Tetragonia tetragonoides (Pall.) Kuntze Restores Blood Perfusion from Hind-Limb Ischemic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tetragonia tetragonoides (Pall.) Kunze Extract
2.2. HUVECs Culture and Cell Proliferation
2.3. Cell Migration Assay and Tubing Formation Assay
2.4. Experimental Animals and Treatments
2.5. Surgeries
2.6. Blood Perfusion Analysis
2.7. Histopathological Analysis
2.8. Immunohistochemical Stain
2.9. Immunoblot Assay
2.10. Statistical Analysis
3. Results
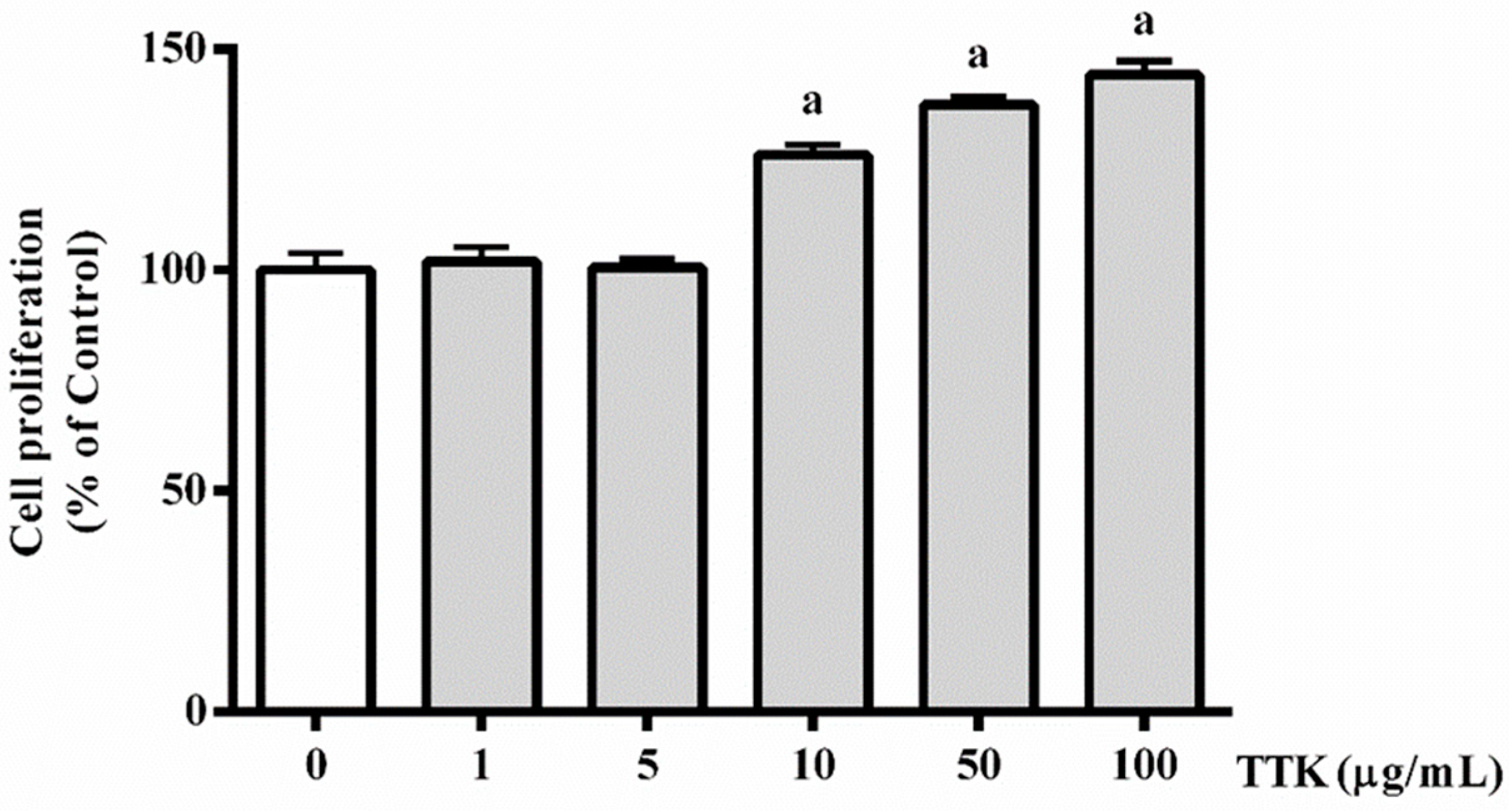
3.1. Effect of TTK on Proliferation of HUVECs
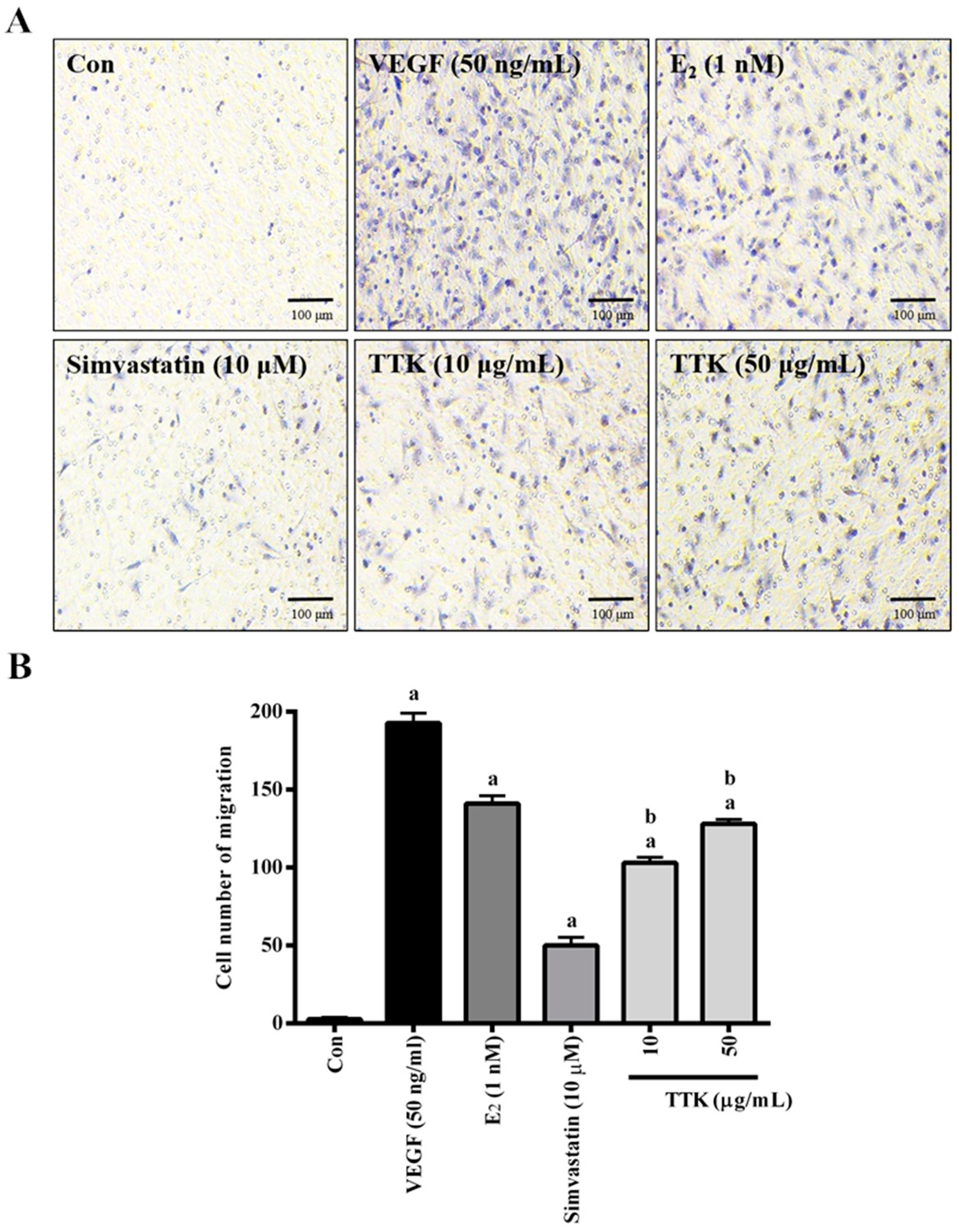
3.2. Effect of TTK on Migration of HUVECs
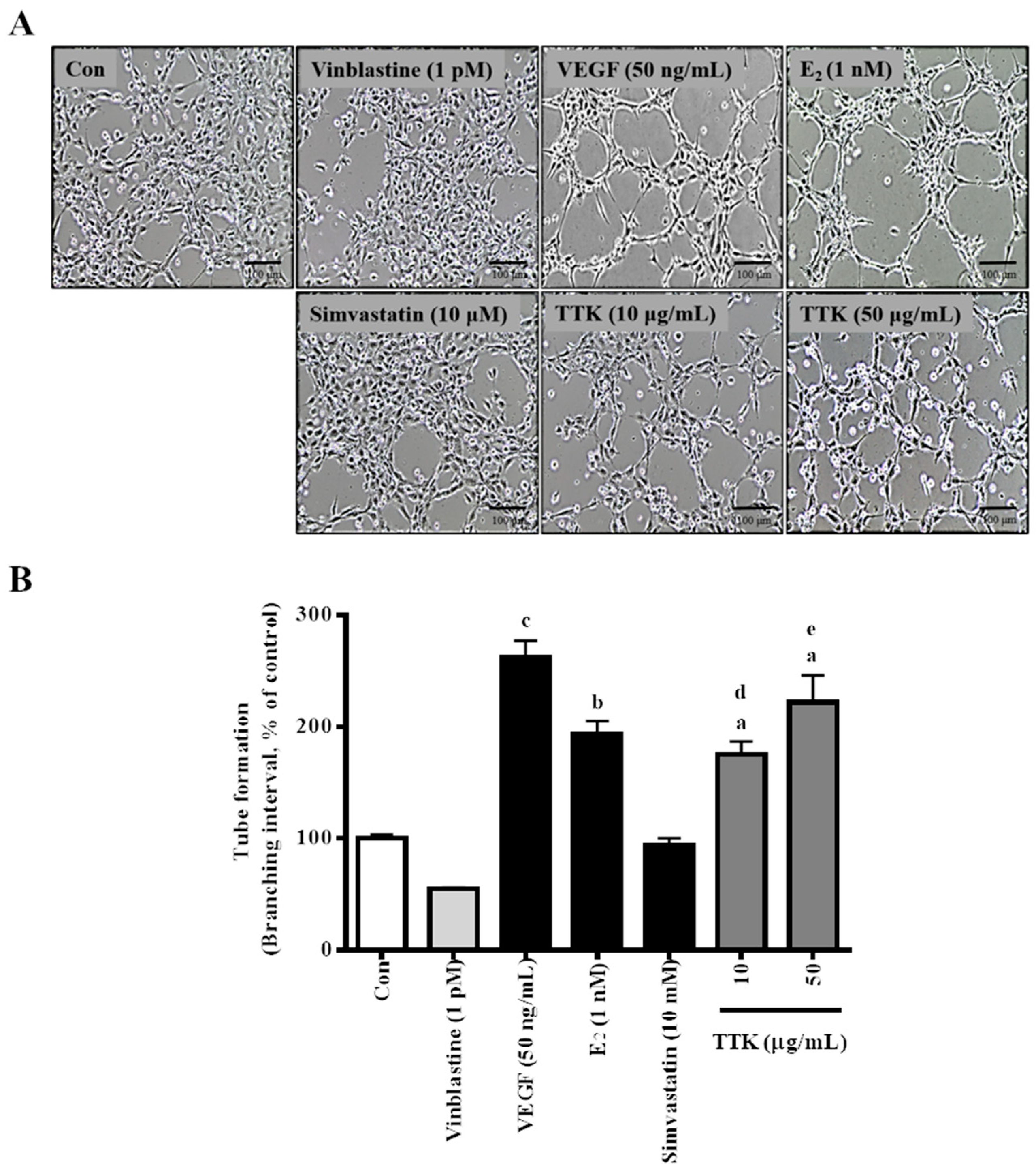
3.3. Effect of TTK on In Vitro tube Formation
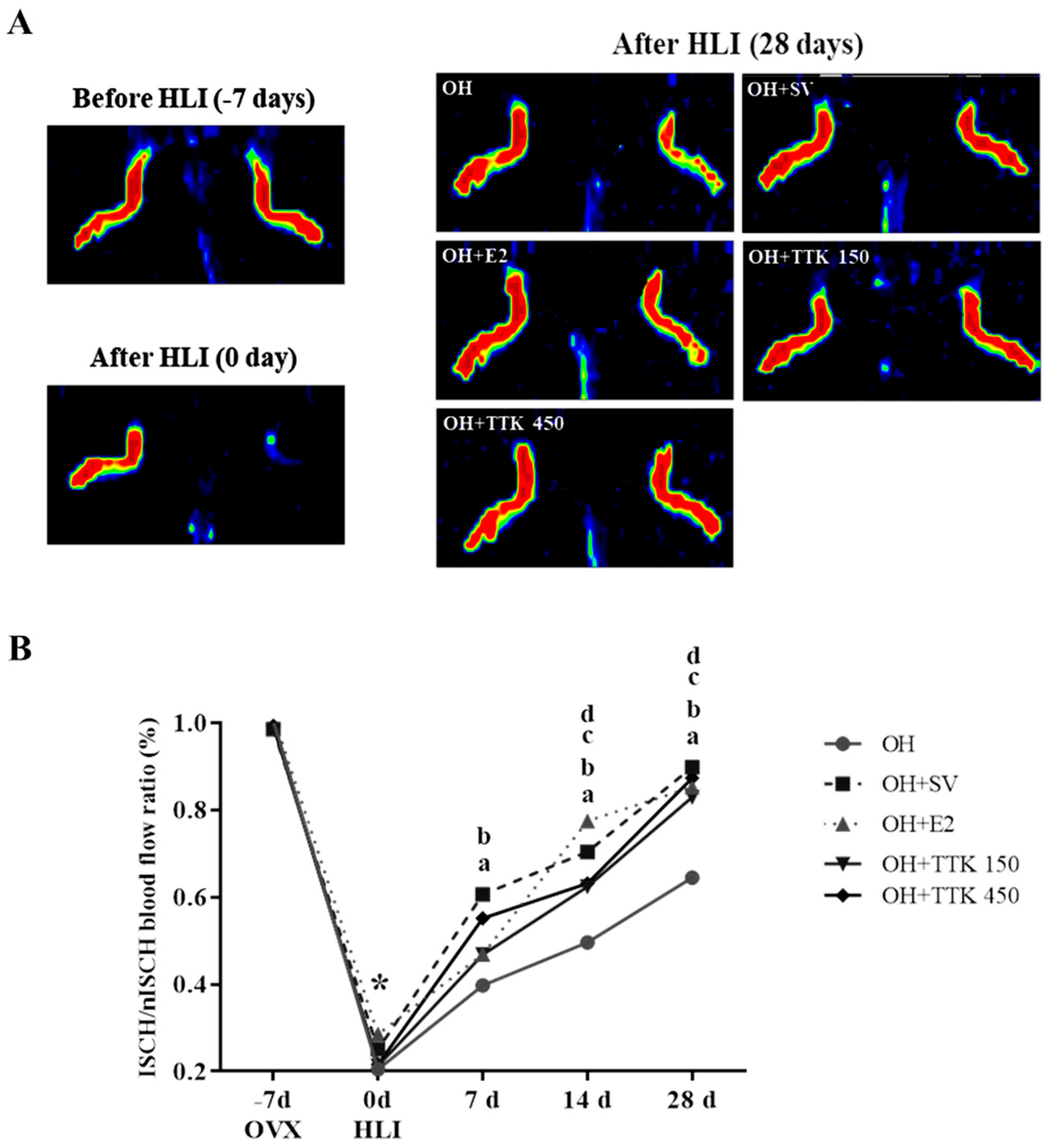
3.4. Blood Perfusion and Animal Condition
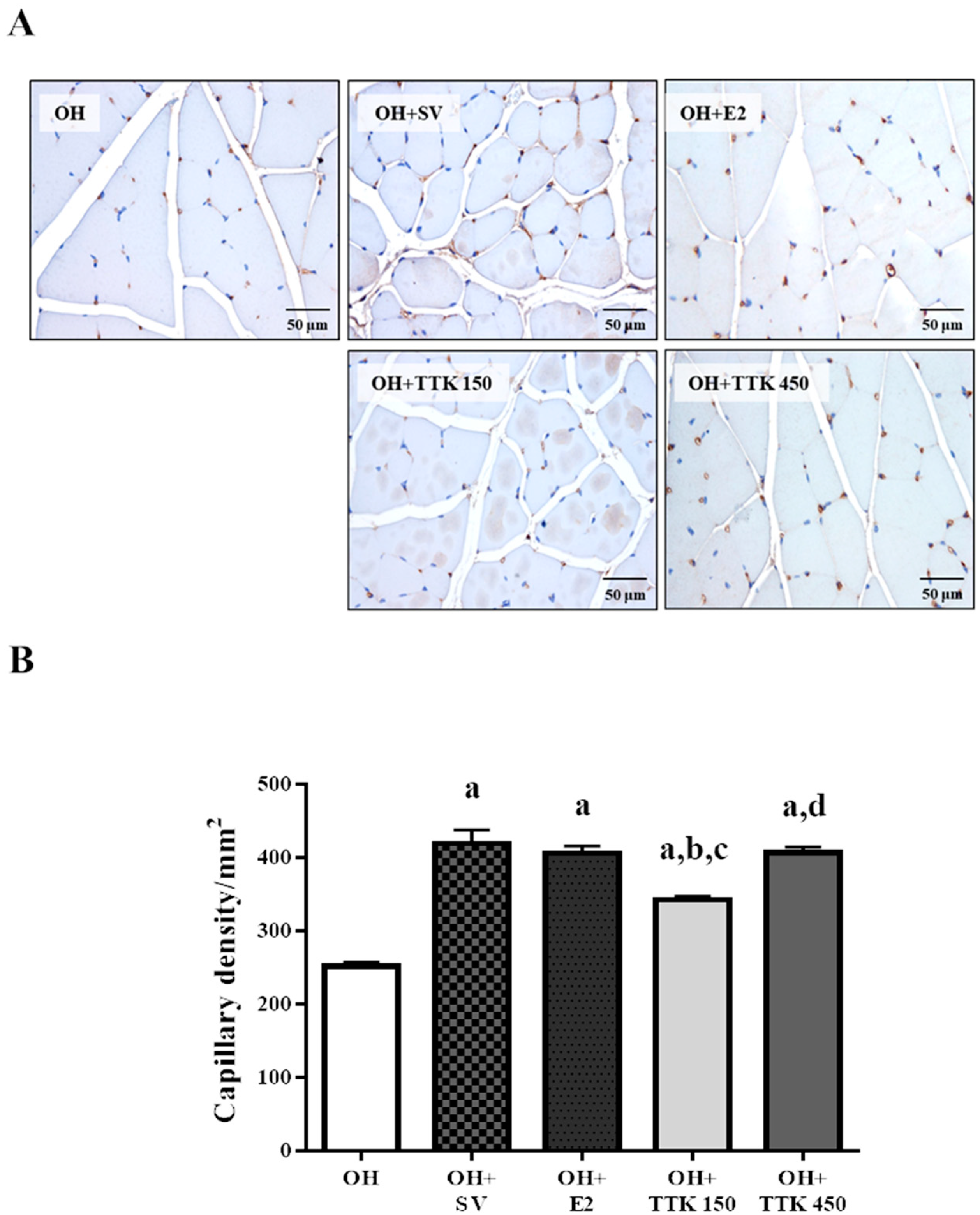
3.5. Capillary Density
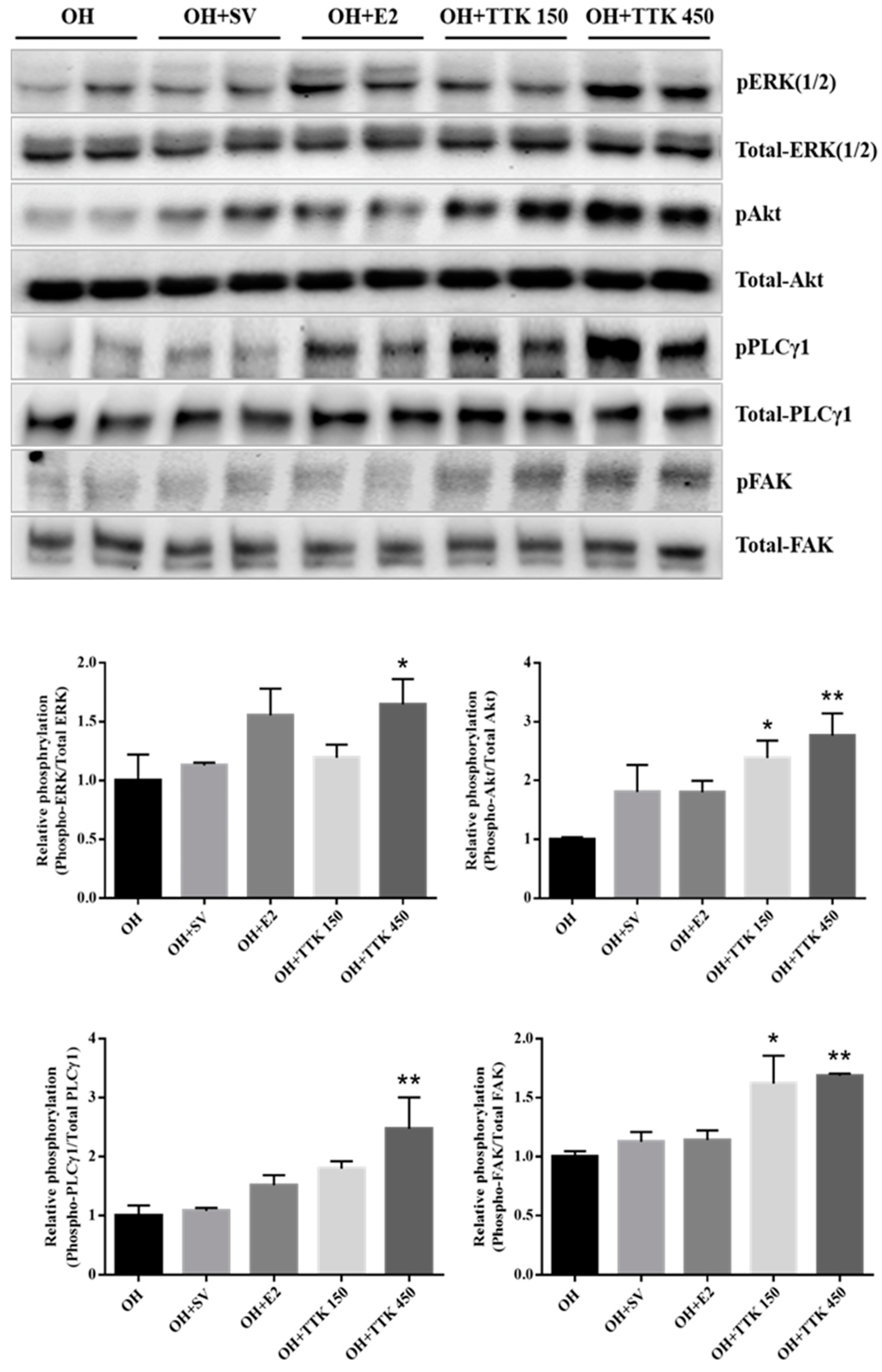
3.6. Expression of Angiogenic Factors In Vivo
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef]
- Nappi, R.E.; Lachowsky, M. Menopause and sexuality: Prevalence of symptoms and impact on quality of life. Maturitas 2009, 63, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Gierach, G.L.; Johnson, B.D.; Merz, C.N.B.; Kelsey, S.F.; Bittner, V.; Olson, M.B.; Rogers, W.J. Hypertension, menopause, and coronary artery disease risk in the Women’s Ischemia Syndrome Evaluation (WISE) Study. J. Am. Coll. Cardiol. 2006, 47, S50–S58. [Google Scholar] [CrossRef] [PubMed]
- Erlik, Y.; Meldrum, D.R.; Judd, H.L. Estrogen levels in postmenopausal women with hot flashes. Obstet. Gynecol. 1982, 59, 403–407. [Google Scholar]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Bush, T.L. Estrogen and coronary heart disease in women. JAMA 1991, 265, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Guetta, V.; Cannon, O.R. Cardiovascular effects of estrogen and lipid-lowering therapies in postmenopausal women. Circulation 1996, 93, 1928–1937. [Google Scholar] [CrossRef]
- Schainfeld, R.M.; Isner, J.M. Critical Limb Ischemia: Nothing to Give at the Office? Ann. Intern. Med. 1999, 130, 442. [Google Scholar] [CrossRef]
- Krishnamurthy, V.; Munir, K.; Rectenwald, J.E.; Mansour, A.; Hans, S.; Eliason, J.L.; Escobar, G.A.; Gallagher, K.A.; Grossman, P.M.; Gurm, H.S.; et al. Contemporary outcomes with percutaneous vascular interventions for peripheral critical limb ischemia in those with and without poly-vascular disease. Vasc. Med. 2014, 19, 491–499. [Google Scholar] [CrossRef]
- Sultan, S.; Hamada, N.; Soylu, E.; Fahy, A.; Hynes, N.; Tawfick, W. Sequential compression biomechanical device in patients with critical limb ischemia and nonreconstructible peripheral vascular disease. J. Vasc. Surg. 2011, 54, 440–446, discussion 446–447. [Google Scholar] [CrossRef]
- Thom, T.; Haase, N.; Rosamond, W.; Howard, V.J.; Rumsfeld, J. Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009, 119, e21–e181. [Google Scholar]
- Farhat, G.N.; Cauley, J.A. The link between osteoporosis and cardiovascular disease. Clin. Cases Miner. Bone Metab. 2008, 5, 19–34. [Google Scholar]
- Rosano, G.M.; Panina, G. Oestrogens and the heart. Therapie 1999, 54, 381–385. [Google Scholar]
- Grodstein, F.; Manson, J.E.; Colditz, G.A.; Willett, W.C.; Speizer, F.E.; Stampfer, M.J. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann. Intern. Med. 2000, 133, 933–941. [Google Scholar] [CrossRef]
- Rosano, G.M.; Vitale, C.; Fini, M. Cardiovascular aspects of menopausal hormone replacement therapy. Climacteric J. Int. Menopause Soc. 2009, 12 (Suppl. 1), 41–46. [Google Scholar] [CrossRef]
- Yang, X.P.; Reckelhoff, J.F. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 133–138. [Google Scholar] [CrossRef]
- Manson, J.E.; Hsia, J.; Johnson, K.C.; Rossouw, J.E.; Assaf, A.R.; Lasser, N.L.; Trevisan, M.; Black, H.R.; Heckbert, S.R.; Detrano, R.; et al. Estrogen plus Progestin and the Risk of Coronary Heart Disease. N. Engl. J. Med. 2003, 349, 523–534. [Google Scholar] [CrossRef]
- Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E.; Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. Randomized Trial of Estrogen Plus Progestin for Secondary Prevention of Coronary Heart Disease in Postmenopausal Women. JAMA 1998, 280, 605–613. [Google Scholar] [CrossRef]
- Hulley, S.; Furberg, C.; Barrett-Connor, E. Cardiovascular Disease Outcomes during 6.8 Years of Hormone Therapy: Heart and Estrogen/Progestin Replacement Study Follow-up (HERS II)—Correction. JAMA 2002, 288, 1064. [Google Scholar] [CrossRef]
- Eaker, E.D.; Chesebro, J.H.; Sacks, F.M.; Wenger, N.K.; Whisnant, J.P.; Winston, M. Cardiovascular disease in women. Circulation 1993, 88, 1999–2009. [Google Scholar] [CrossRef]
- Sunita, P.; Pattanayak, S.P. Phytoestrogens in postmenopausal indications: A theoretical perspective. Pharmacogn. Rev. 2011, 5, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hurn, P.D.; Brass, L.M. Estrogen and stroke: A balanced analysis. Stroke 2003, 34, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, Z.S.; Kremastinos, D.T.; Karayannakos, P. Estrogen stimulates angiogenesis in normoperfused skeletal muscle in rabbits. Circulation 2001, 103, E107–E108. [Google Scholar] [CrossRef] [PubMed]
- Losordo, D.W.; Isner, J.M. Estrogen and angiogenesis: A review. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 6–12. [Google Scholar] [CrossRef]
- Alvarez, R.J., Jr.; Gips, S.J.; Moldovan, N.; Wilhide, C.C.; Milliken, E.E.; Hoang, A.T.; Goldschmidt-Clermont, P.J. 17beta-estradiol inhibits apoptosis of endothelial cells. Biochem. Biophys. Res. Commun. 1997, 237, 372–381. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Greenwell-Wild, T.; Horan, M.A.; Wahl, S.M.; Ferguson, M.W. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am. J. Pathol. 1999, 155, 1137–1146. [Google Scholar] [CrossRef]
- Chen, Z.; Yuhanna, I.S.; Galcheva-Gargova, Z.; Karas, R.H.; Mendelsohn, M.E.; Shaul, P.W. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J. Clin. Investig. 1999, 103, 401–406. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Zhang, X.; Takata, K.; Takahashi, K.; Karas, R.H.; Kurachi, H.; Mendelsohn, M.E. Differential regulation of the inducible nitric oxide synthase gene by estrogen receptors 1 and 2. J. Endocrinol. 2008, 199, 267–273. [Google Scholar] [CrossRef]
- Liao, W.X.; Magness, R.R.; Chen, D.B. Expression of estrogen receptors-alpha and -beta in the pregnant ovine uterine artery endothelial cells in vivo and in vitro. Biol. Reprod. 2005, 72, 530–537. [Google Scholar] [CrossRef]
- Mowa, C.N.; Hoch, R.; Montavon, C.L.; Jesmin, S.; Hindman, G.; Hou, G. Estrogen enhances wound healing in the penis of rats. Biomed. Res. 2008, 29, 267–270. [Google Scholar]
- Ashcroft, G.S.; Mills, S.J.; Lei, K.; Gibbons, L.; Jeong, M.-J.; Taniguchi, M.; Burow, M.; Horan, M.A.; Wahl, S.M.; Nakayama, T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J. Clin. Investig. 2003, 111, 1309–1318. [Google Scholar] [CrossRef]
- Deroo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Ruifrok, W.-P.T.; de Boer, R.A.; Iwakura, A.; Silver, M.; Kusano, K.; Tio, R.A.; Losordo, D.W. Estradiol-induced, endothelial progenitor cell-mediated neovascularization in male mice with hind-limb ischemia. Vasc. Med. 2009, 14, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, S.; Kim, N.; Park, H.; Kim, P.H.; Kwon, O.; Ahn, S. Donguibogam (English Version); Korean Institute of Oriental Medicine: Daejon, Korea, 2008. [Google Scholar]
- Ryuk, J.A.; Ko, B.-S.; Lee, H.W.; Kim, D.S.; Kang, S.; Lee, Y.H.; Park, S. Tetragonia tetragonioides (Pall.) Kuntze protects estrogen-deficient rats against disturbances of energy and glucose metabolism and decreases proinflammatory cytokines. Exp. Biol. Med. 2016, 242, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.K.; Sclafani, A. Comparison of ovarian and hypothalamic obesity syndromes in the female rat: Effects of diet palatability on food intake and body weight. J. Comp. Physiol. Psychol. 1977, 91, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.C.; Silva, A.M.; Santos, M.S.; Sardao, V.A. Phytoestrogens as alternative hormone replacement therapy in menopause: What is real, what is unknown. J. Steroid Biochem. Mol. Biol. 2014, 143, 61–71. [Google Scholar] [CrossRef]
- Amato, P.; Christophe, S.; Mellon, P.L. Estrogenic activity of herbs commonly used as remedies for menopausal symptoms. Menopause 2002, 9, 145–150. [Google Scholar] [CrossRef]
- Bush, T.M.; Rayburn, K.S.; Holloway, S.W.; Sanchez-Yamamoto, D.S.; Allen, B.L.; Lam, T.; So, B.K.; Tran, D.H.; Greyber, E.R.; Kantor, S.; et al. Adverse interactions between herbal and dietary substances and prescription medications: A clinical survey. Altern. Ther. Health Med. 2007, 13, 30–35. [Google Scholar]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Nilsson, R. Endocrine modulators in the food chain and environment. Toxicol. Pathol. 2000, 28, 420–431. [Google Scholar] [CrossRef]
- Rodriguez-Fragoso, L.; Reyes-Esparza, J.; Burchiel, S.W.; Herrera-Ruiz, D.; Torres, E. Risks and benefits of commonly used herbal medicines in Mexico. Toxicol. Appl. Pharmacol. 2008, 227, 125–135. [Google Scholar] [CrossRef]
- Fredriksson, I.; Larsson, M.; Stromberg, T. Measurement depth and volume in laser Doppler flowmetry. Microvasc. Res. 2009, 78, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Gonda, K.; Takeda, M.; Sato, A.; Watanabe, M.; Yambe, T.; Satomi, S.; Ohuchi, N. In vivo imaging of the molecular distribution of the VEGF receptor during angiogenesis in a mouse model of ischemia. Blood 2011, 118, e93–e100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lobo, R.A. Serono Symposia USA: Perimenopause; Springer: New York, NY, USA, 1997. [Google Scholar]
- Novi, J.M.; Ross, H.L. Perimenopause; Informa Healthcare: New York, NY, USA, 2009. [Google Scholar]
- Matsubara, K.; Harada, H.; Ando, N.; Watada, S.; Obara, H.; Matsumoto, K.; Kitagawa, Y. Estrogen deficiency attenuates neovascularization in a murine model of hindlimb ischemia. J. Surg. Res. 2012, 178, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Pavlick, K.P.; Hines, I.N.; Lefer, D.J.; Hoffman, J.M.; Bharwani, S.S.; Wolf, R.E.; Grisham, M.B. Sexual dimorphism in reduced-size liver ischemia and reperfusion injury in mice: Role of endothelial cell nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2003, 100, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yang, Z.; Chen, Y.; Chen, Y.; Huang, Z.; You, B.; Peng, Y.; Chen, J. Estrogen Accelerates Cutaneous Wound Healing by Promoting Proliferation of Epidermal Keratinocytes via Erk/Akt Signaling Pathway. Cell. Physiol. Biochem. 2016, 38, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Bharwani, S.; Pavlick, K.P.; Korach, K.S.; Grisham, M.B. Estrogen Receptor-α, Sexual Dimorphism and Reduced-Size Liver Ischemia and Reperfusion Injury in Mice. Pediatr. Res. 2004, 55, 450–456. [Google Scholar] [CrossRef]
- Powazniak, Y.; Kempfer, A.C.; De La Paz Dominguez, M.; Farias, C.; Keller, L.; Calderazzo, J.C.; Lazzari, M.A. Effect of estradiol, progesterone and testosterone on apoptosis- and proliferation-induced MAPK signaling in human umbilical vein endothelial cells. Mol. Med. Rep. 2009, 2, 441–447. [Google Scholar] [CrossRef]
- Esfahanian, N.; Shakiba, Y.; Nikbin, B. Effect of metformin on the proliferation, migration, and MMP-2 and -9 expression of human umbilical vein endothelial cells. Mol. Med. Rep. 2012, 5, 1068–1074. [Google Scholar] [CrossRef]
- Zhou, J.; Du, T.; Li, B.; Rong, Y.; Verkhratsky, A.; Peng, L. Crosstalk Between MAPK/ERK and PI3K/AKT Signal Pathways during Brain Ischemia/Reperfusion. ASN Neuro 2015, 7, 1759091415602463. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Han, S.J.; Kim, J.I.; Lee, S.; Lipschutz, J.H.; Park, K.M. Activation of ERK accelerates repair of renal tubular epithelial cells, where as it inhibits progression of fibrosis following ischemia/reperfusion injury. Biochim. Biophys. Acta 2013, 1832, 1998–2008. [Google Scholar] [CrossRef]
- Tavora, B.; Batista, S.; Reynolds, L.E.; Jadeja, S.; Robinson, S.D.; Kostourou, V.; Hart, I.; Fruttiger, M.; Parsons, M.; Hodivala-Dilke, K. Endothelial FAK is required for tumour angiogenesis. EMBO Mol. Med. 2010, 2, 516–528. [Google Scholar] [CrossRef]
- Hubbard, S.R.; Till, J.H. Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 2000, 69, 373–398. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wang, Z. Akt binds to and phosphorylates phospholipase C-gamma1 in response to epidermal growth factor. Mol. Biol. Cell 2006, 17, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1999, 13, 9–22. [Google Scholar] [CrossRef]
- Marino, M.; Acconcia, F.; Trentalance, A. Biphasic estradiol-induced AKT phosphorylation is modulated by PTEN via MAP kinase in HepG2 cells. Mol. Biol. Cell 2003, 14, 2583–2591. [Google Scholar] [CrossRef]
| Groups | Body Weight (g) | Body Weight Gain (g) | Daily Food Intake (g) | Uterine Weight (mg) | |
|---|---|---|---|---|---|
| Baseline | After 28 Days | ||||
| OH | 19.80 ± 0.49 | 23.06 ± 1.22 | 3.27 ± 1.36 | 3.72 ± 0.41 | 17.48 ± 3.61 |
| OH + SV | 20.11 ± 0.88 | 23.00 ± 1.68 | 2.89 ± 1.99 | 3.91 ± 0.96 | 16.34 ± 3.31 |
| OH + E2 | 19.98 ± 0.73 | 22.51 ± 0.82 | 2.53 ± 0.63 | 3.84 ± 0.62 | 265.10 ± 176.02 a |
| TTK 150 | 20.48 ± 0.63 | 23.36 ± 1.69 | 2.88 ± 0.88 | 3.39 ± 0.40 | 14.79 ± 3.09 |
| TTK 450 | 20.41 ± 1.26 | 24.19 ± 1.63 | 3.78 ± 1.86 | 3.73 ± 0.52 | 15.83 ± 2.62 |
| Group | Surgery I (OVX) | Surgery II (HLI) | After Surgery II (HLI) | ||
|---|---|---|---|---|---|
| −7d | 0d | 7d | 14d | 28d | |
| OH | 1.01 ± 0.04 | 0.21 ± 0.05 | 0.40 ± 0.12 | 0.50 ± 0.07 | 0.64 ± 0.08 |
| OH + SV | 0.99 ± 0.03 | 0.25 ± 0.06 | 0.61 ± 0.07 a | 0.70 ± 0.11 a | 0.90 ± 0.06 a |
| OH + E2 | 1.02 ± 0.02 | 0.29 ± 0.08 | 0.47 ± 0.08 b | 0.78 ± 0.09 a | 0.85 ± 0.08 a |
| TTK 150 | 1.00 ± 0.02 | 0.21 ± 0.04 | 0.47 ± 0.07 b | 0.62 ± 0.06 ac | 0.83 ± 0.07 a |
| TTK 450 | 0.99 ± 0.04 | 0.22 ± 0.04 | 0.55 ± 0.10 a | 0.63 ± 0.09 ac | 0.87 ± 0.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Jung, D.H.; Lee, H.W.; Lee, D.; Ko, B.S. Tetragonia tetragonoides (Pall.) Kuntze Restores Blood Perfusion from Hind-Limb Ischemic Mice. Appl. Sci. 2020, 10, 8562. https://doi.org/10.3390/app10238562
Yang H, Jung DH, Lee HW, Lee D, Ko BS. Tetragonia tetragonoides (Pall.) Kuntze Restores Blood Perfusion from Hind-Limb Ischemic Mice. Applied Sciences. 2020; 10(23):8562. https://doi.org/10.3390/app10238562
Chicago/Turabian StyleYang, Hyun, Dong Ho Jung, Hye Won Lee, Dongoh Lee, and Byoung Seob Ko. 2020. "Tetragonia tetragonoides (Pall.) Kuntze Restores Blood Perfusion from Hind-Limb Ischemic Mice" Applied Sciences 10, no. 23: 8562. https://doi.org/10.3390/app10238562
APA StyleYang, H., Jung, D. H., Lee, H. W., Lee, D., & Ko, B. S. (2020). Tetragonia tetragonoides (Pall.) Kuntze Restores Blood Perfusion from Hind-Limb Ischemic Mice. Applied Sciences, 10(23), 8562. https://doi.org/10.3390/app10238562






