scholar] [CrossRef]
- López-Maya, E.; Montoro, C.; Rodríguez-Albelo, L.M.; Cervantes, S.D.A.; Lozano-Pérez, A.A.; Cenís, J.L.; Barea, E.; Navarro, J.A.R. Textile/Metal-Organic-Framework Composites as Self-Detoxifying Filters for Chemical-Warfare Agents. Angew. Chem. Int. Ed. 2015, 54, 6790–6794. [Google Scholar] [CrossRef]
- Singh, V.V.; Jurado-Sánchez, B.; Sattayasamitsathit, S.; Orozco, J.; Li, J.; Galarnyk, M.; Fedorak, Y.; Wang, J. Multifunctional Silver-Exchanged Zeolite Micromotors for Catalytic Detoxification of Chemical and Biological Threats. Adv. Funct. Mater. 2015, 25, 2147–2155. [Google Scholar] [CrossRef]
- Son, Y.R.; Kim, M.-K.; Ryu, S.G.; Kim, H.S. Rapid Capture and Hydrolysis of a Sulfur Mustard Gas in Silver-Ion-Exchanged Zeolite, Y. ACS Appl. Mater. Interfaces 2018, 10, 40651–40660. [Google Scholar] [CrossRef]
- Padial, N.M.; Procopio, E.Q.; Montoro, C.; López, E.; Oltra, J.E.; Colombo, V.; Maspero, A.; Masciocchi, N.; Galli, S.; Senkovska, I.; et al. Highly Hydrophobic Isoreticular Porous Metal-Organic Frameworks for the Capture of Harmful Volatile Organic Compounds. Angew. Chem. Int. Ed. 2013, 52, 8290–8294. [Google Scholar] [CrossRef]
- Montoro, C.; Linares, F.; Procopio, E.Q.; Senkovska, I.; Kaskel, S.; Galli, S.; Masciocchi, N.; Barea, E.; Navarro, J.A.R. Capture of Nerve Agents and Mustard Gas Analogues by Hydrophobic Robust MOF-5 Type Metal–Organic Frameworks. J. Am. Chem. Soc. 2011, 133, 11888–11891. [Google Scholar] [CrossRef] [PubMed]
- Doonan, C.J.; Tranchemontagne, D.J.; Glover, T.G.; Hunt, J.R.; Yaghi, O.M. Exceptional ammonia uptake by a covalent organic framework. Nat. Chem. 2010, 2, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Ren, H.; Meng, S.; Yan, Z.; Zhao, H.; Sun, F.; Zhu, G. A 3D microporous covalent organic framework with exceedingly high C3H8/CH4 and C2 hydrocarbon/CH4 selectivity. Chem. Commun. 2013, 49, 9773–9775. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, J.; Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015, 7, 905–912. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Zhang, J.; Wu, X.; Liu, Y.; Cui, Y. Homochiral 2D Porous Covalent Organic Frameworks for Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2016, 138, 12332–12335. [Google Scholar] [CrossRef]
- Colson, J.W.; Woll, A.R.; Mukherjee, A.; Levendorf, M.P.; Spitler, E.L.; Shields, V.B.; Spencer, M.G.; Park, J.; Dichtel, W.R. Oriented 2D Covalent Organic Framework Thin Films on Single-Layer Graphene. Science 2011, 332, 228–231. [Google Scholar] [CrossRef]
- Xu, H.; Tao, S.; Jiang, H.X.S.T.D. Proton conduction in crystalline and porous covalent organic frameworks. Nat. Mater. 2016, 15, 722–726. [Google Scholar] [CrossRef]
- Du, Y.; Yang, H.; Whiteley, J.M.; Wan, S.; Jin, Y.; Lee, S.; Zhang, W. Ionic Covalent Organic Frameworks with Spiroborate Linkage. Angew. Chem. Int. Ed. 2016, 55, 1737–1741. [Google Scholar] [CrossRef]
- Calik, M.; Auras, F.; Salonen, L.M.; Bader, K.; Grill, I.; Handloser, M.; Medina, D.D.; Dogru, M.; Löbermann, F.; Trauner, D.; et al. Extraction of Photogenerated Electrons and Holes from a Covalent Organic Framework Integrated Heterojunction. J. Am. Chem. Soc. 2014, 136, 17802–17807. [Google Scholar] [CrossRef]
- Mitra, S.; Kandambeth, S.; Biswal, B.P.; Khayum, M.A.; Choudhury, C.K.; Mehta, M.; Kaur, G.; Banerjee, S.; A Prabhune, A.; Verma, S.; et al. Self-Exfoliated Guanidinium-Based Ionic Covalent Organic Nanosheets (iCONs). J. Am. Chem. Soc. 2016, 138, 2823–2828. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Yusenko, K.; Fischer, R.A. Metallocenes@COF-102: Organometallic host–guest chemistry of porous crystalline organic frameworks. Chem. Commun. 2011, 47, 8506. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Meng, Q.; Faheem, M.; Yang, Y.; Li, Z.; Wang, Z.; Deng, D.; Sun, F.; He, H.; Huang, Y.; et al. A Molecular Coordination Template Strategy for Designing Selective Porous Aromatic Framework Materials for Uranyl Capture. ACS Central Sci. 2019, 5, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhu, G. Porous Aromatic Frameworks as a Platform for Multifunctional Applications. ACS Central Sci. 2019, 5, 409–418. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Zhu, G. Multifunctional porous aromatic frameworks: State of the art and opportunities. EnergyChem 2020, 2, 100037. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Zhu, G. Molecularly Imprinted Porous Aromatic Frameworks for Molecular Recognition. ACS Central Sci. 2020, 6, 1082–1094. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Hayashi, T. Gold(I)-catalyzed asymmetric aldol reaction of fluorinated benzaldehydes with a-isocyanoacetamide. Tetrahedron Asymmetr. 1994, 5, 1091–1094. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Kirilenko, A.G.; Galushko, S.V.; Kukhar, V.P. Catalytic asymmetric synthesis of b-fluoroalkyl-b-amino acids via biomimetic [1,3]-proton shift reaction. Tetrahedron Lett. 1994, 35, 5063–5064. [Google Scholar] [CrossRef]
- Sorochinsky, A.E.; Katagiri, T.; Ono, T.; Wzorek, A.; Aceña, J.L.; Soloshonok, V.A. Optical Purifications via Self-Disproportionation of Enantiomers by Achiral Chromatography: Case Study of a Series of α-CF3-containing Secondary Alcohols. Chirality 2013, 25, 365–368. [Google Scholar] [CrossRef]
- A Soloshonok, V.; Avilov, D.V.; Kukhar, V.P. Asymmetric aldol reactions of trifluoromethyl ketones with a chiral Ni(II) complex of glycine: Stereocontrolling effect of the trifluoromethyl group. Tetrahedron 1996, 52, 12433–12442. [Google Scholar] [CrossRef]











| Surface area (cm2·g−1) | Vtotal 1 (cm3·g−1) | Vmicro 2 (cm3·g−1) | Vmeso 3 (cm3·g−1) | M0% 4 | M20% 5 | M60% 6 | |
|---|---|---|---|---|---|---|---|
| C-1 | 344 | 0.63 | 0.12 | 0.51 | 8.15% | 10.37% | / |
| C-1-F | 434 | 0.50 | 0.16 | 0.34 | 13.73% | 13.90% | 18.24% |
| Activated carbon | 558 | 0.31 | 0.23 | 0.08 | 22.39% | 22.06% | 24.10% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Kong, W.; Wang, K.; Han, H.; Yang, D.; Zhao, Y.; Zhou, H.; Meng, Q.; Yuan, Y. Hydrophobic Fluorinated Porous Organic Frameworks for Enhanced Adsorption of Nerve Agents. Appl. Sci. 2020, 10, 8789. https://doi.org/10.3390/app10248789
Zhou S, Kong W, Wang K, Han H, Yang D, Zhao Y, Zhou H, Meng Q, Yuan Y. Hydrophobic Fluorinated Porous Organic Frameworks for Enhanced Adsorption of Nerve Agents. Applied Sciences. 2020; 10(24):8789. https://doi.org/10.3390/app10248789
Chicago/Turabian StyleZhou, Shuyuan, Weimin Kong, Kunpeng Wang, Hao Han, Derui Yang, Yue Zhao, Hong Zhou, Qinghao Meng, and Ye Yuan. 2020. "Hydrophobic Fluorinated Porous Organic Frameworks for Enhanced Adsorption of Nerve Agents" Applied Sciences 10, no. 24: 8789. https://doi.org/10.3390/app10248789
APA StyleZhou, S., Kong, W., Wang, K., Han, H., Yang, D., Zhao, Y., Zhou, H., Meng, Q., & Yuan, Y. (2020). Hydrophobic Fluorinated Porous Organic Frameworks for Enhanced Adsorption of Nerve Agents. Applied Sciences, 10(24), 8789. https://doi.org/10.3390/app10248789





