Featured Application
Summer Savory extracts could be a promising pharmacological tool not only in the management of inflammation associated to Inflammatory Bowel Disease (IBD) but also in managing the impaired secondary inflammatory mechanisms related with colorectal cancer progression, with a potential clinical use as a supplement to standard of care therapy.
Abstract
Summer Savory (Satureja hortensis L.) is a plant traditionally used as a food spice in the Mediterranean region. Surprisingly, not much is known about the health beneficial effects of its phenolic-rich extracts. The majority of publications have always focused on the properties of their essential oil. One of the main phenolic compounds of Summer Savory is rosmarinic acid, which has demonstrated anti-inflammatory outcomes in several animal models of inflammatory-mediated diseases. Inflammatory Bowel Disease (IBD) is a chronic inflammatory disease, in addition to Ulcerative Colitis and Crohn’s Disease, frequently related with increased morbidity and even mortality due to the complications associated, including colorectal cancer. Our work has shown, to our knowledge, for the first time, that administration of a phenolic extract of Summer Savory in a mouse model of Ulcerative Colitis led to the reduction of several markers for intestinal injury, including reduction of inducible nitric oxide synthase (iNOS) and Cyclooxygenase-2 (COX-2 or prostaglandin-endoperoxide synthase) expression, two well-known mediators of tissue inflammation and progression to cancer and led also to a reduction of the mortality. Given the chemical constitution found in the extract and the preclinical evidence of a beneficial effect of polyphenols in inflammatory processes, an opportunity arises for pharmacological modulation of pathways relevant for IBD and progression to cancer with phenolic-rich extracts.
1. Introduction
Summer Savory (Satureja hortensis L.) is a plant traditionally used as a food spice in Southern Europe and the Mediterranean region, yet, surprisingly, not much is known about the elucidation of its biological effects given that most of the existing publications focus on its use as food preservative related to its antimicrobial effects [1,2,3,4,5,6].
Satureja (Lamiaceae) species have been mainly used as a food flavoring compounds, and both pharmaceutical and cosmetic industries have demonstrated a growing interest in this plant because of its particular sweet flavor and its straightforward cultivation properties [1]. Although the information regarding medicinal uses of Summer Savory is not as consistent as other aromatic herbs, there are reports for the traditional use to treat myalgia and gastrointestinal (GI) disorders, such as cramps (related to its tonic and carminative compounds), indigestion, nausea, and diarrhea [7,8,9]. The antimicrobial properties of Summer Savory have also increased its use and research in oral and dental infections leading to periodontal inflammation [10,11].
However, the focus of Summer Savory research has been mainly on its essential oil (regarding its use in industrial, culinary, or medicinal uses), with less focus being given to the pharmacological effects of its phenolic compounds [12]. However, there is an increasing trend in researching the pharmacological role of effects of the phenolic content of aromatic plants [13].
Our group has previously studied the effect of phenolic extracts (leaves) of aromatic plants (anti-inflammatory related effect), having described the positive effects of administration of phenolic extracts of pennyroyal and spearmint in a mouse model of inflammatory bowel disease (IBD) [14,15]. Additionally, previous studies have characterized aqueous and alcoholic extracts of Satureja species and identified phenolic acids as main constituents, with a chemical profile similar to the ones studied by our team [16,17,18,19].
Inflammatory Bowel Disease (IBD) is a clinical term that encompasses two intestinal diseases characterized by inflammation: Crohn’s Disease and Ulcerative Colitis [20]. Although these diseases have been extensively studied throughout the years, there is still uncertainty regarding the specific mechanistic and pathophysiological causes that lead to the excessive inflammation [21]. However, it appears to be consensual that the main driver of this process is a genetically-mediated dysregulation of the immune system that leads to an abnormal local immune reaction triggered by environmental factors [22].
One of the primary complications of patients with IBD is the well-known increase of the risk for development of colorectal neoplasia, mainly dysplasia and colorectal cancer (CRC) [23,24,25], which has been ubiquitously pointed out as main consequence of chronic inflammation [24,26,27]. One of the widely-recognized driving factors for inflammatory signaling pathways associated to the link between IBD and CRC is the local generation of reactive oxygen species [28,29]. CRC is one of the cancers with higher incidence worldwide, with the latest data from GLOBOCAN (The Global Cancer Observatory: CANCER TODAY) reporting an estimate of over 1.8 million cases and 881.000 deaths in 2018. These numbers correspond to about 1 in 10 cancer cases and deaths. In the overall ranking of total cancer cases and deaths during 2018, CRC was the third cancer with higher incidence and the second with highest mortality [30].
Given the high impact that CRC has in morbidity and mortality, there is an urgent necessity for strategies to prevent the neoplastic initiation and progression processes. Taking into consideration the crucial role that inflammation plays in these processes, there is an opportunity in modulating chronic inflammation in patients with IBD [31]. Targeting the processes leading to progression to CRC will reduce the risk factors leading to morbidity and mortality [32]. Neoplastic initiation and progression are extremely influenced by a variety of external factors, in particular, the environment and diet. In fact, depending on its constitution, diet can, either decrease or increase CRC risk [33].
Considering the well-known beneficial effect of phenolic acids in the overproduction of reactive oxygen species and inflammatory processes, they may present an adjuvant pharmacological tool acting not only on the reduction of intestinal inflammation of IBD patients. Additionally, they might provide a possible preventive benefit in the impairment of the evolution of dysplastic cells (highly inflammatory and proliferative) into neoplastic cells [34,35,36].
The goal of this work was to characterize the chemical constitution and antioxidant effects of a Summer Savory aerial parts extract and to assess the anti-inflammatory effects (acute and chronic) that might be relevant for a pharmacological intervention in IBD, as well as in the prevention of CRC development.
We, therefore, screened the acute anti-inflammatory effect in a model of paw edema and further assessed the effect of the extract’s administration in a mouse model of colitis induced by TNBS (2,4,6-Trinitrobenzenesulfonic acid).
2. Materials and Methods
2.1. Reagents and Chemicals
Ketamine (Imalgene® 1000) and xilazine (Rompun® 2%) were purchased through the supplier Bio2 Produtos Veterinários (Lisboa, Portugal). Other substances used throughout the experiments were purchased through Sigma-Aldrich, Sintra, Portugal (if not, that will be clearly stated throughout the publication).
2.2. Plant Related Material and Extract Preparation
Fresh samples of Summer Savory (Satureja hortensis L.) were acquired locally from a cultivar (Lisboa, Portugal). Water was used to wash plants (stems and leaves) that afterwards were sliced into thin slices. Consequently, sliced plant material (20 g) was extracted using 100 mL of ethanol (70%, v/v) for 24 h, in dark conditions, at room temperature and under stirring. The resulting extracts were filtered (Whatman, n°1) and rotary evaporator at 40 °C (Heidolph LABOROTA 4001, Heidolph Instruments / Soquimica, Lisbon, Portugal) was used to eliminate ethanol. Finally, extracts were centrifuged (6000 g, 15 min, 4 °C) (Sigma 4K-15C), and the supernatants were split into 1 mL aliquots and were kept storage at −50 °C until future analyses.
2.3. Total Phenolic and Total Flavonoid Content
In order to determine total phenolic compounds the Kosar et al. method was used [37], assessing total phenolic concentration by the Folin–Ciocalteu method. When it comes to total flavonoid content, we followed Barros et al. method [38]. The outcome of total phenolic content was expressed as mg gallic acid equivalents (GAE) per mL extract and per g of dry plant, and total flavonoid content was expressed in µmol equivalents of catechin (CE) per mL of extract.
2.4. High-Performance Liquid Chromatography (HPLC)
HPLC of the extract was performed as previously described [15]. Briefly, the phenolic components of the aqueous extract were isolated using solid phase extraction C18 columns (500 mg/3 mL, Ref. 7020-03, J.T. Baker) in order to eliminate sugars and other non-phenolic components that could affect the chromatographic separation. An aliquot of the aqueous extract was added to the column and the adsorbed phenolic components were eluted with aqueous formic acid (0.1% v/v) and acetonitrile. The obtained extracts were combined concentrated to the initial volume and filtered through a 0.22 µm filter. The chromatographic separation was performed in a HPLC system (SpectraSystem, Thermo, Sigma-Aldrich, Sintra, Portugal), equipped with a diode array detector (DAD), and a Thermo C-18 column, and elution was performed with 0.1% formic acid (solvent A) and a mixture of 90% acetonitrile + 9.9% water + 0.1% formic acid (solvent B) at a flow rate of 0.8 mL/min.
Spectra acquisition was made in the range of 190 nm to 700 nm with selective detection at 280, 320 and 360 nm. Identification of the main functional groups present in the extract was performed by comparison of their UV spectra with those of representative standards analyzed in the same conditions. Standard curves were determined for rosmarinic and ferulic acids by analyzing the corresponding standards in the concentration range of 0.05 to 1 mg/mL.
2.5. Antioxidant Capacity
2.5.1. Assay for Reduction of Cupric Antioxidant Capacity (CUPRAC)
CUPRAC assay followed the normal sample measurement procedure as previously stated [39]. Results were expressed as µmol ascorbic acid equivalents (AAE)/mL of extract.
2.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP assay was performed according to the procedure as previously stated [40]. A calibration curve of ferrous sulphate (0–1.25 mM) was utilized, and results were expressed as µmol Fe2+/mL of extract.
2.5.3. DPPH (2,2-diphenyl-1-picrylhydrazyl) Radical-Scavenging Assay
The DPPH assay was performed according to the procedure previously stated [41]. Results were expressed as mg ascorbic acid equivalents (AAE)/mL of extract.
2.5.4. Superoxide Anion Radical-Scavenging Assay
The superoxide anion radical-scavenging assay was carried according to the procedure previously stated [42]. Results were expressed as μmol equivalents of ascorbic acid/mL of extract.
2.6. Carrageenan-Induced Paw Edema in Rat
2.6.1. Animals
The paw edema protocol was performed using 46 Wistar rats (150–200 g, male) (Instituto de Higiene e Medicina Tropical, Lisbon, Portugal). Ad libitum food and water was guaranteed to all rats up to 12 h prior to the beginning of the protocol.
2.6.2. Edema Induction and Evaluation
Paw edema was induced by sub-plantar (intradermal) injection of 100 µL of a λ-carrageenan solution (1% in saline) into the rat left hind paw as previously described [43]. Paw volume measurements were performed as described: V0 or basal volume is the volume of the hind paw measured immediately after carrageenan injection, and V6 is the volume at 6 h post carrageenan administration. The increase in paw volume was measured as edema and expressed as relative percentage of the increase in the volume at 6 h compared to the initial volume, according to the following formula: % paw volume increase = [(V6 − V0)/V0] × 100.
2.6.3. Experimental Groups
All the animals were split into six groups randomly: (i) Control Group included animals in which edema protocol detailed previously was performed, except for the administration of 100 µL of apyrogenic saline instead of carrageenan. Animals were subjected to administration of water (1 mL/kg) by oral gavage (n = 6); (ii) Carrageenan Group included paw edema animals and administered with 1 mL/kg of water by gastric gavage (n = 8); (iii) Summer Savory group-paw edema animals and administered with Summer Savory extract (15 mg of phenolic acids/kg by gastric gavage) 30 min prior to injection of carrageenan (n = 8); (iv) indomethacin group included paw edema animals with and administered with indomethacin (10 mg/kg by gastric gavage) 30 min prior to injection of carrageenan (n = 8); (v) tempol group included paw edema animals and administered with tempol (30 mg/kg by gastric gavage) 30 min prior to injection of carrageenan (n = 8); and (vi) trolox group included paw edema animals and administered with trolox (10 mg/kg by gastric gavage) 30 min prior to injection of carrageenan (n = 8). The dose of the extract was selected from previous studies by our group related with the evaluation of the beneficial effects of different phenolic extracts in several models of inflammation (including studies with aromatic plants), and the dose of 15 mg/kg of phenolic acids lead to consistent results and also fits into the range of possibility for clinical translation and use, considering a human adult of 70 kg [14,15,44,45,46].
2.7. TNBS-Induced Ulcerative Colitis Model in Mice
2.7.1. Animals
Male CD-1 mice (Crl:CD1(ICR)), with weights ranging from 25–30 g (5–6 weeks of age) (Harlan, Spain), were kept according to the standard protocols. Thus, ad libitum to water and food, they were kept in an automatically controlled temperature/light room (20 °C with a 12 h light/dark cycle) at the Animal Facility of the Faculty of Pharmacy-University of Lisbon.
2.7.2. Induction of Colitis
Induction of colitis was performed by administration of TNBS as previously described (Rocha et al., 2019). Briefly, a 50% ethanolic (EtOH) solution of TNBS (2.5% m/v) was administered by intracolonic administration (4 cm above the anus). At day 4 post-induction, blood samples were collected by cardiac puncture under surgical anesthesia, followed by euthanasia by cervical dislocation and subsequent necropsy. The colon was removed and was observed for classification of diarrhea severity. Furthermore, the colon was washed with (phosphate buffered saline) PBS for a macroscopic observation of lesions and fixed in (paraformaldehyde) PFA for histological studies.
2.7.3. Experimental Groups
Mice were randomized into the experimental groups described below:
- Sham group (n = 6): the colitis induction protocol was performed as stated previously, except for the intracolonic administration that was performed with 100 μL of saline solution. Animals were administered daily with 10 mL/kg of water by gastric gavage until the end of the experiment.
- Ethanol group (n = 6): the colitis induction protocol was performed as stated previously except for the intracolonic administration that was performed with 100 μL of 50% (v/v) ethanol solution. Animals were administered daily with 10 mL/kg of water by gastric gavage until the end of the experiment.
- TNBS group (n = 10): the colitis induction protocol was performed as previously described, with the administration of 100 μL of a TNBS solution (2.5% TNBS in 50% ethanol). Animals were administered daily with 10 mL/kg of water by gastric gavage until the end of the experiment.
- TNBS + Summer Savory group (n = 10): the colitis induction protocol was performed as described previously. Animals were administered daily with Summer Savory extract (15 mg/kg of phenolic acids by oral gavage) until the end of the experiment.
2.7.4. Evaluation of Colitis Severity (Macroscopic Analysis)
Diarrhea severity was classified by an observer blinded to the experimental groups following the classification found in Table 1. A microscopic observation of the tissue was done, followed by colon measurement, as well as measurement of the extent of injury.

Table 1.
Score of diarrhea severity.
2.7.5. Histology/Immunohistochemistry Procedures
Hematoxylin & Eosin (H & E) staining was performed as previously described [44], as well as the immunohistochemistry studies, for measurement of COX-2 and iNOS expression. Colon histological damage was scored as follows: score 0-normal colon with no lesions, the mucosa is of uniform thickness, and the crypts are straight, normal crypt architecture, there is no cellular infiltration, edema, or exudate; score 1-colon with mild lesions, there are mucosal erosion and small superficial ulcers scattered along the length of the colon, with slight crypt loss and mononuclear cell infiltration; score 2- colon with moderate lesions, intestines have extensive erosion and ulceration, with moderate crypt loss and neutrophil infiltration; score 3-colon with very severe ulceration, much of the mucosa is thin with loss of crypts and markedly increased infiltration of neutrophils and acute inflammatory exudate. The intensity of the protein staining is relatable to the level of expression of iNOS and COX-2. The level of iNOS or COX-2 staining was quantitatively evaluated by determining the percentage of tissue area that was stained in brown, using the ImageJ (Fiji Is Just, 2020, NIH, Bethesda, Maryland, USA) software.
2.8. Animal Experiments
Experiments were performed according to the Home Office Guidance in the Operation of Animals (Scientific Procedures) Act 1986, published by Her Majesty’s Stationary Office, London, UK, and the Institutional Animal Research Committee Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication no. 85–23, revised 1996) and according to the most updated EC regulations (Directive 2010/63/EU). ARRIVE Guidelines for Reporting Animal Research summarized at http://www.nc3rs.org.uk were strictly followed and respected during the studies. Protocol was submitted to and approved (CEEE-002/16) by the Ethics Committee for Animal Experiments (CEEA) of the Faculty of Pharmacy—University of Lisboa at February 2016.
2.9. Statistical Analysis
The animal experiment results (in vivo) were represented as mean ± standard error of the mean (SEM) of n observations. In order to compare results among experimental groups, a one-way ANOVA test was performed, followed by a Bonferroni’s test (post-hoc multiple comparisons) using GraphPad Prism version 8.2.1, GraphPad, San Diego, CA, USA). When p < 0.05 (CI of 95%), differences were considered statistically significant. Analysis of mortality onset was accomplished by Kaplan–Meier statistics, followed by a Mantel–Cox Test. When p < 0.05 (CI of 95%), differences were considered statistically significant.
3. Results
3.1. Chemical Characterization
Initial chemical characterization of the Summer Savory extract was done using the Folin-Ciocalteu method. The resulting total phenolic and flavonoid compounds of the extract is displayed below in Table 2.

Table 2.
Total phenolic and flavonoid content of Summer Savory extract.
The chromatographic profile resulted from the phenolic extract of Summer Savory is displayed in Figure 1 and shows that the predominant phenolics in Summer Savory ethanolic extract (Figure 2) were hydroxycinnamic acids (53.5%), flavonol glycosides (23.3%), and flavones and flavanones (13.0%), considering the sum of the corresponding chromatographic areas.
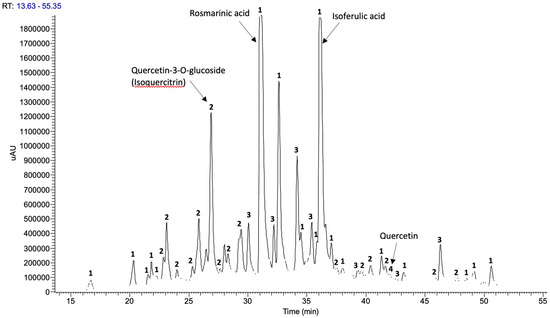
Figure 1.
Phenolic profile of Summer Savory extract determined by HPL-DAD with selective detection at 320 nm, including identification of functional groups (main components). Legend: 1. hydroxycinnamic acids, 2. flavonol glycosides, 3. flavones and flavonones, 4. Flavonols.
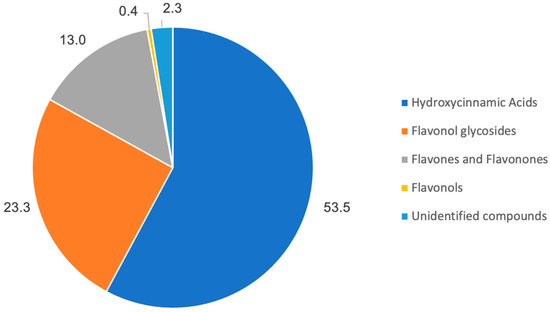
Figure 2.
Chromatographic area relative distribution of the principal phenolic functional groups identified in the Summer Savory extract.
Similar composition is reported for Summer Savory extracts obtained by Boroja et al. [18] using aqueous methanol or by Maškovićc et al. [19] using aqueous ethanol.
The main components found in the ethanolic extract of Summer Savory were rosmarinic acid (1 mg/mL) and isoferulic acid (0.75 mg/mL), corresponding to 19% and 15% of the chromatography area, respectively. These components were also predominant in other Satureja species hydroalcoholic extracts studied by other authors that also detected caffeic, isoferulic, chlorogenic acids, quercetin and apigenin glycosides, naringenin, and apigenin in relevant concentrations [17,18,19].
To determine the antioxidant properties of Summer Savory extract reducing capacity and free radical scavenging activity, several assays were performed (Table 3). The antioxidant capacity of Summer Savory extract was confirmed in the protocols tested. The outcomes match the ones demonstrated in previous studies, as well that ethanolic extracts of Summer Savory exhibit reducing capacity and are able to scavenge reactive oxygen species.

Table 3.
Antioxidant capacity test results.
3.2. Paw Edema Evaluation
Intradermal injection of carrageenan unsurprisingly led to paw edema (evidenced by increased volume) at 6 h post-injection comparing to animals injected with only saline, therefore validating this local inflammation model. Administration of Summer Savory phenolic extract led to a statistically significant reduction of edema formation (Figure 3). Comparison of these results with known anti-inflammatory and antioxidant compounds (indomethacin, trolox, and tempol) led to the conclusion that administration of a dose of 15 mg of phenolic acids/kg of this Summer Savory phenolic extract reduced the formation of paw edema at the same magnitude as the positive controls used.
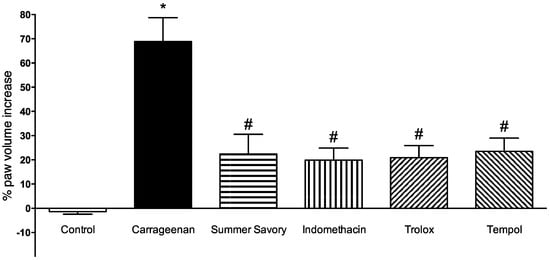
Figure 3.
Effect of Summer Savory extract administration on the paw edema volume induced by carrageenan. * p < 0.001 vs. Control; # p < 0.001 vs. Carrageenan. Control group (n = 8); Carrageenan Group (n = 8); Summer Savory (n = 8); Indomethacin (n = 8); Trolox (n = 8); Tempol (n = 8).
3.3. Macroscopic and Functional Signs of Colitis Injury
Analysis of results from both the Sham and Ethanol Groups showed that animals did not exhibit macroscopic signs of colonic lesion and exhibited also 0% of mortality. Colitis induction in the TNBS/EtOH Group produced statistically significant differences in: the decrease of colon length, injury extent (ulcer) increase, and diarrhea severity increase, associated also with a 40% mortality rate. For groups treated with the Summer Savory extract, all these results of colonic injury were significantly attenuated when compared to untreated animals with colitis (Table 4, Figure 4, Figure 5 and Figure 6).

Table 4.
Assessment of the colonic morphologic and functional characteristics.
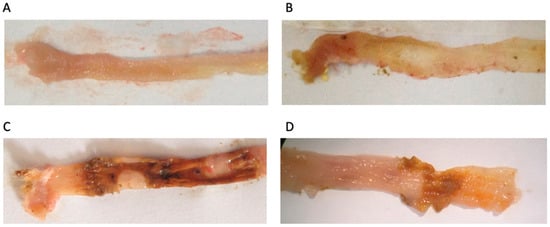
Figure 4.
Effect of Summer Savory extract administration on the macroscopic characteristic of colon. (A) Sham group (n = 6), (B) EtOH group (n = 6), (C) TNBS group (n = 10), (D) TNBS + Summer Savory extract (n = 10).
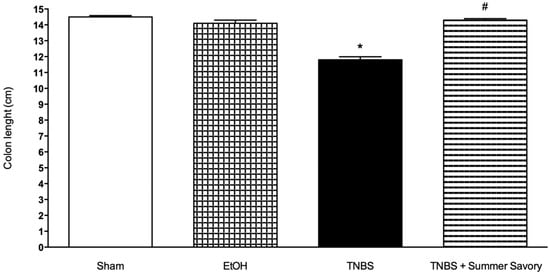
Figure 5.
Effect of Summer Savory extract administration on the length of the colon (cm). * p < 0.001 vs. Sham; # p < 0.001 vs. TNBS. Sham Group (n = 6); EtOH Group (n = 6); TNBS + EtOH Group (n = 10); TNBS + Summer Savory Group (n = 10).
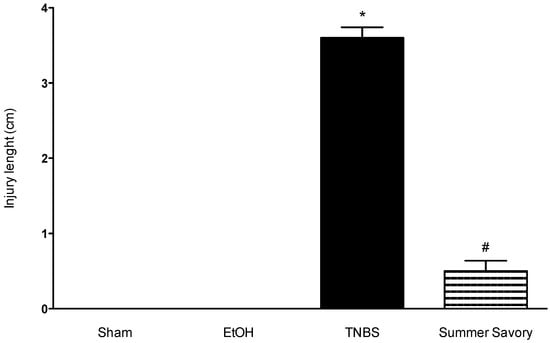
Figure 6.
Effect of Summer Savory extract administration on the colon injury extent (cm). * p < 0.001 vs. Sham; # p < 0.001 vs. TNBS. Sham Group (n = 6); EtOH Group (n = 6); TNBS + EtOH Group (n = 10); TNBS + Summer Savory Group (n = 10).
In addition, mortality was significantly reduced in the group subjected to Summer Savory administration (40% vs. 20%, for TNBS and Summer Savory groups, respectively), as depicted in the Kaplan–Meier analysis in Figure 7.
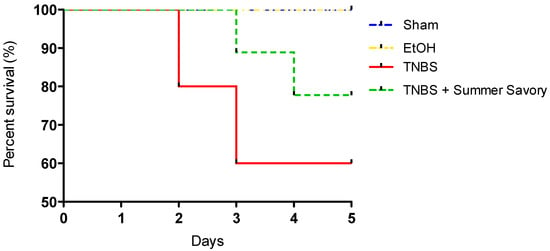
Figure 7.
Kaplan–Meier analysis of survival in the colitis model. Sham Group (n = 6); EtOH Group (n = 6); TNBS + EtOH Group (n = 10); TNBS + Summer Savory Group (n = 10).
3.4. Colon Injury: Histological Features and Inflammatory Markers
The histological analysis (Figure 8) revealed that, even though control samples exhibit colon with no lesions (normal colon), a mucosa with uniform thickness, normal crypt architecture, and no signs of inflammation (score 0), the colitis sample exhibits a strong ulceration where crypts loss and thinner mucosa can easily be identified with a severe neutrophils’ infiltration, consistent with a score of 3. The samples from animals treated with Summer Savory show erosion of the mucosa and small superficial ulcers disseminated across the length of the colon, with partial crypt destruction and only slight mononuclear cell infiltration, indicating a lower damage score of 1.
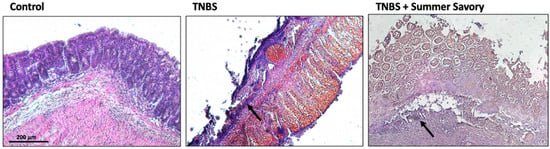
Figure 8.
Histological analysis revealed that specimens from TNBS colitis group exhibited very marked ulceration, thin mucosa with destruction of crypts, and severely increased infiltration of neutrophils (score 3, black-arrow), while specimens from animals treated with Summer Savory extract exhibited colon with erosion of the mucosa and small superficial ulcers disseminated across the length of the colon, with crypt loss and slight mononuclear cell infiltration (score 1, black-arrow). Sham Group (n = 6); EtOH Group (n = 6); TNBS + EtOH Group (n = 10); TNBS + Summer Savory Group (n = 10). Original magnification ×100. Scale bar equals 200 µm.
As shown in Figure 9 and Figure 10, colitis treatment led to a marked increase of COX-2 and iNOS expression (1.96-fold, p < 0.05 and 1.71-fold, p < 0.01, respectively) across the remaining crypts highlighted by brown color when compared with control. Curiously, samples treated with Summer Savory show no expression of COX-2 or iNOS (1.04- and 1.26-fold, p < 0.05 vs. colitis, respectively) indicative of a reduced inflammatory status, further supporting the histological observations.
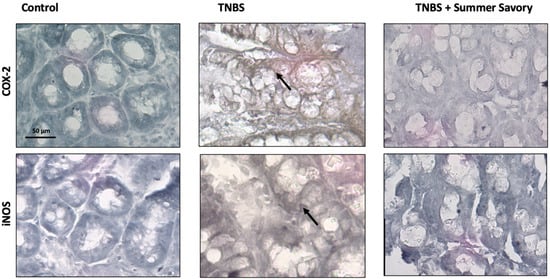
Figure 9.
Evaluation of COX-2 and iNOS expression in the colitis model revealed that specimens from the TNBS colitis group exhibited severe expression of COX-2 and iNOS (brown staining, arrow), while specimens from the treated animals with Savory showed no expression of COX-2 and iNOS. Sham Group (n = 6); EtOH Group (n = 6); TNBS + EtOH Group (n = 10); TNBS + Summer Savory Group (n = 10). Original magnification ×100. Scale bar equals 50 µm.
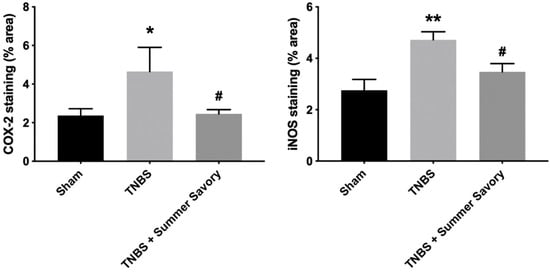
Figure 10.
Evaluation of COX-2 and iNOS activation in the TNBS colitis model reveals that specimens from TNBS colitis group exhibits severe expression of COX-2 and iNOS (brown staining, arrow), while specimens from the animals treated with Summer Savory extract exhibited no expression of COX-2 and iNOS. Sham Group (n = 6); EtOH Group (n = 6); TNBS + EtOH Group (n = 10); TNBS + Summer Savory Group (n = 10). Original magnification ×100. Scale bar equals 50 µm.
4. Discussion
Even though Summer Savory (Satureja hortensis) has been listed for many years as a medicinal herb and is a well-known herb in the food and culinary industry, scientific-based literature evaluating the potential beneficial effects of Summer Savory in health is surprisingly scarce, especially when compared to other aromatic herbs and even more obvious when particularizing for the study of the aerial parts as opposed to considerably more information regarding essential oil properties [1]. However, some studies, mainly from Mediterranean region countries in which Summer Savory is used commonly, have attributed antimicrobial, anti-inflammatory, antioxidant, antidiabetic, and antitumor effects to both seed (essential oil) and aerial parts extract of Summer Savory [47,48].
One of the few studies focusing on an alcoholic extract of the aerial parts of Summer Savory was a study from Boroja et al. in 2018 that evaluated the beneficial effects of administration of a methanolic extract of the aerial parts of Summer Savory in a cisplatin-induced model of oxidative kidney, liver, and testicular [18]. Injury was induced by injection of cisplatin (7.5 mg/kg, sigle dose i.p.) to wistar rats and Summer Savory extract was administered orally (doses of 50, 100, and 200 mg/kg/day for 10 days). Extract’s administration ameliorated tissue morphology, impaired the rise in serum level of liver, renal and testes injury markers and reduced tissue oxidative stress with reduction of apoptosis evidenced by an increased Bcl-2/Bax ratio. In this study, and similarly to the situation described in our study, the principle constituent of the phenolic extract identified was rosmarinic acid.
In fact, several studies report high rosmarinic acid content in aromatic plants from the Lamiaceae family [49,50,51], and, although Satureja hortensis is not one of family members with higher concentration of rosmarinic acid, it normally has concentrations higher than plants, such as Rosmarinus officinalis and Lavandula angustifolia [52]. When compared to our study with spearmint extract, results from our present study shows that not only the concentration of rosmarinic acid is equivalent (1.0 mg/mL vs. 1.4 mg/mL, for Summer Savory and spearmint extracts, respectively) but also the antioxidant capacities, as well [15]. Given that antioxidant capacity is usually appointed as a main responsible for an alti-inflammatory activity, one of our previous work aimed to prove that anti-inflammatory activity is often independent of the anti-oxidant effect. In a study of the anti-inflammatory effect of an extract of Rosmarinus officinalis and of rosmarinic acid, our team proved that the anti-inflammatory effect of both was equivalent for the same dose of rosmarinic acid, although the anti-oxidant activity was much higher for the extract [43]. Therefore, in the present work, characterization of the anti-oxidant effect of the extract was important to show anti-oxidant equivalence with other aromatic plants [14,15], but evaluation of its specific effect on inflammatory models was necessary to assess the anti-inflammatory activity.
Therefore, in the present work, in order to do a preliminary evaluation of the anti-inflammatory effect of the Summer Savory extract, we evaluated a single-dose effect of Summer Savory oral administration in an acute model of paw edema. Measurement of paw volumes after edema induction by carrageenan revealed that administration of Summer Savory extract impaired the increase in paw volume by 46.5% when compared to animals administered with vehicle. Additionally, the magnitude of that effect was not statistically different when compared to positive control groups administered with known antioxidant and anti-inflammatory properties (49% for indomethacin, 48% for Trolox, and 45.5% for tempol), indicating a potential benefit of this extract in inflammatory-mediated diseases.
The analgesic and anti-inflammatory effects of Summer Savory seed essential oil and hydroalcoholic and polyphenolic extracts was evaluated by Hajhashemi and colleagues [9]. Anti-nociceptive activity was evaluated in the mouse models of acetic acid and formalin-induced pain. Appraisement of anti-inflammatory activity was performed in the carrageenan-induced rat paw edema model was. Animals were pretreated with 50, 100, or 200 mg/kg, i.p. and 100 or 200 µL/kg, p.o, in the anti-nociceptive and anti-inflammatory models, respectively. Administration of hydroalcoholic and polyphenolic extracts or essential oil significantly decreased acetic acid-induced abdominal twitches, while hydroalcoholic extract additionally decreased pain responses in both early and late phases of the formalin test. In this test, the polyphenolic extract and essential oil only demonstrated beneficial effect in the late phase of the formalin test, while all three fractions reduced paw edema in the carrageenan model. However, even though one of the fractions tested was a polyphenolic extract, it was a seed extract which corresponds to a very different chemical constitution from a leave extract.
After the anti-inflammatory effect of Summer Savory was confirmed in an acute local model of inflammation, we aimed to access the positive effect of the extract in an experimental colitis mouse model. Administration of Summer Savory extract to animals with TNBS-induced colitis drove to a decrease of multiple markers of colon injury and inflammation: impairment of colon length decrease, reduction of injury extent, decrease of diarrhea severity, and reduction of mortality rate. Additionally, both macroscopical and histological signs of colon inflammation were decreased in animals administered with Summer Savory extract compared to colitis animals.
Surprisingly, even though no preclinical animal study was published until now that evaluated the beneficial effects of Summer Savory in experimental IBD, there is one publication reporting the results of a small clinical trial studying the administration of a commercial preparation of Summer Savory in Ulcerative Colitis patients [53]. This trial was a randomized, double-blind, placebo-controlled study that assessed the clinical benefit of commercial tablets of dried leaves of Summer Savory (500 mg daily) in Ulcerative Colitis. After 4 months, 85% (12/14) of patients that received supplementation with Summer Savory extract were in complete remission when compared to 46.2% (6/13) of patients on placebo. The flare up frequency was also statistically lower for patients treated with Summer Savory extract. This trial was very limited in terms of dimension, given that included only 27 patients total; nevertheless, it suggests a beneficial effect of Summer Savory in inflammatory bowel disease patients.
In our study, a relatively low dose phenolic extract of 15 mg/kg was used, which was derived from previous studies from our group that have been assessing the positive effects of different phenolic extracts in multiple models of inflammation with this dose of phenolic acids having generated matching results and is also within the dose-range of clinical translation [44,45,46,54].
Lately, we have been evaluating the protective effect of phenolic extracts originating from aromatic herbs and have demonstrated a beneficial effect of the administration of spearmint and pennyroyal phenolic extracts in this animal model of TNBS-induced Ulcerative Colitis [14,15]. Curiously, we demonstrated that rosmarinic acid was the principal compound in both of these experiments, like what we observed in the present study, further suggesting a relevant role of this phenolic acid in the beneficial effect. In addition, in the past, we have compared the beneficial role of an extract of rosemary and isolated rosmarinic acid in a local model of inflammation and established that rosmarinic acid was the principal responsible for the anti-inflammatory effect (Rocha et al., 2015). We have also identified in that study an important function of rosmarinic acid in the modulation of several critical pathways of inflammation in liver and lung inflammation models, in which administration of rosmarinic acid led to reduction of nuclear factor-kappa B (NF-kB) activation, as well as inhibition of the phosphatidylinositol 3-kinase (PI3k)/protein kinase B (Akt) and Glycogen synthase kinase 3beta (GSK-3beta) pathways.
In fact, Jin and colleagues have published their work in 2017 where they studied the effect of rosmarinic acid in dextran sulfate sodium (DSS)-induced experimental colitis and possible underlying mechanisms [55]. In this experiment, rosmarinic acid (30 or 60 mg/kg/day, p.o.) significantly reduced the severity of colitis, associated with decrease of inflammatory-related cytokines production (interleukins IL-6, IL-1β, and IL-22) and protein levels of COX-2 and iNOS. Furthermore, rosmarinic acid also inhibited NF-kB and signal transducer and activator of transcription 3 (STAT3) activation, with subsequent reduction of the activity of pro-survival genes.
Similarly, in our present study, we found that experimental colitis led to the induction of both iNOS and COX-2 expression and that administration of Summer Savory extract was able to impair that increase in expression, leading to statistically similar expression levels compared to control animal.
The effects of Summer Savory in COX-2 and iNOS expression were also characterized by Farzaneh and colleagues, who reported that Satureja hortensis extracts inhibited lipopolysaccharide (LPS)-induced inflammatory responses by J774.1 macrophages [56]. This study demonstrated that dichloromethane and hexane extracts od Summer Savory reduced nitric oxide (NO) production with more efficacy than other extracts, correlated with a decreased gene expression of iNOS, COX-2, IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α). Incubation of macrophages with the extracts also reduced IL-6 and IL-1β production by macrophages and reduced the expression of intercellular adhesion molecule 1 (ICAM-1) [56].
The beneficial effect of a Summer Savory aqueous extract (250 mg/kg) as anti-inflammatory was evaluated in a rabbit model of rhinosinusitis by measurement of nitric oxide (NO) metabolites and histological changes [57]. The results of this study demonstrated that both activity of NOS and concentration of NO metabolites were significantly reduced by topical administration of Summer Savory extract. Additionally, this study showed a reduction of edema and inflammation in the treated group when comparing to control [57].
Knowing that one of the main long-term consequences of IBD patients is the well-known increase of the risk for development of CRC [23,24,25] and its important link to chronic inflammation [24,26,27], it highlights even more the beneficial effects reported by our work. Research has actually highlighted the continuous effect of chronic inflammation and its association with the severity, extent and duration of IBD as the principal link between IBD and the elevated CRC risk [58].
Some studies have reported antiproliferative effects of S. hortensis extracts on leukemic cell lines [59]; however, we have previously evaluated the effect of Summer Savory extract in an in vitro based model with human colon adenocarcinoma HT-29 cells and concluded that this extract does not have any effect of HT-29 cell proliferation, although it partially inhibited cell invasion (unpublished data). Additionally, we further evaluated the effect on matrix metallopeptidases (MMP) activity, with no difference being observed when compared to controls (unpublished data), and, although a study has previously shown that MMP-2 and MMP-9 activity was inhibited by S. hortensis, those assays were only performed with the essential oil [60].
Rather than the more traditional mechanisms for cancer initiation and progression, inflammatory processes seem to be the main regulation of colitis-associated cancer, an imbalance in intestinal microbiota, and a crosstalk between various signaling pathways [61]; therefore, a pleiotropic approach seems to present itself as better pharmacological tool, not only targeting inflammation associated with colitis but also associated with the mechanism controlling cancer development and progression.
Several studies have already identified that extracts of plants known to possess high concentrations of rosmarinic acid exhibit anticancer effects in different in vitro and in vivo models of various types of cancer [62,63,64].
One study evaluated rosmarinic acid administration in a rat model of 1,2 dimethylhydrazine (DMH)-induced CRC [65]. Rosmarinic acid was administered (5 mg/kg) through the whole period of CRC induction and exhibited tumor reduction, reduction of stress oxidative markers, reduction mucosal bacterial enzymes activity, regulation of xenobiotic metabolizing enzymes, and up-regulation of apoptotic factors. In another study, rosmarinic acid antagonized activator protein-1-(AP-1)dependent activation of COX-2 expression in HT-29 colon cancer cells [66].
Given the well-known relevance of these two crucial inflammatory enzymes, the impairment of COX-2 and iNOS expression in our work confirmed the beneficial effect of Summer Savory not only on IBD pathogenesis but also on disease progression and the possible link to cancer proliferation. Their roles in angiogenesis, apoptosis, and metastatic processes and resistance to chemotherapy/radiation are well understood and of significant importance for IBD and CRC patients [67,68,69,70,71,72]. In fact, the role of COX-2 and iNOS in intestinal inflammation has pointed them out as great targets for chemoprevention of colon cancer [72].
5. Conclusions
For the first time, to our knowledge, our results show a beneficial effect of Summer Savory phenolic extract on the amelioration of experimental IBD, with reduction of its severity and mortality, as well as reduction of several injury markers and inflammatory mediators, including COX-2 and iNOS, known to have critical roles in intestinal inflammation and progression to CRC. Taking into consideration the role of inflammatory processes in the link between Ulcerative Colitis and progression to CRC, these results suggest that the beneficial effects evidenced by Summer Savory extract might provide an interesting pharmacological tool not only in the management of inflammation associated to IBD but also in the management of the impaired secondary inflammatory mechanisms related with CRC progression.
Author Contributions
Conceptualization, J.R., B.S., and M.-E.F.; Methodology, formal analysis, investigation and data curation, J.R., R.L., R.D., M.G., M.P.D., A.F., B.S., and M.-E.F.; Writing—original draft preparation, J.R., B.S., and M.-E.F.; Writing—review and editing, J.R., B.S., and M.-E.F.; Supervision, J.R., B.S., and M.-E.F.; Funding acquisition, J.R., R.L., R.D., M.G., M.P.D., A.F., B.S., and M.-E.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support from FCT—Fundação para a Ciência e Tecnologia within the R & D Units Project Scope: UIDB/04077/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fierascu, I.; Dinu-Pirvu, C.E.; Fierascu, R.; Velescu, B.S.; Anuta, V.; Ortan, A.; Jinga, V. Phytochemical Profile and Biological Activities of Satureja hortensis L.: A Review of the Last Decade. Molecules 2018, 23, 2458. [Google Scholar] [CrossRef]
- Güllüce, M.; Sökmen, M.; Daferera, D.; Aǧar, G.; Özkan, H.; Kartal, N.; Polissiou, M.; Sökmen, A.; Şahi̇n, F. In Vitro Antibacterial, Antifungal, and Antioxidant Activities of the Essential Oil and Methanol Extracts of Herbal Parts and Callus Cultures of Satureja hortensis L. J. Agric. Food Chem. 2003, 51, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Lee, S.-C.; Huh, M.-J.; Seo, S.-M.; Kwon, J.H.; Park, I.-K. Fumigant Antifungal Activity via Reactive Oxygen Species of Thymus vulgaris and Satureja hortensis Essential Oils and Constituents Against Raffaelea quercus-mongolicae and Rhizoctonia solani. Biomolecules 2019, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Şahin, F.; Karaman, I.; Güllüce, M.; Öğütçü, H.; Şengül, M.; Adıgüzel, A.; Öztürk, S.; Kotán, R. Evaluation of antimicrobial activities of Satureja hortensis L. J. Ethnopharmacol. 2003, 87, 61–65. [Google Scholar] [CrossRef]
- Sharifi, A.; Mohammadzadeh, A.; Salehi, T.Z.; Mahmoodi, P. Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J. Appl. Microbiol. 2018, 124, 379–388. [Google Scholar] [CrossRef]
- Gomes, F.; Dias, M.I.; Lima, Â.; Barros, L.; Rodrigues, M.E.; Ferreira, I.; Henriques, M. Satureja montana L. and Origanum majorana L. Decoctions: Antimicrobial Activity, Mode of Action and Phenolic Characterization. Antibiotics 2020, 9, 294. [Google Scholar] [CrossRef]
- Tepe, B.; Cilkiz, M. A pharmacological and phytochemical overview onSatureja. Pharm. Biol. 2015, 54, 375–412. [Google Scholar] [CrossRef]
- Popovici, R.A.; Vaduva, D.; Pinzaru, I.; Dehelean, C.A.; Farcas, C.G.; Coricovac, D.; Danciu, C.; Popescu, I.; Alexa, E.; Lazureanu, V.; et al. A comparative study on the biological activity of essential oil and total hydro-alcoholic extract of Satureja hortensis L. Exp. Ther. Med. 2019, 18, 932–942. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Sadraei, H.; Ghannadi, A.R.; Mohseni, M. Antispasmodic and anti-diarrhoeal effect of Satureja hortensis L. essential oil. J. Ethnopharmacol. 2000, 71, 187–192. [Google Scholar] [CrossRef]
- Zeidán-Chuliá, F.; Keskin, M.; Könönen, E.; Uitto, V.-J.; Söderling, E.; Moreira, J.C.F.; Gursoy, U.K. Antibacterial and Antigelatinolytic Effects of Satureja hortensis L. Essential Oil on Epithelial Cells Exposed to Fusobacterium nucleatum. J. Med. Food 2015, 18, 503–506. [Google Scholar] [CrossRef]
- SSharifzadeh, A.; Khosravi, A.R.; Ahmadian, S. Chemical composition and antifungal activity of Satureja hortensis L. essentiall oil against planktonic and biofilm growth of Candida albicans isolates from buccal lesions of HIV+ individuals. Microb. Pathog. 2016, 96, 1–9. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G. New Tricks for Old Guys: Recent Developments in the Chemistry, Biochemistry, Applications and Exploitation of Selected Species from the Lamiaceae Family. In Chemistry and Biodiversity; Wiley-VCH Verlag: Weinheim, Germany, 2020. [Google Scholar] [CrossRef]
- Delgado, A.M.; Issaoui, M.; Chammem, N. Analysis of main and healthy Phenolic compounds in foods. J. AOAC Int. 2019, 102, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Direito, R.; Lima, A.; Mota, J.; Gonçalves, M.; Duarte, M.P.; Solas, J.; Peniche, B.F.; Fernandes, A.; Pinto, R.; et al. Reduction of inflammation and colon injury by a Pennyroyal phenolic extract in experimental inflammatory bowel disease in mice. Biomed. Pharmacother. 2019, 118. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J.; Lima, A.I.G.; Gonçalves, M.; Duarte, M.P.; Mateus, V.; Sousa, C.; Fernandes, A.; Pinto, R.M.A.; Ferreira, R.B.; et al. Reduction of Inflammation and Colon Injury by a Spearmint Phenolic Extract in Experimental Bowel Disease in Mice. Medicines 2019, 6, 65. [Google Scholar] [CrossRef]
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.P.; Troganis, A.; Boskou, D. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J. Agric. Food Chem. 2002, 50, 5294–5299. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Choulitoudi, E.; Bimpilas, A.; Mitropoulou, G.; Kourkoutas, Y.; Oreopoulou, V. Exploitation of the biological potential of Satureja thymbra essential oil and distillation by-products. J. Appl. Res. Med. Aromat. Plants 2017, 4, 12–20. [Google Scholar] [CrossRef]
- Boroja, T.; Katanić, J.; Rosić, G.; Selaković, D.; Joksimović, J.; Mišić, D.; Stanković, V.; Jovičić, N.; Mihailović, V. Summer savory (Satureja hortensis L.) extract: Phytochemical profile and modulation of cisplatin-induced liver, renal and testicular toxicity. Food Chem. Toxicol. 2018, 118, 252–263. [Google Scholar] [CrossRef]
- Mašković, P.; Veličković, V.; Mitić, M.N.; Đurović, S.; Zeković, Z.; Radojković, M.; Cvetanović, A.; Švarc-Gajić, J.; Vujić, J. Summer savory extracts prepared by novel extraction methods resulted in enhanced biological activity. Ind. Crop. Prod. 2017, 109, 875–881. [Google Scholar] [CrossRef]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef]
- Corridoni, D.; Arseneau, K.O.; Cominelli, F. Inflammatory bowel disease. Immunol. Lett. 2014, 161, 231–235. [Google Scholar] [CrossRef]
- Salaritabar, A.; Darvishi, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.M.; Fitzpatrick, L.R.; Bishayee, A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gastroenterol. 2017, 23, 5097. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Shah, S.C. Diagnosis and management of inflammatory bowel disease-associated neoplasia: Considera. In Therapeutic Advances in Gastroenterology; SAGE Publications Ltd.: Thousand Oaks, CA, USA, 2020. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016, 22, 4794. [Google Scholar] [CrossRef] [PubMed]
- McDowell, C.; Haseeb, M. Bowel, Inflammatory Disease (IBD). StatPearls 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470312/ (accessed on 26 November 2020).
- Nasef, N.A.; Mehta, S. Role of inflammation in pathophysiology of colonic disease: An update. Int. J. Mol. Sci. 2020, 21, 4748. [Google Scholar] [CrossRef] [PubMed]
- Caprara, G.; Allavena, P.; Erreni, M. Intestinal Macrophages at the Crossroad between Diet, Inflammation, and Cancer. Int. J. Mol. Sci. 2020, 21, 4825. [Google Scholar] [CrossRef]
- Romano, M.; De Francesco, F.; Zarantonello, L.; Ruffolo, C.; Ferraro, G.A.; Zanus, G.; Giordano, A.; Bassi, N.; Cillo, U. From Inflammation to Cancer in Inflammatory Bowel Disease: Molecular Perspectives. Anticancer Res. 2016, 36, 1447–1460. [Google Scholar]
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 345, 235–241. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef]
- Shawki, S.; Ashburn, J.; Signs, S.A.; Huang, E. Colon Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 269–287. [Google Scholar] [CrossRef]
- Donovan, M.G.; Selmin, O.I.; Doetschman, T.C.; Romagnolo, D.F. Mediterranean Diet: Prevention of Colorectal Cancer. Front. Nutr. 2017, 4, 59. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Kapinova, A.; Kubatka, P.; Golubnitschaja, O.; Kello, M.; Zubor, P.; Solar, P.; Pec, M. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Koşar, M.; Göger, F.; Can Başer, K.H. In Vitro Antioxidant Properties and Phenolic Composition of Salvia virgata Jacq. from Turkey. J. Agric. Food Chem. 2008, 56, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Ramful, D.; Bahorun, T.; Bourdon, E.; Tarnus, E.; Aruoma, O.I. Bioactive phenolics and antioxidant propensity of flavedo extracts of Mauritian citrus fruits: Potential prophylactic ingredients for functional foods application. Toxicology 2010, 278, 75–87. [Google Scholar] [CrossRef]
- Miceli, N.; Trovato, A.; Dugo, P.; Cacciola, F.; Donato, P.A.E.; Marino, A.; Bellinghieri, V.; La Barbera, T.M.; Güvenç, A.; Taviano, M.F. Comparative Analysis of Flavonoid Profile, Antioxidant and Antimicrobial Activity of the Berries of Juniperus communis L. var. communis and Juniperus communis L. var. saxatilis Pall. from Turkey. J. Agric. Food Chem. 2009, 57, 6570–6577. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidant activity of Centaurium erythraea infusion evidenced by its superoxide radical scavenging and xanthine oxidase inhibitory activity. J. Agric. Food Chem. 2001, 49, 3476–3479. [Google Scholar] [CrossRef]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-inflammatory effect of rosmarinic acid and an extract of rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 398–413. [Google Scholar] [CrossRef]
- Direito, R.; Lima, A.; Rocha, J.; Ferreira, R.B.; Mota, J.; Rebelo, P.; Fernandes, A.; Pinto, R.M.A.; Alves, P.C.; Bronze, R.; et al. Dyospiros kaki phenolics inhibit colitis and colon cancer cell proliferation, but not gelatinase activities. J. Nutr. Biochem. 2017, 46, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Figueira, M.-E.; Camara, M.B.; Direito, R.; Rocha, J.; Serra, A.-T.; Duarte, C.M.; Fernandes, A.; Freitas, M.; Fernandes, E.; Marques, M.C.; et al. Chemical characterization of a red raspberry fruit extract and evaluation of its pharmacological effects in experimental models of acute inflammation and collagen-induced arthritis. Food Funct. 2014, 5, 3241–3251. [Google Scholar] [CrossRef] [PubMed]
- Figueira, M.-E.; Oliveira, M.; Direito, R.; Rocha, J.; Alves, P.; Serra, A.-T.; Duarte, C.; Bronze, R.; Fernandes, A.; Brites, D.; et al. Protective effects of a blueberry extract in acute inflammation and collagen-induced arthritis in the rat. Biomed. Pharmacother. 2016, 83, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Abou Baker, D.H.; Al-Moghazy, M.; ElSayed, A.A.A. The in vitro cytotoxicity, antioxidant and antibacterial potential of Satureja hortensis L. essential oil cultivated in Egypt. Bioorg. Chem. 2020, 95. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M.; Sohraby, M. Summer savory: From the selection of traditional applications to the novel effect in relief, prevention, and treatment of a number of serious illnesses such as diabetes, cardiovascular disease, Alzheimer’s disease, and cancer. J. Tradit. Complement. Med. 2014, 4, 140–144. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004, 87, 307–311. [Google Scholar] [CrossRef]
- Fatiha, B.; Didier, H.; Naima, G.; Khodir, M.; Martin, K.; Léocadie, K.; Caroline, S.; Mohamed, C.; Pierre, D. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae). Ind. Crop. Prod. 2015, 74, 722–730. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: Thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008, 108, 1039–1053. [Google Scholar] [CrossRef]
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.R.; Hamedani, M.P. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn. Mag. 2012, 8, 37–41. [Google Scholar] [CrossRef]
- Rastegarpa, M.; Omidzohour, N.; Vahedi, H.; Malekzadeh, R.; Hashemian, F.; Safarnavad, T.; Abdollahi, M. Management of human ulcerative colitis by saturexTM: A randomized controlled trial. Int. J. Pharmacol. 2011, 7, 516–521. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J.; Serra, A.T.; Fernandes, A.; Freitas, M.; Fernandes, E.; Pinto, R.M.A.; Bronze, M.D.R.; Sepodes, B.; Figueira, M.-E. Anti-inflammatory Effects of Persimmon (Diospyros kaki L.) in Experimental Rodent Rheumatoid Arthritis. J. Diet. Suppl. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.-R.; Chung, K.-S.; Cheon, S.-Y.; Lee, M.; Hwang, S.; Hwang, S.N.; Rhee, K.-J.; An, H.-J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, Z.; Kalantar, K.; Iraji, A.; Amirghofran, Z. Inhibition of LPS-induced inflammatory responses by Satureja hortensis extracts in J774.1 macrophages. J. Immunoass Immunochem. 2018, 39, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Uslu, C.; Karasen, R.M.; Sahin, F.; Taysi, S.; Akcay, F. Effects of aqueous extracts of Satureja hortensis L. on rhinosinusitis treatment in rabbit. J. Ethnopharmacol. 2003, 88, 225–228. [Google Scholar] [CrossRef]
- Kraus, S.; Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 2009, 9, 405–410. [Google Scholar] [CrossRef]
- Esmaeilbeig, M.; Kouhpayeh, S.A.; Amirghofran, Z. An investigation of the growth inhibitory capacity of several medicinal plants from Iran on tumor cell lines. Int. J. Cancer Manag. 2015, 8. [Google Scholar] [CrossRef]
- Zeidán-Chuliá, F.; De Oliveira, B.-H.N.; Gursoy, M.; Könönen, E.; Moreira, J.C.F.; Gursoy, U.K.; Uitto, V.-J. MMP-REDOX/NO Interplay in Periodontitis and Its Inhibition with Satureja hortensis L. Essential Oil. Chem. Biodivers. 2013, 10, 507–523. [Google Scholar] [CrossRef]
- Jurjus, A.; Eid, A.; Al Kattar, S.; Zeenny, M.N.; Gerges-Geagea, A.; Haydar, H.; Hilal, A.; Oueidat, D.; Matar, M.; Tawilah, J.F.; et al. Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: The links. BBA Clin. 2016, 5, 16–24. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Salvia fruticosa, salvia officinalis, and rosmarinic acid induce apoptosis and inhibit proliferation of human colorectal cell lines: The role in MAPK/ERK pathway. Nutr. Cancer 2009, 61, 564–571. [Google Scholar] [CrossRef]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Gunasekaran, S.; Jesudoss, V.A.S.; Namasivayam, N. The effect of rosmarinic acid on 1,2-dimethylhydrazine induced colon carcinogenesis. Exp. Toxicol. Pathol. 2013, 65, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Scheckel, K.A.; Degner, S.C.; Romagnolo, D.F. Rosmarinic acid antagonizes activator protein-1-dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J. Nutr. 2008, 138, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.K.; Wilson, K.T. Nitric oxide in inflammatory bowel disease. Inflamm. Bowel Dis. 2003, 9, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Hellström, P.M.; Fagerhol, M.K.; Weitzberg, E.; Roseth, A.G. Technology Insight: Calprotectin, lactoferrin and nitric oxide as novel markers of inflammatory bowel disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef]
- Azer, S.A. Overview of molecular pathways in inflammatory bowel disease associated with colorectal cancer development. Eur. J. Gastroenterol. Hepatol. 2013, 25, 271–281. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Xie, Z.; Zhou, S.; Li, Y.; Zhou, Y.; Sun, M.-Y. Nitric oxide (NO) and NO synthases (NOS)-based targeted therapy for colon cancer. Cancers 2020, 12, 1881. [Google Scholar] [CrossRef]
- Watanabe, K.; Kawamori, T.; Nakatsugi, S.; Wakabayashi, K. COX-2 and iNOS, good targets for chemoprevention of colon cancer. BioFactors 2000, 12, 129–133. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).