Abstract
The purpose of this study was to verify the effect of deep-sea water thalassotherapy (DSWTT) on recovery from fatigue and muscle damage. The same exercise program is conducted in general underwater and deep-sea water to confirm the characteristics of deep-sea water through fatigue recovery and muscle damage enzymes. A total of 30 male college students were studied, including 10 belonging to the control group (CG), 10 in the water exercise group (WEG), and 10 in the deep-sea water exercise group (DSWEG). The DSWTT treatment consists of three components—preheating, treatment, and cooling—and the DSWTT program stretches and massages the entire upper body, lower body, back, and the entire body for a total of 25 min in a deep-sea tank. After the DSWTT program, blood tests were conducted to confirm the level of fatigue-related parameters and muscle damage enzymes. Fatigue-related parameters including glucose, lactate, ammonia, and lactate dehydrogenase (LDH), and the levels of muscle damage enzymes such as creatinine kinase (CK) and aspartate aminotransferase (AST) were measured. The results revealed that fatigue had a primary effect (p < 0.001) and exhibited strongly significant interaction (p < 0.001) with lactate, ammonia, and LDH levels, whereas the glucose level remained unchanged. The post hoc results showed a significant decrease in these parameters among DSWEG compared to CG and WEG (p < 0.01). Muscle damage enzymes showed a main effect (p < 0.001) and significant interaction (p < 0.001) with CK and AST (p < 0.001). The post hoc results showed a significant decrease in DSWEG compared with CG and WEG (p < 0.01). In conclusion, the DSWTT program applied to this study showed significant effects on muscle fatigue and muscle damage recovery. When the DSWTT program is applied in hot springs, it can have a positive effect on muscle fatigue and muscle damage recovery and can contribute to improving national health and quality of life. Further studies are needed to investigate DSWTT programs with various research subjects at different program temperatures, exercise times, and frequencies of treatment and exercise.
1. Introduction
In recent years, both men and women have become interested in effective physical activity and healing while pursuing a healthy life. As a result of the increase in interest, studies on physical recovery programs such as fatigue recovery and muscle damage due to various sports and physical activities are actively being conducted [1,2]. Fatigue is a decline in mental and physical functions that occur accompanied by continuous or repeated mental and physical work and is an indicator of physiological stress, defensive response, or pathological precursor [3]. Therefore, fatigue and muscle damage caused by physical and mental activity are common early symptoms of many diseases [4]. In addition, fatigue may degrade the quality of life of daily life [5], cause physiological homeostasis disorder, and develop into a disease or a chronic disease, which may result in decreased physical and mental functions [6].
Fatigue is triggered by a peripheral signal from the brain, which inhibits the motor system, and thereby the mobilization of exercise units, and it is controlled by accumulation of metabolites or energy depletion [7]. The accumulation of these fatigue substances is important to prevent exercise-related injuries caused by fatigue, depending on the intensity, time, shape, and environment [8,9].
Methods to promote recovery from fatigue include static measures such as rest or sleep and dynamic elements such as bathing, massage, and gymnastics. Among the bathing methods performed underwater, the recovery in hot water (37~38 °C) is known to activate blood circulation by reducing the imbalance of the upper and lower limbs of the body [10]. It can also increase muscle fatigue and resilience to muscle damage by not only adding pressure to the muscles but also carrying heat to the muscle tissue [11]. Ice fomentation at low temperature (15 °C) is known to relieve excitement of the central nervous system and promote the removal of metabolites [12,13]. The detailed mechanism underlying recovery from fatigue using water therapy has yet to be reported.
However, regular exercise has a positive effect on prevention and treatment of lifestyle diseases by decreasing the risk factors of cardiovascular disease and metabolic syndrome, but it also has a negative effect depending on exercise intensity. In particular, high-intensity exercise causes muscle damage and damage to human tissues due to upper respiratory tract infection, inhibition of immune system function, increased lipid peroxidation, and reduction in antioxidant enzymes [14].
The exercise-induced muscle damage is caused by one-time or long-term muscle cell and tissue damage, which can be indirectly predicted by the blood concentration of enzymes such as creatinine kinase (CK) and lactate dehydrogenase (LDH) released from muscle tissue following exercise [15,16]. According to Yeom’s [17] study, treadmill exercise was performed until the time when the exercise intensity consumed energy of 40, 60, and 80% to 200, 400, and 600 kcal of VO2max, and all creatine phosphokinase(CPK) was significantly higher; the CPK concentration changed according to the exercise intensity and momentum.
These changes in CPK serve as biochemical variables of exercise and physical strength. They are used as indirect indicators of muscle damage and inflammation such as cell membrane destruction and tissue necrosis in long-term exercise or high-intensity exercise. The increase in LDH and CPK levels in the blood is attributed to damaged muscle fibers due to excessive exercise, resulting in muscle pain and muscle fatigue, which can lead to declining performance and exercise-related injuries.
The underwater treatment program reduces the load on the body weight and leads to greater joint movement than the ground treatment program [18], which is predicted to improve the range of motion (ROM) quickly and effectively [19] by promoting recovering from muscle damage after vigorous exercise. In recent years, the application of thalassotherapy (TT; derivation of Greek Thalassa, which means the sea) using deep-sea water (DSW), characterized by low temperature stability, eutrophic, clean, and anti-aging properties [20], has become a hot topic in the field of water therapy.
The progress of the study, which restores fatigue and muscle damage quickly after physical activity, can be a great help in restoring physical condition and improving exercise ability. The underwater treatment program increases blood flow to the muscle, which leads to recovery from muscle tension [21] and facilitates the elimination of metabolic byproducts by improving venous reflux rate [22]. However, most studies have shown that there are treatments in the water, but the treatments using hot spring water have not been reported yet [23], and the effect of DSW-based fatigue and muscle injury recovery programs on exercise physiology has yet to be demonstrated.
Therefore, this study is to verify the effect on fatigue and muscle damage recovery through an exercise program based on deep-sea water thalassotherapy (DSWTT) and to confirm the characteristics of DSW.
2. Materials and Methods
2.1. Subjects
The subjects of this study were a selected group of Korean male college students in their 20s. Subjects with a history of neurosurgical and orthopedic issues within 6 months of the measurement date were excluded from the study if they were associated with musculoskeletal problems interfering with the water treatment and DSWTT programs. In addition, participants were randomly selected from numbers 1 to 30, and participants were randomly assigned to each group, and the experiment was conducted in compliance with the ethical principles of the Helsinki Declaration. During the DSWTT program, the participants were photographed, and the photographs taken were agreed to be used in the thesis. A total of 30 participants were classified equally as 10 as control group (CG), 10 as water exercise group (WEG), and 10 as deep-sea water exercise group (DSWEG). The general physical characteristics of the subjects are shown in Table 1.

Table 1.
General characteristics of subjects.
2.2. DSWTT Treatment Program
The water therapy and DSWTT programs administered in this study are presented in Table 2. After fatigue induced by exercise program progression, the subjects were asked to identify the effects of DSWTT program on fatigue recovery and muscle damage enzymes.

Table 2.
Thalassotherapy using deep-sea water.
The subjects in WEG actively participated in a water treatment program involving a tap-water bath (standard 3 × 3 × 1.5 m, horizontal × vertical × height) maintained at 34 ± 1 °C. The subjects in the DSWEG actively performed the same DSWTT program similar to the WEG but in a deep-sea water bath, which was maintained at 34 ± 1 °C (Figure 1). The subjects in the CG underwent dynamic recovery on the ground. The subjects of the study, which were first conducted by research director by group, were thoroughly educated in advance to perform the program with an accurate attitude on the DSWTT program (lower body, upper body, back body, whole body).
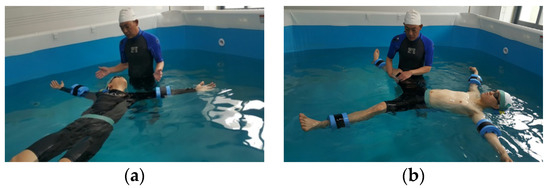
Figure 1.
Deep-sea water thalassotherapy (DSWTT) treatment program: (a) floating; (b) lower body.
The DSWTT program was used by reconstructing the items used by Kim et al. The program was organized in the order of warm-up, treatment (lower body, upper body, back, body stretching and massage treatment), and cool-down phases. The time required for each phase was 2~5 min, lasting a total of 25 min. The DSWTT program consists of 7 lower body exercises, 8 upper body exercises, and 2 full body exercises. Each action was performed by an expert. The composition of the DSWTT program, treatment sites, and the time are shown in Table 2.
In the DSWTT program environment, DSWEG was warmly heated with a regenerative boiler using the unique characteristics and properties of DSW with 34.03% salinity and pH 7.81 water depth of 0.5 m. The main elements included magnesium 1270 mg/L, calcium 367 mg/L, potassium 357 mg/L, sodium 11,033 mg/L, manganese 1.9 mg/L, zinc 0.68 mg/L, and iron 018 mg/L. WEG used tap water supplied by K-water to warm up. Considering the characteristics of this study, a special bathtub was installed in the room 3 × 3 × 1.5 m (horizontal × vertical × height), and the program was applied while maintaining a depth of 1.2 ± 0.1 m.
2.3. Measurement Parameters and Method
2.3.1. Fatigue Inducement Test
The fatigue inducement test was conducted using the 20 m shuttle run test (20 m multi-shuttle run test; 20 m-MST) designed by Leger and Lambert [24] and conducted by the “National Fitness Award 100 Center” of Korea, C university. The 20 m-MST method involves long-distance driving at a length of 20 m and speeds up every minute. The 20 m section was initially measured using a cassette that started to walk at a speed of 8.5 km/h and was set to increase the beep interval by 0.5 km/h every minute. The subjects were started by a beep. It was impossible to continue running according to the beep after running for 20 m based on a regularly accelerating audio rhythm, and when the rhythm could not be followed more than twice (about 3 m behind), the shuttle run test was completed for each individual (Figure 2). After the shuttle run, the recovery program started immediately without a time interval. The number of round trips to and from the finished 20 m section was recorded, and the number of recorded numbers is in Table 3 [25]. The data designed by the study were used, and using Table 3, we calculated the acceleration by section, and the performance ability of the subjects was the difference according to individual difference; so, when the two times did not follow the standard, it was selected as the maximum exercise.

Figure 2.
Fatigue inducement test (20 m shuttle run).

Table 3.
Sectional acceleration of shuttle run test.
2.3.2. Blood Test
Blood tests were performed to ensure the reliability of the test data, and blood was collected by clinical pathologists 3 times in total (Figure 3). The first test was conducted at the laboratory 30 min before the start of the experiment and was collected under a stable time. The second test was performed immediately after the artificial fatigue inducement exercise (20 m shuttle run test). The third test was performed after 25 min of treatment program.

Figure 3.
Blood test.
Blood samples (10 mL each) were collected from the subjects’ upper veins and were centrifuged at 3000 rpm for 5 min and stored at −70 °C until analysis. The samples were collected by the Clinical Pathology Department of the E Medical Foundation (Seoul, Korea), and the fatigue parameters and muscle damage enzymes were analyzed. The fatigue parameters included lactate, ammonia, glucose, and LDH. The muscle damage enzymes included CK and aspartate aminotransferase (AST).
2.3.3. Statistical Analysis
The statistical differences were calculated using the SPSS Version 18.0 program, and the average difference between groups was measured by 3 × 3 repeated measurements of the group and the measurement time as independent variables (two-way ANOVA with the measurements). Measurements were taken three times pre-exercise, post-exercise, and post-treatment. A two-way repeated measures ANOVA was performed when the main effect and the interaction effects between the group and the measurement time were significant. The significance level of statistical analysis was set to α = 0.05.
3. Results
3.1. Fatigue Recovery
The changes in fatigue recovery after the DSWTT program are shown in Table 4.

Table 4.
Changes in fatigue recovery parameters.
Glucose was not significantly different in the analysis of interaction effects between the groups and the measurement time, the main effect according to the measurement time. Lactic acid showed a significant difference in the interaction (p < 0.001) between the group and the measurement time (p < 0.01). However, there was no difference between the groups at the time of measurement. The post hoc analysis showed that DSWEG significantly decreased after program following maximum exercise (p < 0.01), and WEG significantly decreased after CG (p < 0.05).
Ammonia showed a significant difference in the main effect of measurement (p < 0.001) between groups within the measurement time (p < 0.05), and the interaction effect (p < 0.01) between the group and the measurement time. The post hoc analysis showed that DSWEG significantly decreased after the program following maximum exercise (p < 0.01), and WEG significantly decreased after CG (p < 0.05). LDH showed a significant difference in the main effect of measurement (p < 0.001) between groups within the measurement time (p < 0.01), and the interaction (p < 0.01) between the group and the measurement time. The post hoc analysis revealed that DSWEG significantly decreased after the maximum exercise program in the CG and WEG (p < 0.01).
3.2. Enzymes Released in Muscle Damage
The changes in enzymes released in muscle damage following the DSWTT program are shown in Table 5.

Table 5.
Changes in enzyme levels during exercise-induced muscle damage.
CK showed a significant difference in the interaction (p < 0.001) between the groups and the measurement time (p < 0.001). However, there was no difference between the groups at the time of measurement. The post hoc analysis showed that DSWEG significantly decreased after program following maximum exercise (p < 0.01), and WEG significantly decreased after CG (p < 0.05). AST showed a significant difference in the interaction (p < 0.001) between the group and the measurement time (p < 0.001). However, there was no difference between the groups at the time of measurement. The post hoc analysis showed that DSWEG significantly decreased after program-induced loss after maximum exercise (p < 0.01), and WEG significantly decreased after CG (p < 0.05). In this study, subjects in the DSWEG recovered significantly faster than those of the WEG and CG, suggesting that DSW was effective in ameliorating muscle damage as well as promoting fatigue recovery. Thus, the DSWTT program was found to be relevant and necessary not only in elite sports but also to enhance the performance of sports activity.
Elevated serum AST level is a marker of liver or cardiac muscle damage. AST is also known as glutamic oxaloacetic transaminase (GOT), an enzyme present in various cells of the living body facilitating synthesis of amino acids. It increases after drinking or exercising, and it is one of the enzymes associated with muscle damage, which increases in liver disease or muscle disease [26]. AST is present not only in the liver but also in the muscles. It increases when the muscles are damaged after exercise and, therefore, can be used to evaluate exercise-induced stress. The concentration of AST is proportional to exercise intensity or exercise duration [27]. Therefore, DSWEG showed a significant recovery compared to WEG and CG, which may have facilitated the recovery of muscle damage by promoting relaxation of the body through floating, stretching, and massage during the DSWTT program and gently coordinating various movements.
4. Discussion
This study demonstrates that fatigue and muscle damage are induced by a shuttle run test, and following the DSWTT program, the DSWEG showed a significant decrease in lactate, ammonia, LDH, CK, and AST compared to WEG and CG. WEG showed a significant decrease in lactate, ammonia, CK, and AST compared to CG, but CG did not show any difference compared to other groups.
Recently, DSW treatment was used to demonstrate recovery from fatigue and muscle damage using hot spring water [28]. DSW is a resource that maintains a cold temperature at seabeds below a depth of 200 m. DSW is clean seawater that is not contaminated by Escherichia coli or common bacteria. When DSW is applied as an underwater motion, DSW shows buoyancy and water pressure higher than normal water due to eutrophication and salt concentration, and it acts on the human body in a more favorable environment than the general water in the floating posture. Therefore, in this study, DSWTT was applied to the exercise environment in the floating posture and the recovery of fatigue and muscle damage was verified.
Fatigue, a physiological phenomenon that is induced by high-intensity training and long-term exercise, significantly reduces muscle glycogen and blood glucose, which reduces the function of organs and tissues in the human body [29]. In general, the blood–glucose concentration is 80~110 mL/dL when the adult is stabilized. During the exercise, the muscle glycogen is rapidly depleted, resulting in fatigue. If exercise reduces blood sugar, insulin secretion is reduced and blood–glucose level is maintained [30]. In order to determine the changes in the secretion of glucose concentration, this study compared the glucose concentration before, immediately after exercise, and 30 min of exercise. Based on the results of group comparison, the effects were significantly higher in DWEG than in CG.
These results attributed to a decrease in blood sugar level due to the increased demand for energy sources during the one-time exercise. The muscle glycogen levels depleted immediately after exercise reduced the blood–glucose level, as the glucose entered the muscle to supplement the glycogen reserves [31]. Boer and Armstrong [32] reported an increase in the use of blood sugar immediately after exercise, and this study was consistent. Another study [33] showed that the underwater environment strongly facilitated blood flow, human body metabolism, and fatigue parameters by inducing changes in the physiology of the human body depending on the differences between water temperature, water viscosity, density, depth, body parts, and individual. This study also showed that the effect of DSW on fatigue recovery was highly favorable. These results suggest that DSWTT can prevent glycogen depletion in the body and lower the blood lactate acid and ammonia concentration, which are private fatigue substances when recovering and have a positive effect on the improvement of recovery ability after high-intensity fatigue induction training. Recovery from muscle fatigue to improve physical activity quickly eliminates lactate accumulated by energy metabolism and enzyme action in muscles and plays a very important role in improving activities of daily life and control conditions [34]. The accumulation and elimination of blood lactate determines the limitations of muscle movement directly linked to muscle fatigue [35]. Among them, the weight load is reduced due to buoyancy and water pressure. During the underwater exercise, the movement of the joints is larger than on the land, and the effect of stability and stretching is increased [19]. Based on this principle, this study, which verified the effectiveness of DSW, showed that the DSWTT program was effective in eliminating lactate. DSWTT may have increased blood flow to the muscle and improved muscle function. Especially, in the recovery process, it was found that DSWTT was very effective in the recovery of muscle tension and the removal of byproducts of metabolic processes through improvement of varicose reflux rate.
Water therapy has various effects on the physiology of the human body by providing an environment different from the atmosphere such as buoyancy, resistance, water pressure, and water temperature [33]. The results of this study, compared with previous studies [28,36], suggest that the underwater treatment facilitates circulation of blood and lymph to the skin or damaged muscle tissue and ameliorated the pain by relieving muscle spasms. In addition, DSW promotes metabolism and helps blood circulation, such that lactate or waste is discharged more quickly to the outside of the body, and the body is replenished with the required oxygen or nutrients.
Ammonia, one of the fatigue-inducing substances, increases the central fatigue and motor coordination [37]. However, the supply of proteins as energy supplements has been shown to inhibit serotonin production, thereby reducing central fatigue and improving endurance performance [38]. In this study, subjects in the WEG recovered faster than those of the CG group, and the DSWG recovered significantly faster than the WEG and CG. In accordance with Marybetts’ [39] findings that heat takes 15 min to transfer to the muscles, the exposure to 25 min of underwater flooding at 34 ± 1 °C in this study is likely to induce muscle relaxation and eliminate lactate and ammonia. In this study, we discussed the impact of DSWTT program on ammonia level, which is a strong exercise-induced fatigue substance, known to decrease the exercise performance. Participation in the DSWTT program led to rapid excretion of accumulated ammonia. Studies on DSWTT and energy supplementation are needed when BCAA intake induced by fatigue and OKG or albumin are reported to inhibit ammonia accumulation [40].
LDH regulates the formation and conversion of lactate in muscle cells during muscle activity. LDH regulates the formation of lactate by reducing polysaccharide synthesis during anoxic metabolism in muscles and liver [41]. LDH activity in the blood is very low at rest, but if muscle cells are damaged by high-intensity exercise, LDH in the cell is released out of the cell, and LDH activity in the blood is high. Muscle LDH catalyzes the reduction of pyruvate into lactate, which occurs at higher rates when the glycolysis flux increases, such as during muscle contraction. LDH is an intra-blood specific enzyme that can be used to evaluate the energy system in various exercise situations. It represents the degree of adaptation of metabolic function during energy metabolism, exercise intensity, muscle stiffness, fatigue recovery, and excessive training and histological damage analysis [42,43]. In this study, the subjects in DSWEG recovered significantly faster than those in WEG and CG, which confirmed that the DSWTT program using DSW resolved fatigue. When high-intensity fatigue-induced exercise is performed, the muscle is overloaded and affected by cell membrane destruction or tissue necrosis, which increases blood CK and LDH concentrations. LDH is mainly present in red cell and muscle cells, and it is an essential enzyme that produces ATP via the lactic acid system. It plays a role of balancing glucose physicochemical and assimilation by using pyruvate at the final stage of the lactic acid system.
However, an appropriate level of physical activity in various exercises improves performance, but excessive physical activity has a negative effect [44,45]. Typical negative effects include muscle damage due structural damage of muscle fiber [46], followed by protein leakage in muscle tissue [47], and acute inflammation reaction [48]. Muscle damage is accompanied by a decrease in maximum strength, delayed onset muscle soreness (DOMS) [49], and leakage of muscle proteins such as CK, myoglobin (Mb), and AST into the blood [50].
CPK is a non-platelet-specific enzyme that affects muscle damage and inflammation rather than muscle metabolism. CPK concentration increases proportionally as muscle damage increases after high-intensity exercise [51]. Therefore, rapid recovery from accumulated fatigue and muscle damage is an important factor in exercise performance. Body changes due to muscle damage have a negative effect on the participation of the general public in exercise programs and increase psychological discomfort. In order to recover muscle damage quickly, this study is consistent with previous studies [28,33,36], suggesting that water treatment was effective in inducing a psychological sense of stability and a refreshing mood.
In this study, subjects in the DSWEG recovered significantly faster than those of the WEG and CG, suggesting that DSW was effective in ameliorating muscle damage as well as promoting fatigue recovery. Thus, the DSWTT program was found to be relevant and necessary not only in elite sports but also to enhance the performance of sports activity. In the fatigue-inducing 20 m-MST test, the mobilization of the type Ⅰ was greater, and the expression of the type Ⅱ was greater in the weight test. Based on this, it can be explained that if the mobilization of the type Ⅱ is greater than the mobilization of the type Ⅰ, the fatigue induction and the muscle itself are more damaged.
Elevated serum AST level is a marker of liver or cardiac muscle damage. AST is also known as glutamic oxaloacetic transaminase (GOT), an enzyme present in various cells of the living body facilitating synthesis of amino acids. It increases after drinking or exercising, and it is one of the enzymes associated with muscle damage, which increases in liver disease or muscle disease [26]. AST is present not only in the liver but also in the muscles. It increases when the muscles are damaged after exercise and, therefore, can be used to evaluate exercise-induced stress. The concentration of AST is proportional to exercise intensity or exercise duration [27]. Therefore, DSWEG showed a significant recovery compared to WEG and CG, which may have facilitated the recovery of muscle damage by promoting relaxation of the body through floating, stretching, and massage during the DSWTT program and gently coordinating various movements.
In conclusion, the DSWTT program had a significant effect on fatigue and muscle damage recovery, suggesting that application of the program in hot springs can contribute to national health care and promotion and quality of life. Further studies are needed to investigate DSWTT programs with various research subjects at different program temperatures, exercise times, and frequencies of treatment and exercise. In addition, it is necessary to analyze the effects of DSWTT program to maintain the refreshing state of various classes, especially the weak and the elderly.
DSWTT application is a useful seawater resource with low temperature, cleanliness, stability, eutrophicity, minerality, and aging properties in the mechanism that affects the recovery of fatigue of human body. It is a huge clean resource that is generated from the material circulation system with solar energy as a source of energy and is produced as seawater and recycled as sea water. These DSW show higher buoyancy and hydrostatic pressure than normal water due to eutrophicity and salinity concentration, and stretching and massage performance in underwater floating posture can be interpreted as more effective in restoring human fatigue than normal water. In addition, when stretching or massage activities in DSW compared to the normal water, it means that the fatigue material generated in the muscle recovered more quickly.
The most important characteristic of DSW in the mechanism that DSWTT application affects the recovery of muscle damage in human body is that it maintains stable low temperature and has little organic matter such as bacteria and pathogens, and it is applied to the treatment of muscle damage by applying artificial heat, as sea water is rich in minerals such as nutrients and minerals essential for the growth of marine plants. This suggests that warm DSW is more stable than normal water in DSWTT program, and that the relaxation of the body through stretching and muscle massage provides conditions for muscle damage recovery.
There is a limitation of not comparing other evaluation parameters in this study. However, in this study, a study was conducted to evaluate factors such as glucose, lactate acid, ammonia, LDH, CK, and AST, which are very important for physiological fatigue. It is considered to require a study to evaluate the different parameters mentioned later.
Author Contributions
Conceptualization, W.-s.S.; Data curation, S.-J.K.; Formal analysis, A.-R.C.; Investigation, J.-H.J. and H.-j.E.; Methodology, B.K.; Writing—original draft, N.-I.K.; Writing—review & editing, S.-S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was funded by the Ministry of Oceans and Fisheries in 2016 and supported by the Korea Institute of Marine Science & Technology Promotion (Task Number: 20160300).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kang, S.R.; Jeong, G.Y.; Bae, J.J.; Min, J.Y.; Yu, C.H.; Kim, J.J.; Kwon, T.K. Effect of muscle function and muscular reaction of knee joint in the twenties on the whole body vibration exercise. J. Korean Soc. Precis. Eng. 2013, 30, 762–768. [Google Scholar] [CrossRef]
- Cho, J.S.; Kwon, T.K.; Hong, J.P. A study of evaluation index development of healthcare rehabilitation device design. Korea Soc. Emot. Sensib. 2014, 17, 129–142. [Google Scholar] [CrossRef]
- Kathryu, A.; Lentz, M.J.; Taylor, D.L. Fatigue as a response to environmental demands in women’s lives image. J. Nurs. Scholarsh. 1994, 26, 149–154. [Google Scholar]
- Blesch, K.S.; Paice, J.A.; Wickham, R.; Harte, N.; Schnoor, D.K.; Purl, S.; Rehwal, T.M.; Kopp, P.L.; Manson, S.; Coveny, S.B. Correlates of fatigue in people with breast or lung cancer. Oncol. Nurs. Forum 1991, 18, 81–87. [Google Scholar]
- Youn, B.B.; Kang, H.C.; Shin, K.K.; Lee, K.S. An analysis of fatigue among outpatients. Korean J. Fam. Med. 1999, 20, 978–990. [Google Scholar]
- Larun, L.; Brurberg, K.G.; Odgaard-Jensen, J.; Price, J.R. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst. Rev. 2017, 25, 1–4. [Google Scholar]
- Kay, D.; Marino, F.E.; Cannon, J.; Gibson, A.S.C.; Lambert, M.I.; Noakes, T.D. Evidence for neuromuscular fatigue during high-intensity cycling in warm, humid conditions. Eur. J. Appl. Physiol. 2001, 84, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ohkuwa, T.; Miyamura, M. Plasma LDH activity and LDH isozymes after 400m and 3,000m runs in sprint and long distance runners. J. Sports Med. Phys. Fit. 1986, 26, 362–368. [Google Scholar]
- Kim, J.K.; Moon, H.W. Effect of Blood fatigue factors following eccentric exercise on delayed muscle damage. Exerc. Sci. 2004, 13, 251–262. [Google Scholar]
- Kim, I.G. The effect of sauna and half-bath participation on systolic blood pressure, heart rate and vascular elasticity of middle-aged men. Korean Sports Res. 2006, 17, 319–327. [Google Scholar]
- Cha, S.W.; Shin, S.K.; Lim, I.S. The effect of passive recovery, massage, cold & hot bath and aroma therapy on fatigue metabolic substrate after 10km running. J. Exerc. Nutr. Biochem. 2006, 10, 37–42. [Google Scholar]
- Darryl, J. Alternating hot and cold water immersion for athlete recovery: A review. Phys. Ther. Sports 2004, 5, 26–32. [Google Scholar]
- Mang, H.J. Cortisol and testosterone changes in cold therapy after muscle fatigue induced. Korean J. Phys. Educ. 2002, 41, 317–323. [Google Scholar]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Buonauro, R.; Limongelli, F.M. Serum enzyme monitoring in sports medicine. Clin. Sports Med. 2008, 27, 1–18. [Google Scholar] [CrossRef]
- Lippi, G.; Schena, F.; Salvagno, G.L.; Montagnana, M.; Gelati, M.; Tarper, I.C.; Banfi, G.; Guidi, G.C. Acute variation of biochemical markers of muscle damage following a 21-km, half-marathon run. Scand. J. Clin. Lab. Investig. 2008, 68, 667–672. [Google Scholar] [CrossRef]
- Yeom, I.H. Effect of Exercise Intensity on Inflammatory Marker and Immunoglobulin. Master’s Thesis, Incheon National University, Incheon, Korea, 2011. Unpublished. [Google Scholar]
- Lee, S.K.; Chung, E.Y. The effect of Ai Chi aquatic exercise to the level of human stress and muscle activities. Korean J. Wellness 2014, 9, 131–137. [Google Scholar]
- Hay, L.; Wylie, K. Towards evidence-based emergency medicine: Best BETs from the Manchester Royal Infirmary. BET 4: Hydrotherapy following rotator cuff repair. Emerg. Med. J. 2011, 28, 634–635. [Google Scholar]
- Jun, S.Y.; Lee, K.S.; Nam, K.S. Efficacy testing for tarasotherapy in deep sea water. In Proceedings of the Korean Marine Environmental Engineering Society, Seoul, Korea, 21 May 2017. [Google Scholar]
- Weinberg, R.; Jackson, A. The relationship of massage and exercise to mood enhancement. Sports Psychol. 1988, 2, 202–211. [Google Scholar] [CrossRef]
- Dubrovsky, V.I. Changes in muscle and venous blood flow after massage. Sov. Sports Rev. 1982, 4, 56–57. [Google Scholar]
- Mooenthan, A.; Nivethitha, L. Scientific evidence-based effects of hydrotherapy on various systems of the body. N. Am. J. Med. Sci. 2014, 6, 199–209. [Google Scholar] [CrossRef]
- Leger, L.A.; Lambert, J. A maximal multistage 20m shuttle run test to predict VO2max. Eur. J. Appl. Physiol. 1982, 49, 1–12. [Google Scholar] [CrossRef]
- Brewer, J.; Ramsbottom, R.; Williams, C. Multistage Fitness Test; National Coaching Foundation: Leeds, UK, 1988. [Google Scholar]
- Armstrong, R.B. Initial event in exercise induced muscular injury. Med. Sci. Sport Exerc. 1990, 22, 429–435. [Google Scholar]
- Barranco, T.; Tvarijonaviciute, A.; Tecles, F.; Carrillo, J.M.; Sánchez-Resalt, C.; Jimenez-Reyes, P.; Rubio, M.; García-Balletbó, M.; Cerón, J.J.; Cugat, R. Changes in CK, LDH and AST in saliva samples after an intense exercise: A pilot study. J. Sports Med. Phys. Fit. 2017, 5, 2441–2455. [Google Scholar]
- Lee, S.S.; Kim, J.T.; Shin, W.S.; Kim, N.I.; Ryu, O.S.; Jang, J.H. Changes of trunk ROM and maximal muscular strength after deep sea water thalassotherapy. Korean Soc. Growth Dev. 2017, 25, 353–361. [Google Scholar]
- Gibson, H.; Edwards, R.H.T. Muscular exercise and fatigue. Sports Med. 1985, 2, 120–132. [Google Scholar] [CrossRef]
- Heath, G.W.; Gavin, J.R., III; Hinderliter, J.M.; Hagberg, J.M.; Bloomfield, S.A.; Hollozy, J.O. Effect of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J. Appl. Physiol. 1983, 55, 512–517. [Google Scholar] [CrossRef]
- Praet, S.F.; van Loon, L.J. Exercise therapy in type 2 diabetes. Acta Diabetol. 2009, 46, 263–278. [Google Scholar] [CrossRef]
- Borer, J.; Armstrong, P. Proceedings of the 99th meeting of the Food and Drug Administration Cardiovascular and Renal Drugs Advisory Committee. Circulation 2003, 107, e9052. [Google Scholar] [CrossRef]
- Jung, B.K. Understanding of aquatic rehabilitation movement. Korean Assoc. Certif. Exerc. Prof. Annu. Meet. 2002, 1, 13–17. [Google Scholar]
- Aslan, A.; Acikada, C.; Güvenç, A.; Gören, H.; Hazir, T.; Ozkara, A. Metabolic demands of match performance in young soccer players. J. Sports Sci. Med. 2012, 11, 170–179. [Google Scholar]
- Park, H.S.; Kim, M.K.; Shim, B.C.; Chae, J.R.; Cho, S.C.; Jun, H.Y.; Kim, H.J. A comparative analysis of blood lactate, LDH, and glucose before and after treadmill exercise in athletics. J. Dongui Physiol. 2006, 20, 1254–1260. [Google Scholar]
- Park, J.O. The Effect of Sports Massage on the Recovery of Fatigue and Injury Prevention of Dancers. Master’s Thesis, Kyungseung University, Busan, Korea, 2001. Unpublished. [Google Scholar]
- Meneguello, M.O.; Mendonca, J.R.; Lancha, A.H., Jr.; Costa Rosa, L.F. Effect of arginine, ornithine and citrulline supplementation upon performance and metabolism of trained rats. Cell Biochem. Funct. 2003, 21, 85–91. [Google Scholar] [CrossRef]
- Blomstrand, E.; Celsing, F.; Newshorme, E.A. Changes in concentration of aromatic and branched chain amino acid during sustained exercise in man and their possible role in fatigue. Acta Physiol. Scand. 1998, 33, 115–121. [Google Scholar] [CrossRef]
- Marybetts, S. Modern Hydrotherapy for the Massage Therapist; Lippincott Williams & Wilkins, Inc.: Philadelphia, PA, USA, 2008. [Google Scholar]
- Cho, S.Y.; Paik, I.Y.; Woo, J.H.; Kim, K.S. The effects of BCAA and additional OKG or albumin supplements on blood fatigue factors and energy substrates. Korean J. Sport Sci. 2004, 15, 1–10. [Google Scholar]
- Everse, J.; Kaplan, N.O. Mechanism a of action and biological function of various dehydrogenease isozymes. In Isozymes Physiological Function; Markert, C.L., Ed.; Academic Press: New York, NY, USA, 1975; pp. 29–44. [Google Scholar]
- Hooloszy, J.O.; Booth, F.W. Biochemical adaptation to endurance exercise in muscle. Ann. Rev. Physiol. 1976, 22, 623–627. [Google Scholar] [CrossRef]
- Apple, P.F.; Rogers, M.A. Skeletal muscle lactate dehydrogenase isozyme alterations in men and women marathon runners. J. Applex Physiol. 1986, 61, 477–481. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Witt, E.; Matsumoto, M.; Packer, L. Vitamin E inhibits protein oxidation in skeletal muscle of resting and exercise rats. Biochem. Biophys. Res. Commun. 1992, 189, 801–806. [Google Scholar] [CrossRef]
- Jun, Y.K.; Lee, K.H. Effect of different methods of recovery after high strength aerobic exercise on antioxidant enzymes. Korean J. Phys. Educ. Sci. 2014, 23, 1127–1135. [Google Scholar]
- Jaworski, C.A. Medical concerns of marathons. Curr. Sports Med. Rep. 2005, 4, 137–143. [Google Scholar] [CrossRef]
- Sorichter, S.; Puschendorf, B.; Mair, J. Skeletal muscle injury induced by eccentric muscle action: Muscle proteins as markers of muscle fiber injury. Exerc. Immunol. Rev. 1999, 5, 5–21. [Google Scholar]
- McIntyre, K.W.; Shuster, D.J.; Gillooly, K.M.; Warrier, R.R.; Connaughton, S.E.; Hall, L.B.; Arp, L.H.; Gately, M.K.; Magram, J. Reduced incidence and severity of collagen-induced arthritis in interleukin-12-deficient mice. Eur. J. Immunol. 1996, 26, 2933–2938. [Google Scholar] [CrossRef]
- Howatson, G.; Hoad, M.; Goodall, S.; Tallent, J.; Bell, P.G.; French, D.N. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: A randomized, double-blind, placebo controlled study. J. Int. Soc. Sports Nutr. 2013, 9, 20. [Google Scholar] [CrossRef]
- Matsumoto, K.; Koba, T.; Hamada, K.; Sakurai, M.; Higuchi, T.; Miyata, H. Branched-chain amino acid supplementation attenuates muscle soreness, muscle damage and inflammation during an intensive training program. J. Sports Med. Phys. Fit. 2009, 49, 424–431. [Google Scholar]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).